The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process
The TFIIH-associated kinase Cdk7 is found to switch androgen receptor fate and function: AR phosphorylation promotes recruitment of the ubiquitin ligase Mdm2 and cyclic transcription, while lack of AR phosphorylation recruits another ubiquitin ligase, CHIP, into the transactivation complex.
Keywords: androgen receptor, E3 ligases, phosphorylation, proteasome, TFIIH
Abstract
In response to hormonal stimuli, a cascade of hierarchical post-translational modifications of nuclear receptors are required for the correct expression of target genes. Here, we show that the transcription factor TFIIH, via its cdk7 kinase, phosphorylates the androgen receptor (AR) at position AR/S515. Strikingly, this phosphorylation is a key step for an accurate transactivation that includes the cyclic recruitment of the transcription machinery, the MDM2 E3 ligase, the subsequent ubiquitination of AR at the promoter of target genes and its degradation by the proteasome machinery. Impaired phosphorylation disrupts the transactivation, as observed in cells either overexpressing the non-phosphorylated AR/S515A, isolated from xeroderma pigmentosum patient (bearing a mutation in XPD subunit of TFIIH), or in which cdk7 kinase was silenced. Indeed, besides affecting the cyclic recruitment of the transcription machinery, the AR phosphorylation defect favourizes to the recruitment of the E3 ligase CHIP instead of MDM2, at the PSA promoter, that will further attract the proteasome machinery. These observations illustrate how the TFIIH phosphorylation might participate to the transactivation by regulating the nuclear receptors turnover.
Introduction
Following induction by ligand, nuclear hormone receptors (NR) target their responsive elements to initiate the formation of a large preinitiation complex, including co-activators, histone-modifying enzymes, mediator, RNA polymerase II (RNA pol II) and the general transcription factors TFII-A, -B, -D, -E, -F and -H (Lemon and Tjian, 2000). TFIIH, also known as a DNA repair factor, is a multiprotein complex composed of two subcomplexes: the core (containing the helicase XPB, p62, p52, p44, p34 and p8) and the cdk-activating kinase complex CAK (containing MAT1, cyclin H and the cdk7 kinase). Whereas the XPB helicase is devoted to the promoter opening (Holstege et al, 1996; Coin et al, 1999), cdk7 phosphorylates the C-terminal domain of the largest subunit of RNA pol II (Lu et al, 1992) thereby facilitating the promoter clearance, as well as NRs, which is an essential step for transactivation of target genes (Rochette-Egly et al, 1997).
Mutations in some of the transcriptional components disrupt protein/protein interaction and/or reversible post-translational modifications (PTMs) that are essential for complex maintenance and its further activation. This situation is well illustrated for TFIIH. Indeed, mutations in the C-terminal domain of the XPD subunit of TFIIH disturbs the architecture of TFIIH and its molecular communication with the retinoic acid receptor α (RARα) (Keriel et al, 2002), the peroxysome proliferator-activated receptors (PPARs) (Compe et al, 2005), the estrogen receptor (Chen et al, 2000) or the thyroid hormone receptors (Compe et al, 2007), leading to the dysregulation of cdk7-related phosphorylation. Defects in the expression of these NRs responsive genes might explain part of the broad range of clinical features of the human genetic disorders such as xeroderma pigmentosum (XP) or trichothiodystrophy (TTD) (Lehmann, 2001). Some of the symptoms such as hypogonadism, sterility, growth retardation, hypoplasy of the adipose tissue and bone abnormalities could be related to an androgen response deficiency.
Androgen steroid hormones that induce the androgen receptor (AR) activity are involved in the development and maintenance of male reproductive organs but also in adipose tissue, skeletal muscle and the bone homeostasis (Mooradian et al, 1987; Brinkmann, 2001; Matsumoto et al, 2008; Vanderschueren et al, 2008). AR and its co-regulators exert their action during the development of normal prostate and in the initiation and progression of prostate cancer, the most common cancer in men in western countries (Heinlein and Chang, 2004; Balk and Knudsen, 2008). As most of the steroid hormone receptors, AR is characterized by a conserved structural and functional organization (Mangelsdorf et al, 1995; Kumar et al, 2004): a heterogeneous N-terminal A/B domain that contains a ligand-independent transactivation domain (AF-1 domain), a highly conserved DNA-binding domain, a homo- and heterodimerization domain, and a large C-terminal ligand-binding domain that harbours a ligand-inducible transactivation function (AF-2 domain). All these domains are subjected to PTMs, such as phosphorylation, acetylation, sumoylation and ubiquitination (Faus and Haendler, 2006).
The main function of these PTMs is to regulate either positively or negatively the interaction between the various biomolecules present in the cell thus providing signals to initiate a given mechanism that is essential for transactivation. Besides being targeted by several protein kinases, NRs are also substrates for E3 ubiquitin ligases (Gaughan et al, 2005; Bour et al, 2007) that engages the ubiquitin–proteasome process during the waves of transcription complex formation (Kodadek et al, 2006). Such process is initiated by protein ubiquitination involving an activating enzyme (E1) and a conjugating enzyme (E2), which relays ubiquitin to the substrate in the presence of an E3 ligase (Hochstrasser, 2009). Polyubiquitinated proteins next bind to the 19S regulatory particle of the proteasome and are then unfolded in an ATP-dependent manner through the action of the ATPases that sit atop the opening to the 20S core particle cavity part of the 26S proteasome (Baumeister et al, 1998). One prominent intersection between phosphorylation and ubiquitination is likely to regulate the turnover of NRs. Whether a crosstalk between these two PTMs occurs and how it might regulate the AR transcriptional activity remains unclear.
In this study, we show how the phosphorylation of AR (at position S515), by the cdk7 kinase of TFIIH, influences the specific recruitment of the mouse homologue of double minute 2 protein (MDM2) E3 ligase and the proteasome to the prostate-specific antigene PSA promoter, one of the most studied AR responsive genes and participates in the regulation of its turnover. Impairing AR/S515 phosphorylation, results in transactivation defect due to an abnormal recruitment of the transcription machinery. In this case, we found that the recruitment of CHIP, another E3 ligase, at the promoter of AR responsive gene is favourized. This results in the dysregulation of the turnover of AR and the cyclic transactivation of its target gene PSA, explaining at least partially some of the mechanistic defects leading to XP or TTD patients.
Results
Specificity in the TFIIH/AR interaction during transactivation
We first investigated the potential effect of XPD mutations on AR transactivation using human primary fibroblasts isolated from XP and TTD patients. The PSA promoter including its androgen responsive elements (AREs) was cloned upstream the luciferase reporter gene (pGL3. PSA-Luc) and transfected together with a vector expressing AR (pSV.AR), and the β-galactosidase encoding vector as an internal control. Following 5α-dihydrotestosterone (DHT) induction, we observed that AR transactivation was largely reduced in XPD/R683W cells in which XPD is mutated at position R683W but not in cells carrying mutations at position XPD/R722W and XPD/R112H. In the latter cell lines, the androgen response reached the same level as in XPD/WT cells (Figure 1A, left panel). We also analysed the effect of these mutations on the expression of a luciferase reporter gene under the control of RARα (Keriel et al, 2002). Here, XPD/R683W as well as XPD/R722W mutations affected RARα transactivation (Figure 1A, right panel), suggesting that XPD mutations differentially disrupted the transactivation mediated by a given nuclear receptor.
Figure 1.
The transactivation and the phosphorylation of AR are disrupted by the XPD/R683W mutation. (A) GM03348D (XPD/WT), TTD12PV (XPD/R112H), XPJCLO (XPD/R683W) and TTD8PV (XPD/R722W) fibroblasts were transiently co-transfected with 100 ng of pGL3.PSA-Luc, pCMV.β-gal and either pSG5.AR (AR) or pSG5.RARα (RARα) or the corresponding empty vector. The cells were then treated with a specific ligand for AR (DHT, 10−7 M) or RARα (t-RA, 10−8 M). Luciferase activity was measured 24 h later and normalized to β-galactosidase activity. The results are the mean of three different experiments. (B) In vivo phosphorylation of AR was investigated in HeLa (WT), HD2 (XPD) and XPD cells overexpressing XPD/WT upon AR immunoprecipitation, autoradiography ([32P]-AR) and western blotting (AR). Quantitative analysis of AR phosphorylation in WT and XPD cells represents the ratio autoradiography/western blot signals ([32P]-AR/AR). (C) AR interacts with TFIIH. (Left panel) Endogenous AR was immunoprecipitated (IP:AR, lane 5) from LNCaP cells and after washes (150 mM NaCl), co-precipitated TFIIH was detected using an anti-XPB antibody. The control IP (IP neg, lane 4) was performed with a non-specific antibody. The input (lane 1) represents 10% of the total volume of LNCaP extract used for the immunoprecipitation. His-AR (lane 2) and purified XPB (lane 3) were used as size markers for the immunodetection of the endogeneous AR and XPB in LNCaP cells. (Right panel) Highly purified-recombinant AR was incubated with Sf9 cell extracts overexpressing TFIIH complex containing a FLAG-tagged p34, in the presence or absence of DHT (10−6 M). Immunoprecipitation was performed using an anti-FLAG antibody (IP:IIH) and after extensive washes (300 mM NaCl) and SDS–PAGE, bound proteins were detected with an anti-AR, XPD, p62, p44 and cdk7 antibodies. (D) Equal amounts of highly purified-recombinant AR was incubated with Sf9 cell extracts overexpressing separately the subunit XPB, XPD, p44 or cdk7 of TFIIH in the presence or absence of DHT (10−6 M). After AR immunoprecipitation, co-precipitated TFIIH subunits were detected by western blot. The control IP (lane 1) was performed without AR. (E) AR, A/B.AR, ARΔA/B, PPARα or A/B.AR S515A were incubated with purified-recombinant TFIIH in the presence or absence of DHT (10−6 M) as indicated. After SDS–PAGE, each protein was resolved by Coomassie blue staining (Stain.) and radioactive labelling was analysed by autoradiography (Auto.). The complete Coomassie blue stainings and western blots of the different purified-recombinant proteins are shown in Supplementary Figure S1A. (F) WT (HeLa) and XPD (HD2) cells were transiently co-transfected with 100 ng of pGL3.PSA-Luc, pCMV.β-gal and pSG5 AR/WT, /S515A, or /S515E before DHT (10−7 M) treatment. The results were obtained as described in A.
We then investigated the phosphorylation status of AR during transactivation (Figure 1B). AR-transfected WT and XPD/R683W (named XPD) cells were incubated with [32P]-orthophosphate and collected over time after DHT treatment. AR was then immunoprecipitated (IP) from the corresponding cell extracts, resolved by SDS–PAGE and analysed by autoradiography and western blot. We repeatedly observed that AR phosphorylation was weaker in XPD cells compared with WT cells (Figure 1B, lanes 1–6; see also the histogram depicting the ratio ([32P]-AR/AR). This defect was compensated in XPD cells, by overexpressing XPD/WT (Figure 1B, compare lane 9 with lanes 6 and 3), indicating a role for XPD and by extension of TFIIH in the phosphorylation of AR.
We then asked whether AR could functionally and physically interact with TFIIH in a relevant physiological context (Figure 1C). After immunoprecipitation of AR from human prostate adenocarcinoma LNCaP cells (which endogenously express AR), we clearly detected the presence of TFIIH (Figure 1C, left panel, lane 5). Non-specific antibodies were unable to immunoprecipitate AR/TFIIH (Figure 1C, left panel, lane 4). In parallel, we also found that AR co-immunoprecipitates in a ligand-independent manner with recombinant TFIIH, as detected by antibodies directed against XPD, p62, p44 or cdk7 subunits of TFIIH (Figure 1C, right panel). We further investigated which TFIIH subunits interact with AR. Each of the 10 subunits of TFIIH was separately overexpressed in insect cells and incubated with the recombinant AR. We found that XPB, XPD and p44 interacted with AR (Figure 1D); the interaction between AR and the other TFIIH subunits was either much weaker (as observed for cdk7) or absent (data not shown). Interestingly, the pattern of interaction between AR and the TFIIH subunits differs from that observed for other NRs, underlying how specific was the interaction between an NR and TFIIH (Supplementary Table I).
The above results prompted us to ask whether AR could be a substrate for the cdk7 kinase of TFIIH. In vitro kinase assays showed that TFIIH, via its cdk7 kinase subunit, phosphorylated AR (Figure 1E, lanes 1–4), an event that was also observed for another NR such as PPARα (lanes 12 and 13). TFIIH phosphorylated A/B domain of AR (A/B.AR) but not the truncated form of AR lacking the A/B domain (ARΔA/B, compare lanes 8–11 with lanes 6–7). A careful screening of the 560 residues of the A/B domain followed by systematic mutagenesis (data not shown) suggested that among the various serine/threonine candidates, serine 515 (S515) is a potential phosphorylation site for cdk7, a proline-directed kinase (Morgan, 1997). Accordingly, when we mutated the S515 into alanine (AR/S515A), the phosphorylation of A/B.AR was largely reduced (compare lanes 16 and 18).
To determine the role of the S515 residue in AR-mediated transcription, the pGL3.PSA-Luc luciferase reporter plasmid together with either pSV.AR/S515A or pSV.AR/S515E (in which S515 was mutated into an alanine or a glutamic acid that mimics a non-phosphorylated or a constitutive phosphorylated AR, respectively), was co-transfected in both WT and XPD cells (Figure 1F). Following DHT induction, AR/S515A did not accurately transactivate in either WT or XPD cells. On the contrary, we observed in XPD cells that AR/S515E compensated the transactivation defect observed with AR/WT.
In addition to demonstrating that the cdk7 kinase of TFIIH specifically phosphorylates AR at position S515 that promote transactivation, the above data also show that the interaction between the NRs and TFIIH is receptor specific, rather than ‘universal' and that the effect of a given mutation in TFIIH during NRs transactivation depends on both the nature of the mutation and the nature of the NRs.
Phosphorylation of AR regulates its turnover
Phosphorylation has a major role in the regulation of steroid receptor stability (Weigel and Moore, 2007). We were therefore wondering whether a defect in the phosphorylation of the activation domain of AR would affect its stability. We examined the turnover of AR protein by conventional pulse chase at different times following ligand exposure. Twenty-four hours after transfection with the various AR expression vectors, WT- and XPD-deficient cells were metabollically labelled with 35S-methionine for 1 h and then treated with the AR ligand. Cells were collected at different times of treatment and AR was IP before being resolved by SDS–PAGE and autoradiographied. Newly synthesized 35S-AR/WT was detected until the first hour in WT cells while in XPD cells, AR labelling was visible until 2/4 h post-DHT induction (Figure 2A and B, lower panels). To localize 35S-AR/WT, western blots were performed in parallel (Supplementary Figure S2). A control pulse chase realized in WT and XPD cells, which have not been transfected, is also presented (Figure 2A and B, upper panels). Interestingly, the transfection in XPD cells of either AR/S515E or XPD/WT (together with AR/WT), that reestablished both the phosphorylation status and the transactivation process of AR (Figure 1B and F), restored the half-life of AR to a similar level of that observed in normal cells for AR/WT (compare Figure 2D and F with Figure 2A). Strikingly, we repeatedly observed that in WT cells, the labelling of 35S-AR/S515A (in which the phosphorylated serine site was abrogated) was visible past 2 h post-DHT treatment (Figure 2C).
Figure 2.
Pulse chase of AR protein in WT and XPD cells. (A–F) WT (A, C) and XPD cells (B, D) were transiently transfected in order to overexpress AR/WT (A, B, lower panels), AR/S515A (C) or AR/S515E (D). XPD cells were also co-transfected in order to simultaneously overexpress AR/WT and XPD/WT (F). Control experiments were performed with cells transfected with empty vectors (A, B, upper panels). Following a [35S] pulse, cells were maintained in the presence of DHT (10−7 M) for the indicated time points (0, 0.5, 1, 2, 4 and 8 h). After immunoprecipitation, AR was resolved by SDS–PAGE and [35S] labelling quantified with a phosphoimager. Western blots were performed in parallel to localize AR (see Supplementary Figure S2). Arrows indicate the position of the radiolabelled [35S]-AR. Graphs depict AR protein levels normalized to that observed in absence of DHT treatment (arbitrary units, au). The values are the mean±s.e.m. of three independent experiments. (E) Summary of AR turnover following DHT treatment in WT cells overexpressing either AR/WT (black curve) or AR/S515A (blue curve), and in XPD cells overexpressing either AR/S515E (yellow curve) or AR/WT in the absence (red curve) or presence of XPD/WT (green curve). The [35S]-AR levels are presented as percentages, 100% being the [35S]-AR levels in absence of DHT treatment. Data are the mean±s.e.m. of three independent experiments.
Altogether, our results suggest that a deficiency in the AR phosphorylation, resulting from either mutation in the XPD subunit of TFIIH or abrogation of the AR/S515 phosphorylation site, prolongs the turnover of AR following ligand induction (Figure 2E).
Selective recruitment of the ubiquitin ligase at the PSA promoter
The above results prompted us to analyse the expression of endogenous gene known to be under the control of AR in WT and XPD cells. Following DHT treatment, PSA mRNA cyclically peaked at 2 and 16 h in WT cells (Figure 3A), while in XPD cells, the PSA mRNA synthesis only peaked at 1 h and then slowly decreased (Figure 3B). Strikingly, the transfection in XPD cells of either XPD/WT (Figure 3C) or AR/S515E (Figure 3D) restored the profile of the mRNA synthesis observed in WT cells (Figure 3A).
Figure 3.
AR phosphorylation status selectively promotes the recruitment of ubiquitin–proteasome components at the PSA promoter. WT and XPD cells were transiently transfected to overexpress either AR/WT, AR/WT together with XPD/WT or AR/S515E (as indicated at the top of each panel). (A–D) Expression of the PSA gene: RT–qPCR analysis was performed at indicated times after DHT (10−7 M) treatment. The values were normalized relative to the GAPDH mRNA expression. The results of three independent experiments are presented as n-fold induction relative to non-treated cells. (E–H) After DHT treatment, the recruitment of RNA pol II (yellow curve), TFIIH (via its XPB and cdk7 subunits, blue and green curve, respectively) and AR (red curve) were analysed by ChIP assays at the PSA proximal promoter. The results are presented as percentage of DNA immunoprecipitated relative to the input (% input). (I–L) Recruitment of the MDM2 (green curve) and CHIP (yellow curve) E3 ligases on the PSA promoter. The recruitment of the ubiquitinated AR-containing fraction (brown curve) was also analysed by ChIP/re-ChIP assays using first an anti-ubiquitin antibody and second with an anti-AR antibody. (M–P) Recruitment of SUG1 (red curve), β5 (light blue) and S1 (dark blue) subunits of the proteasome at the PSA promoter was analysed by ChIP assays. Note that the recruitment of S1 has not been analysed in XPD cells transiently co-transfected with plasmids encoding AR/WT and XPD/WT (O).
The transactivation of NR-target genes involves phosphorylation by specific kinases, ubiquitination by E3 ligases and degradation by the proteasome (Kodadek et al, 2006; Bour et al, 2007). We thus studied the dynamic recruitment of the various components of the transcriptional complex at the PSA promoter over time by chromatin immunoprecipitation (ChIP) assays analysed by quantitative PCR. In WT cells, AR, TFIIH (as visualized by the presence of its XPB and cdk7 subunits) as well as RNA pol II, were recruited to the PSA promoter at 2 and 16 h post-ligand induction (Figure 3E); their concomitant recruitments paralleled the PSA mRNA synthesis (Figure 3A). Conversely, in XPD cells, AR strongly accumulated after 2 h of DHT treatment to progressively decrease during the following 20 h (Figure 3F). Here, the recruitment of TFIIH paralleled the one observed for AR. It is worthwhile to mention that the recruitment pattern of RNA pol II (Figure 3F) as well as of p300 (a co-activator of AR, data not shown) (Popov et al, 2007) were strongly disturbed in XPD cells when compared with that observed in WT cells (compare Figure 3F and E). Interestingly, when overexpressing either XPD/WT (together with AR/WT) or AR/S515E in XPD cells, we observed the restoration of the biphasic recruitment of AR, RNA pol II and TFIIH (compare Figure 3G and H with Figure 3E).
We also investigated whether defects in the AR phosphorylation impact its targeting by the ubiquitin–proteasome pathway. We particularly focused our attention on the MDM2 (the human homologue of the ‘Murine double minute') and CHIP (C-terminus of Hsc70-interacting protein) E3 ligases, which are known to interact with AR in a phosphorylation-dependent manner (Lin et al, 2002; Rees et al, 2006) and to be associated with active PSA promoter (Kang et al, 2002; Gaughan et al, 2005). We found that MDM2 was mainly recruited with AR after DHT induction in WT cells (Figure 3I). Surprisingly, in XPD cells, in addition to the MDM2 recruitment, we also observed the recruitment of CHIP, which was hardly detected in WT cells (Figure 3J and I). It should be noticed that in both WT and XPD cells, the cellular concentration of MDM2 and CHIP was similar (unpublished results). Furthermore, upon expression in XPD cells, of either AR/WT (+XPD/WT) or the constitutive phosphorylated AR/S515E, the preferential MDM2 cyclic recruitment was restored (Figure 3K and L). ChIP/re-ChIP analysis using first an anti-ubiquitin antibody and second an anti-AR antibody showed that the AR-containing fraction that was ubiquitinated, paralleled the recruitment of the E3 ligases (Figure 3I–L).
ChIP assays next revealed that the presence of S1 and SUG1, two regulatory subunits of the 19S proteasome and the β5 catalytic subunit of the 20S proteasome (Baumeister et al, 1998; Gianni et al, 2002; Kang et al, 2002) was concomitant to the presence of the MDM2 or CHIP E3 ligases (Figure 3M–P), when AR is at the PSA promoter.
The AR/E3 ligases relationship
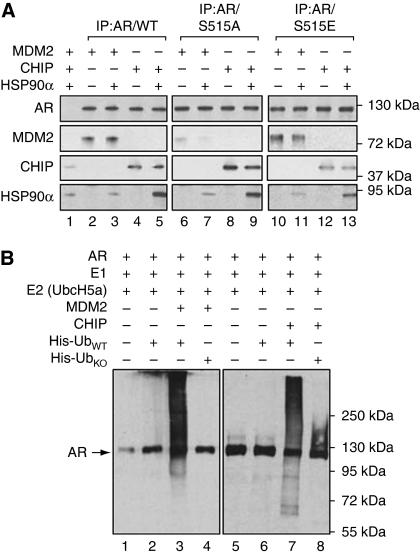
The above data underline a link between the phosphorylation status of AR and the selective recruitment of E3 ligases that direct the ubiquitination and the recruitment of the proteasome machinery at the PSA promoter. To address the effect of protein interactions in these settings, we first overexpressed AR/WT, /S515A or /515E in DHT-treated HeLa cells. After immunoprecipitation and extensive washes (300 mM KCl), the different forms of AR were incubated with either MDM2 or CHIP E3 ligases (see Materials and methods). We found that MDM2 exhibits a higher affinity for AR/WT and AR/S515E contrary to what we observed for AR/S515A (Figure 4A, compare lanes 2–3 and 10–11 with lanes 6–7). Conversely, CHIP is able to interact with the three forms of AR although to a lower extent with AR/S515E (compare lanes 8–9 with lanes 4–5 and 12–13). In parallel, incubations of AR and E3 ligases were also performed in the presence of HSP90α. We observed that HSP90α specifically co-IP with AR in the presence of CHIP (lanes 5, 9 and 13), which is in accordance with previous observations, suggesting that HSP90α is a molecular chaperone preferentially associated to U-box-type ubiquitin protein ligases such as CHIP (Murata et al, 2003; Hatakeyama et al, 2004; Yan et al, 2010).
Figure 4.
AR interacts with MDM2 and CHIP and is ubiquitinated by both E3 ligases. (A) AR/WT, /S515A or /S515E, immunoprecipitated (IP:AR/) from DHT (10−7 M) treated HeLa cells, were incubated with either purified-recombinant MDM2 or CHIP E3 ligases, in the presence or absence of HSP90α, as indicated. After extensive washes (300 mM KCl), western blot analyses were performed with AR-, MDM2-, CHIP- and HSP90α-specific antibodies. The control IP (lane 1) was performed using a non-specific antibody. Inputs are shown in the Supplementary Figure S3. (B) Recombinant purified AR was incubated with the ubiquitin-activating enzyme E1 (UBE1) and E2 (UbcH5a) in the presence of either MDM2 (1 μM, lanes 3 and 4) or CHIP (1 μM, lanes 7 and 8) E3 ligase as indicated on top of the panel. His-UbWT (wild type) or His-UbK0 (in which all seven lysine residues critical for polyubiquitination are replaced with alanine) have been added to the reaction, when indicated. Western blot analyses were then performed with Ubiquitin and AR-specific antibodies.
We next performed an in vitro ubiquitination assay in which AR/WT was incubated with either MDM2 or CHIP, the suitable E1/E2 enzymes and His-Ubwt. AR was equally polyubiquitinated with both E3 ligases (Figure 4B, lanes 3 and 7). Knowing that MDM2 and CHIP can distinctly mono- or polyubiquitinate their substrates (Li et al, 2003, 2007), we also analysed the AR monoubiquitination by replacing His-UbWT by His-UbK0 (a mutated Ubiquitin, with all the lysine residues replaced by alanine). Interestingly, contrary to that observed with MDM2, AR monoubiquitination was clearly detected in the presence of CHIP (compare lanes 8 and 4), which might be explained by our in vitro experimental conditions (see Discussion).
The above data indicate that MDM2 and CHIP are able to equally polyubiquitinate AR. Strikingly, these results demonstrate that AR phosphorylation status contributes to a selective recruitment of E3 ligases.
CHIP is preferred to MDM2 when the AR phosphorylation is impaired
To further investigate the role of TFIIH in the recruitment of the ubiquitin–proteasome machinery at the PSA promoter upon AR phosphorylation, we have generated stable HeLa cell lines by using a lentiviral system producing AR and have performed silencing experiments. In si-cdk7-treated cells, in which the cdk7 protein level was abrogated (Supplementary Figure S4A), ChIP assays showed the co-presence of AR as well as RNA pol II at the PSA promoter during the first 4 h post-DHT ligand treatment (Figure 5E and F). Strikingly, in si-cdk7-treated cells, in which the phosphorylation of AR was deficient (Supplementary Figure S4B) and its turnover much longer (compare Figure 5R with Q), we noticed that the CHIP recruitment was prominent while MDM2 was hardly detected (compare Figure 5J with I). The preference for CHIP recruitment in the si-cdk7 cells did not affect the recruitment of the proteasome, as clearly illustrated by the presence of the S1, SUG1 and β5 subunits at the PSA promoter (Figure 5N and M). However, contrary to that was observed in si-ctl, SUG1 was highly detected in si-cdk7-treated cells; this was likely due to a differential accessibility of the SUG1 antibody (Figure 5N).
Figure 5.
Silencing either cdk7 or MDM2 promotes CHIP recruitment at the PSA promoter. WT cells stably expressing AR/WT were transfected with either a pool of non-targeting si-RNAs (used as control, si-ctl, panels A, E, I, M, Q), si-cdk7 (panels B, F, J, N, R), si-MDM2 (panels C, G, K, O) or si-CHIP (panels D, H, L, P). (A–D) PSA gene expression was analysed by RT–qPCR at indicated times after DHT (10−7 M) treatment in cells transfected with si-ctl (A), si-cdk7 (B), si-MDM2 (C) or si-CHIP (D). The values were normalized relative to the GAPDH mRNA expression. The results of three independent experiments are presented as n-fold induction relative to non-treated cells. (E–H) The recruitment of RNA pol II (yellow bars) and AR (orange bars) at the PSA promoter was analysed by ChIP assays. The results are presented as percentage of DNA immunoprecipitated relative to the input (% input). (I–L) Recruitment of the E3 ligases MDM2 (green bars) and CHIP (yellow bars) at the PSA promoter was next investigated. (M–P) ChIP assays analysed the recruitment of the proteasomal subunits β5 (green bars), S1 (blue bars) and SUG1 (red bars). (Q–R) [35S] Pulse chase of AR protein in cells stably expressing AR/WT and transfected with either si-ctl (Q) or si-cdk7 (R). Experiments were performed as described in Figure 2. The values are the mean±s.e.m. of three independent experiments.
We also analysed whether silencing MDM2 might favourize the CHIP recruitment. In si-MDM2 cells, in which AR and RNA pol II were recruited to the PSA promoter (Figure 5G), CHIP (Figure 5K) as well as the proteasome machinery (Figure 5O) were present. These data prompted us to investigate whether the absence of CHIP might affect the transactivation process. Whereas silencing CHIP impaired PSA induction, the recruitment of RNA pol II and AR was conserved at the promoter (Figure 5H). In such case, MDM2 is not found, suggesting a co-requirement of both CHIP and MDM2 E3 ligases during the normal transactivation process (Figure 5L). We also failed to detect a recruitment of either SUG1 or β5 proteasome subunits upon DHT treatment (Figure 5P).
Altogether, the above data show that in case of impaired AR phosphorylation and/or MDM2 gene expression abrogation, CHIP replaces MDM2, to further allow the recruitment of the proteasome/ubiquitin machinery at the promoter of activated genes. Regardless, the absence of cdk7, MDM2 or CHIP leads to a defect in the transactivation machinery, which impedes the DHT-induced PSA gene expression (Figure 5A–D).
Discussion
The basic mechanism of cyclic transactivation of nuclear receptors following ligand induction relies on their translocation into the nucleus, their binding to their cognate sequences, and the recruitment of the transcription machinery to the promoter of a given gene. The transactivation process mediated by the nuclear receptors may require PTMs, such as acetylation, sumoylation, ubiquitination or phosphorylation. Although it became clear that the TFIIH-dependent phosphorylation of NRs controls their transactivation capacity (Keriel et al, 2002; Compe et al, 2005), many questions remain related to other potential consequences of these PTMs.
The present work proposes a model in which the phosphorylation of AR by TFIIH can induce its polyubiquitination, the recruitment of the proteasome and therefore the regulation of the expression of the AR responsive gene (Figure 6). We identified the serine S515 of AR as a phosphorylation substrate site for the cdk7 kinase of TFIIH as a key step of the transactivation process. Contrary to other phosphorylation of AR that modulate either the expression of its responsive genes (Weigel and Moore, 2007) or its cellular localization (Lin et al, 2002; Shank et al, 2008), the phosphorylation of AR/S515 is not required for its translocation into the nucleus (see Supplementary Figure S5A) and its subsequent binding to its responsive elements (Figure 3) and therefore seems to exhibit a specificity towards the transactivation process per se.
Figure 6.
TFIIH-mediated phosphorylation of AR regulates its turnover by triggering its degradation by the ubiquitin/proteasome pathway. Upon the DHT ligand induction, the transactivation complex is formed once AR homodimer has targeted its responsive element (ARE) at the PSA promoter; co-factors are assembled at the promoter together with RNA pol II and the general transcription factors (GTFs) including TFIIH. In WT cells, the AR/S515 phosphorylation by TFIIH (via its cdk7 subunit) promotes the recruitment of both MDM2 E3 ligase that helps for AR polyubiquitination and the proteasome. In XPD cells (bearing the XPD/R683W mutation), AR/S515 phosphorylation is strongly inhibited, preventing the recruitment of MDM2. The E3 ligase CHIP is thus preferentially recruited, which allows with a lesser efficiency the AR polyubiquitin/proteasome process resulting in a much slower turnover.
Following ligand induction, we observed that the AR/S515 phosphorylation contributes to the accurate enrolment of components required for the active and cyclic transactivation of target gene such as the PSA gene (Figure 3). Even more interestingly, we here show that the S515 phosphorylation status of AR dictates its turnover via an ubiquitin/proteasome pathway, which implies either MDM2 or CHIP E3 ligases. Indeed, once bound to its responsive element, the phosphorylated AR/S515 provides a specific recognition signal for the recruitment of the MDM2 E3 ligase (Figures 3I and 4A) that might use AR as a substrate (Figure 4B). Conversely, in the absence of AR/S515 phosphorylation, as noticed in both si-cdk7-treated and XPD cells or upon transfection of AR/S515A (unpublished results), we observed a greater and sustained accumulation of AR at the PSA promoter. In this situation, the cyclic recruitment of the transcription machinery cannot take place properly and CHIP E3 ligase is preferentially recruited at the PSA promoter (Figures 3J and 5J). Here, the recruitment of CHIP does result from both the absence of the AR/S515 phosphorylation and the MDM2 recruitment defect. Indeed when MDM2 is silenced, CHIP that was showed to physically and functionally interact with AR (Cardozo et al, 2003; He et al, 2004; Rees et al, 2006; Adachi et al, 2007; DaSilva et al, 2009) is enrolled at the PSA promoter (Figure 5K). It is noteworthy that the in vitro association of AR with MDM2 is more sensitive than with CHIP to the phosphorylation status of AR (Figure 4A). In a cellular context, when the AR/S515 phosphorylation is disrupted, we cannot exclude that other PTMs would contribute to attract CHIP E3 ligase. However, providing a ‘phosphorylated AR' by transfecting either AR/S515E or AR/WT together with XPD/WT into XPD cells restored the recruitment and the transactivation of AR similarly to that observed in WT cells (Figure 3).
Overall, our observations illustrate the crosstalk between two PTMs for example phosphorylation and ubiquitination during AR transactivation. The interplay between these two processes was shown for the recycling of other nuclear receptors, such as the glucocorticoid receptor GR or the RARγ2, which is crucial for the control of their transactivation activity (Wallace and Cidlowski, 2001; Bour et al, 2007). Here, we show that in the absence of an efficient phosphorylation by TFIIH, the recruited CHIP E3 ligase could still polyubiquitinate AR although the ubiquitin/proteasome process seems to be less efficient (Figure 6). This is illustrated by the abnormal accumulation of polyubiquitinated AR, which is observed in MG132-treated XPD cell extracts compared with WT cell extracts (Supplementary Figure S5B). Our in vitro ubiquitination assays showed that AR is polyubiquitinated by either MDM2 or CHIP (Figure 4B). Interestingly, whereas these E3 ligases can distinctly mono- or polyubiquitinate their substrates (Li et al, 2003, 2007), AR monoubiquitination has been only observed in the presence of CHIP (Figure 4B). As illustrated for p53 (Li et al, 2003), it is likely that AR monoubiquitination might be observed depending on the MDM2 concentration. Investigations should be undertaken to further define the selective role (if any) of both E3 ligases, in the AR ubiquitination process. It could not be excluded that both MDM2 and CHIP might work together to recruit the proteasome machinery for an optimal AR degradation process (Figures 3 and 5). Such cooperation is illustrated by the absence of MDM2 on the active PSA promoter when CHIP is silenced, which leads to a recruitment defect of the proteasome (Figure 5L and P).
In addition to the E3 ligases located at the promoter of activated genes, we also detected subunits of the 26S proteasome associated to the transcription machinery. This suggests that both the ubiquitination and the degradation of the nuclear receptor could already be engaged at the PSA promoter. We should mention that non-proteolytic activities of the ubiquitin–proteasome pathway could have a role in AR-mediated transcription. On many occasions, such non-proteolytic activities of the proteasome was observed (Kodadek et al, 2006; Kodadek, 2010), as illustrated by our previous results showing that the SUG1 subunit of the 26S proteasome interacted with the XPB subunit of TFIIH without being proteolysed (Fraser et al, 1997; Weeda et al, 1997).
Disruption of the AR phosphorylation by mutations into TFIIH might have consequences at a physiological level. However, it is difficult to establish a precise genotype/phenotype relationship for XP and TTD patients because they develop a broad range of clinical features (Lehmann, 2003; Kraemer et al, 2007), resulting from the combination of defects in both DNA repair and transcription. Recent work has revealed how certain mutations in XPD (Keriel et al, 2002) or XPG, a partner of TFIIH (Ito et al, 2007), disturb hormonal responses in a way that the phosphorylation of NRs by TFIIH was hampered and resulted in an impaired expression of their responsive genes. Clinical features such as hypogonadism, cachexia, growth retardation, kyphosis or bone loss, which are related to an AR deficiency (Mooradian et al, 1987; Matsumoto et al, 2008; Vanderschueren et al, 2008) are only developed by certain XP and TTD patients (Lehmann, 2001; Cleaver et al, 2009). Interestingly, some TFIIH mutations do not disrupt the transactivation mediated by AR (Figure 1A). Moreover, AR as well as other NRs has a specific and unique pattern of interaction with TFIIH (Supplementary Table I), suggesting that each TFIIH mutation might differently affect the transactivation process according to the NRs. Taken together, these observations suggest that the pleiotropic nature of the XPD and TTD phenotypes might result from various defects of NRs (Keriel et al, 2002; Compe et al, 2007), which have different consequences at a transcriptional level depending on the nature of the target genes since the organization of the transactivation process for each gene is unique (Brivanlou and Darnell, 2002). Among the effect of TFIIH mutations, we demonstrated here that disruption of the AR/S515 phosphorylation affects the turnover of AR. It is likely that mutations close to the AR/S515 site (Takahashi et al, 1995), as found in several prostate cancer can also modify the half-life of AR thereby preventing it to properly transactivate its responsive genes (Heinlein and Chang, 2004).
By investigating the defects leading to XP and TTD phenotypes, the present work contributes to explain the complex and peculiar mechanism that regulates the nuclear receptor transactivation. We demonstrate how a single PTM of a nuclear receptor, for example the phosphorylation of AR at serine 515, regulates its ubiquitination and subsequently its proteolysis as part of the transactivation process. In addition, the simultaneous presence of the transcription and the ubiquitination/proteasome machineries on the promoter of a given gene indicates how this later process has an impact on the transactivation. Taken together, the present study furthers our understanding of how the AR activity is regulated.
Materials and methods
Plasmids and construction of AR mutants
For luciferase reporter assays, the full-length PSA promoter (−5025 to +25) was amplified from human placental genomic DNA and cloned into pGL3.basic (Promega) using the Gateway technology (Invitrogen) giving rise to pGL3.PSA-Luc.
For recombinant his-AR expression in Sf9 cells, PCR product for the entire coding sequence of human AR (aa 1–919) was cloned into pTriEx vector (Novagen) using the appropriated restriction endonucleases. For recombinant protein expression in bacteria, PCR product for the AR truncated form without the A/B domain (his-ARDA/B; aa 559–919) or the A/B domain alone (GST-A/B.AR-his; aa 1 to 558) were, respectively, cloned into pET15b (Novagen) and pGEX.4T3 (Pharmacia) vectors using appropriated restriction endonucleases. Serine residue changes to alanine or glutamic acid were introduced using site-directed mutagenesis kit (Stratagen).
Antibodies
Monoclonal antibodies against the TFIIH subunits XPB (1B3), XPD (2F6), p62 (3C9), p44 (1H5), RNA polymerase II (7C2), SUG1 (2SU 1B8) were produced at the IGBMC facility. Polyclonal antibodies against TFIIH subunit cdk7 (C-19), AR (C-19), CHIP (H-231), Ubiquitin (FL-76), MDM2 (SMP14), β-tubulin and 20S proteasome β5 (C-19) were purchased from Santa Cruz Biotechnology. Rabbit antibodies against proteasome S1 (Rpn2) subunit were purchased from Abcam.
Stable expression of AR in HeLa cells
HeLa lenti-AR cell line was produced through infection with a recombinant lentivirus. The human AR gene was cloned and inserted into the vector plasmid pTripgatewayCMV using the Gateway technology (Invitrogen). Vector particles were produced as previously described (Zennou et al, 2000). Infection efficiency was measured by immunofluorescence using a specific antibody for AR and positive cells were clonally selected and amplified. Finally, stabilization of infection was measured after several passages by western blot.
Cell culture and transfection
HeLa (WT), HeLa lenti-AR and HD2 (XPD), which results from the fusion between human fibroblasts (harbouring the XPD/R683W mutation) and HeLa cells (Johnson et al, 1985), were grown in Dulbecco's modified Eagle medium (DMEM; 1 g glucose/l; GIBCO-BRL) containing 10% foetal calf serum (FCS) and 40 μg/ml gentamicine. Normal fibroblasts (GM03348D; Coriel Cell Repository), XPD-mutated fibroblasts (XPJCLO, XPD/R683W) (Taylor et al, 1997), TTD8PV (XPD/R112H) and TTD12PV (XPD/R722W) (Botta et al, 1998) were grown in DMEM supplemented with 10% FCS and 40 μg/ml gentamicine. Human prostate adenocarcinoma LNCaP cells were grown in RPMI Media 1640 (Invitrogen) supplemented with 10 mM Hepes, 10% FCS, 1 mM sodium pyruvate and 40 μg/ml gentamicine. All cell types were plated at ∼60% confluency and pGL3.PSA-Luc, pSG5.AR, ARS515A, ARS515E, pCDNA3.XPD/WT and pCMV.β-gal, as indicated, were transiently transfected in WT or XPD cells using JetPei reagent (Polyplus transfection). After 6 h of transfection, cells were treated with phenol red-free medium containing 5% charcoal-treated FCS and 40 μg/ml gentamicine. Following 16 h of incubation, appropriate ligand for RARα or AR (respectively, t-RA and DHT) was added and the mixture was incubated for 24 h. Cell extracts were analysed for luciferase and β-galactosidase activities as previously described (Keriel et al, 2002). The results are the mean of three different experiments done in duplicate.
Short interfering RNA
The short interfering RNA (si-RNA) corresponding to human cdk7, MDM2, CHIP and the non-targeting si-RNA (si-ctl) were purchased from Dharmacon and transiently transfected at a final concentration of 100 nM using Lipofectamine 2000 reagent (Invitrogen). After 24 h of transfection, the cells were treated with DHT (10−7 M) and prepared for analysis of RNA, chromatin or protein. si-RNA sequences are available on the Dharmacon web site (http://www.dharmacon.com).
Purification of recombinant proteins
Full-length recombinant his-AR was produced in Sf9 cells grown in medium containing 10−7 M DHT. Cells were harvested in a lysis buffer (20 mM Tris–HCl (pH 6.8), 20% glycerol, 150 mM NaCl, 0.1% NP40, 5 mM β-mercaptoethanol, 500 mM 1-(3-sulphopropyl) pyridinium betain, 10 mM imidazol) before ultracentrifugation. His-AR was then purified using Ni-NTA agarose (Qiagen) according to the manufacturer's instructions. GST-A/B.AR-his was produced in Escherichia coli BL21.RARE strain and purified using first Glutathione Sepharose 4B (Amersham Biosciences) and second Ni-NTA agarose. His-ARΔAB was produced in E. coli BL21.RARE strain grown in medium containing DHT (10−7 M) and purified using Ni-NTA agarose.
ChIP assays
Cells were transiently transfected with each indicated vector or si-RNA. After DHT (10−7 M) treatment, ChIP experiments were carried out as previously described (Compe et al, 2005). Chromatin was prepared and sonicated on ice 20 min using a Bioruptor (Diagenode) in 10 s pulse followed by 20 s cooling. Samples were IP with antibodies at 4°C overnight and Protein G-Sepharose beads (Upstate) were added, incubated 4 h at 4°C and sequentially washed. For ChIP/re-ChIP experiments, after the first immunoprecipitation and washes, protein–DNA complexes were eluted with a 10-mM DTT solution and diluted before addition of antibodies and protein G-sepharose beads for the immunoprecipitation. The complexes were eluted and the crosslinking was heat reversed. DNA fragments were purified using QIAquick PCR purification kit (Qiagen) and analysed by real-time quantitative PCR using sets of primers, available upon request, amplifying the region of interest in the PSA promoter. Results are expressed relative to the amount of input DNA per ChIP.
In vivo and in vitro phosphorylation of AR
Equal amounts of cells were transiently transfected with indicated expression vectors or si-RNA. Next, DHT (10−7 M) treatment, [32P]-orthophosphate labelling and immunoprecipitation were done as previously described (Keriel et al, 2002). After washes, AR was resolved by SDS–PAGE and transferred to a nitrocellulose filter. AR radioactive labelling was then evaluated by exposing an autoradiographic film to the filter. The western blot analysis of AR was then performed on the same filter.
Equal amounts (1 μg) of recombinant purified AR, ARΔAB, A/B.AR, A/B.AR.S515A and PPARα were incubated with highly purified HeLa TFIIH, [γ-32P] ATP (0.14 μM) in the presence or absence of DHT (10−7 M) and reaction was carried out as described (Rossignol et al, 1997).
Co-immunoprecipitation assays
For in vivo co-IPs, total extracts of LNCaP cells treated with DHT (10−7 M) were prepared. After AR immunoprecipitation, followed by extensive washes (150 mM NaCl), TFIIH co-precipitation was detected using an antibody specific for XPB.
For in vitro co-IPs, Sf9 cells were infected with a virus expressing all TFIIH subunits with a FLAG-tagged version of p34 (Tirode et al, 1999). Whole-cell extracts were then incubated with recombinant purified AR in the presence or absence of DHT (10−6 M) before a FLAG immunoprecipitation was carried out. After washes, bound proteins were resolved by SDS–PAGE and detected by western blot. Alternatively, Sf9 cells were infected with each subunits of TFIIH separately (Tirode et al, 1999) and whole-cell extracts were incubated with recombinant purified AR in the presence or absence of DHT (10−6 M). Whole-cell extracts were also prepared from DHT-treated (10−7 M) HeLa cells overexpressing AR/WT, AR/S515A or AR/S515E. AR were IP (using Dynabeads protein A/G, Invitrogen) and incubated in the presence of DHT (10−6 M) with purified MDM2, CHIP (Boston Biochem) or HSP90α (Assay Designs) in an interaction buffer (20 mM Hepes, 100 mM KCl, 0.2 mM EDTA, 10% glycerol, 0.05% NP40, 0.2 mM DTT). After extensive washes (300 mM KCl), bound proteins were resolved by SDS–PAGE and detected by western blot.
Reverse transcription and real-time quantitative PCR
Total RNA was isolated using a GenElute Mammalian Total RNA Miniprep kit (Sigma) and reverse transcribed with SuperScript II reverse transcriptase (Invitrogen). The quantitative PCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen) and the Lightcycler 480 (Roche). The primer sequences for PSA and GAPDH genes used in real-time qPCR are available upon request. PSA mRNA levels were normalized against the GAPDH mRNA.
Half-life measurements
Pulse-chase analysis were carried out in cells transfected with expression vectors or si-RNA, as indicated, that were pretreated during 2 h in DMEM cys−/met− medium and metabolically labelled with 100 μCi/ml 35S-methionine for 1 h in DMEM cys−/met− medium. Cells were then washed with growth medium consisting of phenol red-free DMEM with 5% charcoal-treated FCS. Cells were then treated with DHT (10−7 M) for the indicated times. Whole-cell extracts were prepared using RIPA buffer (0.01 M Tris–HCl pH 8.0, 0.14 M NaCl, 1% Triton X-100, 0.1% Na-Deoxycholate, 0.1% SDS) and 35S-AR protein was IP using an anti-AR antibody and resolved by SDS–PAGE. 35S-AR bands were quantified by phosphoimager analysis using ImageJ software.
In vitro AR ubiquitination
His-AR was purified using Ni-NTA beads (Qiagen) that were extensively washed before ubiquitination reactions. Beads were then mixed with 100 nM of E1, 500 nM of E2 (GST-UbcH5a), 100 mM of His-Ubiquitin wild type (His-UbWT) or with all the lysines mutated to arginine (His-UbK0), 2 mM of ATP and Ub buffer 5 × (Tris–HCl 250 mM, MgCl2 25 mM, DTT 1 mM). All recombinant proteins were purchased from Boston Biochem. Recombinant E3 ligase, either MDM2 (1 μM, Boston Biochem) or CHIP (1 μM, Upstate), was also added to the mix that was incubated 1 h at 37°C. The reaction was stopped with 10 mM of EDTA and bound proteins were resolved by SDS–PAGE and revealed by western blot using antibodies against Ub (FK2, Enzo Life Sciences) and AR.
Supplementary Material
Acknowledgments
We thank Pascal Drané for fruitful discussion and his help for setting the experiments, Astrid Lunkes for critical reading of the manuscript and C Braun for her technical expertise. Work in the JM Egly's laboratory is supported by a European Research Council Advanced Grant 2009, the French National Research Agency (No ANR-05-PCOD-032-03; No ANR-06-BLAN-0141-01; ANR-08-MIEN-02203; ANR-08GENOPAT042), and the Association pour la Recherche sur le Cancer (No 3153). PC was supported by a grant from the Fondation pour la Recherche Médicale, NLM was sponsored by A CDD INSERM young investigator grant.
Author contributions: Pchy, NLM, EC and JME conceived and designed the experiments. PCha produced the lentiviral particles to establish the stable cell lines. Pchy, NLM and EC carried out the experiments. Pchy, NLM, EC and JME analysed the data. Pchy, NLM, EC and JME wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adachi H, Waza M, Tokui K, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G (2007) CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J Neurosci 27: 5115–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SP, Knudsen KE (2008) AR, the cell cycle, and prostate cancer. Nucl Recept Signal 6: e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zuhl F, Seemuller E (1998) The proteasome: paradigm of a self-compartmentalizing protease. Cell 92: 367–380 [DOI] [PubMed] [Google Scholar]
- Botta E, Nardo T, Broughton BC, Marinoni S, Lehmann AR, Stefanini M (1998) Analysis of mutations in the XPD gene in Italian patients with trichothiodystrophy: site of mutation correlates with repair deficiency, but gene dosage appears to determine clinical severity. Am J Hum Genet 63: 1036–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour G, Lalevee S, Rochette-Egly C (2007) Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol 17: 302–309 [DOI] [PubMed] [Google Scholar]
- Brinkmann AO (2001) Lessons to be learned from the androgen receptor. Eur J Dermatol 11: 301–303 [PubMed] [Google Scholar]
- Brivanlou AH, Darnell JE Jr (2002) Signal transduction and the control of gene expression. Science 295: 813–818 [DOI] [PubMed] [Google Scholar]
- Cardozo CP, Michaud C, Ost MC, Fliss AE, Yang E, Patterson C, Hall SJ, Caplan AJ (2003) C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch Biochem Biophys 410: 134–140 [DOI] [PubMed] [Google Scholar]
- Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, Ali S (2000) Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell 6: 127–137 [PubMed] [Google Scholar]
- Cleaver JE, Lam ET, Revet I (2009) Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet 10: 756–768 [DOI] [PubMed] [Google Scholar]
- Coin F, Bergmann E, Tremeau-Bravard A, Egly JM (1999) Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. EMBO J 18: 1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compe E, Drane P, Laurent C, Diderich K, Braun C, Hoeijmakers JH, Egly JM (2005) Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mol Cell Biol 25: 6065–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compe E, Malerba M, Soler L, Marescaux J, Borrelli E, Egly JM (2007) Neurological defects in trichothiodystrophy reveal a coactivator function of TFIIH. Nat Neurosci 10: 1414–1422 [DOI] [PubMed] [Google Scholar]
- DaSilva J, Gioeli D, Weber MJ, Parsons SJ (2009) The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer Res 69: 7402–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faus H, Haendler B (2006) Post-translational modifications of steroid receptors. Biomed Pharmacother 60: 520–528 [DOI] [PubMed] [Google Scholar]
- Fraser RA, Rossignol M, Heard DJ, Egly JM, Chambon P (1997) SUG1, a putative transcriptional mediator and subunit of the PA700 proteasome regulatory complex, is a DNA helicase. J Biol Chem 272: 7122–7126 [DOI] [PubMed] [Google Scholar]
- Gaughan L, Logan IR, Neal DE, Robson CN (2005) Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res 33: 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni M, Bauer A, Garattini E, Chambon P, Rochette-Egly C (2002) Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. EMBO J 21: 3760–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S, Matsumoto M, Yada M, Nakayama KI (2004) Interaction of U-box-type ubiquitin-protein ligases (E3 s) with molecular chaperones. Genes Cells 9: 533–548 [DOI] [PubMed] [Google Scholar]
- He B, Bai S, Hnat AT, Kalman RI, Minges JT, Patterson C, Wilson EM (2004) An androgen receptor NH2-terminal conserved motif interacts with the COOH terminus of the Hsp70-interacting protein (CHIP). J Biol Chem 279: 30643–30653 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C (2004) Androgen receptor in prostate cancer. Endocr Rev 25: 276–308 [DOI] [PubMed] [Google Scholar]
- Hochstrasser M (2009) Origin and function of ubiquitin-like proteins. Nature 458: 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege FC, van der Vliet PC, Timmers HT (1996) Opening of an RNA polymerase II promoter occurs in two distinct steps and requires the basal transcription factors IIE and IIH. EMBO J 15: 1666–1677 [PMC free article] [PubMed] [Google Scholar]
- Ito S, Kuraoka I, Chymkowitch P, Compe E, Takedachi A, Ishigami C, Coin F, Egly JM, Tanaka K (2007) XPG stabilizes TFIIH, allowing transactivation of nuclear receptors: implications for Cockayne syndrome in XP-G/CS patients. Mol Cell 26: 231–243 [DOI] [PubMed] [Google Scholar]
- Johnson RT, Squires S, Ellion GC, Koch GLE, Rainbow AJ (1985) Xeroderma pigmentosum D-HeLa hybrids with low and high ultraviolet sensitivity associated with normal and diminished DNA repair ability, respectively. J Cell Sci 76: 115–133 [DOI] [PubMed] [Google Scholar]
- Kang Z, Pirskanen A, Janne OA, Palvimo JJ (2002) Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 277: 48366–48371 [DOI] [PubMed] [Google Scholar]
- Keriel A, Stary A, Sarasin A, Rochette-Egly C, Egly JM (2002) XPD mutations prevent TFIIH-dependent transactivation by nuclear receptors and phosphorylation of RARalpha. Cell 109: 125–135 [DOI] [PubMed] [Google Scholar]
- Kodadek T (2010) No Splicing, no dicing: non-proteolytic roles of the ubiquitin-proteasome system in transcription. J Biol Chem 285: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodadek T, Sikder D, Nalley K (2006) Keeping transcriptional activators under control. Cell 127: 261–264 [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ (2007) Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience 145: 1388–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Johnson BH, Thompson EB (2004) Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essays Biochem 40: 27–39 [DOI] [PubMed] [Google Scholar]
- Lee DK, Duan HO, Chang C (2000) From androgen receptor to the general transcription factor TFIIH. Identification of cdk activating kinase (cak) as an androgen receptor nh(2)-terminal associated coactivator [in process citation]. J Biol Chem 275: 9308–9313 [DOI] [PubMed] [Google Scholar]
- Lehmann AR (2001) The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases. Genes Dev 15: 15–23 [DOI] [PubMed] [Google Scholar]
- Lehmann AR (2003) DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 85: 1101–1111 [DOI] [PubMed] [Google Scholar]
- Lemon B, Tjian R (2000) Orchestrated response: a symphony of transcription factors for gene control. Genes Dev 14: 2551–2569 [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W (2003) Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302: 1972–1975 [DOI] [PubMed] [Google Scholar]
- Li RF, Shang Y, Liu D, Ren ZS, Chang Z, Sui SF (2007) Differential ubiquitination of Smad1 mediated by CHIP: implications in the regulation of the bone morphogenetic protein signaling pathway. J Mol Biol 374: 777–790 [DOI] [PubMed] [Google Scholar]
- Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C (2002) Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J 21: 4037–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zawel L, Fisher L, Egly JM, Reinberg D (1992) Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature 358: 641–645 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83: 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Shiina H, Kawano H, Sato T, Kato S (2008) Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol 109: 236–241 [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG (1987) Biological actions of androgens. Endocr Rev 8: 1–28 [DOI] [PubMed] [Google Scholar]
- Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13: 261–291 [DOI] [PubMed] [Google Scholar]
- Murata S, Chiba T, Tanaka K (2003) CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int J Biochem Cell Biol 35: 572–578 [DOI] [PubMed] [Google Scholar]
- Popov VM, Wang C, Shirley LA, Rosenberg A, Li S, Nevalainen M, Fu M, Pestell RG (2007) The functional significance of nuclear receptor acetylation. Steroids 72: 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees I, Lee S, Kim H, Tsai FT (2006) The E3 ubiquitin ligase CHIP binds the androgen receptor in a phosphorylation-dependent manner. Biochim Biophys Acta 1764: 1073–1079 [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Adam S, Rossignol M, Egly JM, Chambon P (1997) Stimulation of RAR alpha activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell 90: 97–107 [DOI] [PubMed] [Google Scholar]
- Rossignol M, Kolb-Cheynel I, Egly JM (1997) Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J 16: 1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank LC, Kelley JB, Gioeli D, Yang CS, Spencer A, Allison LA, Paschal BM (2008) Activation of the DNA-dependent protein kinase stimulates nuclear export of the androgen receptor in vitro. J Biol Chem 283: 10568–10580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Furusato M, Allsbrook WC Jr, Nishii H, Wakui S, Barrett JC, Boyd J (1995) Prevalence of androgen receptor gene mutations in latent prostatic carcinomas from Japanese men. Cancer Res 55: 1621–1624 [PubMed] [Google Scholar]
- Taylor E, Broughton B, Botta E, Stefanini M, Sarasin A, Jaspers N, Fawcett H, Harcourt S, Arlett C, Lehmann A (1997) Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc Natl Acad Sci USA 94: 8658–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirode F, Busso D, Coin F, Egly JM (1999) Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Mol Cell 3: 87–95 [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Gaytant J, Boonen S, Venken K (2008) Androgens and bone. Curr Opin Endocrinol Diabetes Obes 15: 250–254 [DOI] [PubMed] [Google Scholar]
- Wallace AD, Cidlowski JA (2001) Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem 276: 42714–42721 [DOI] [PubMed] [Google Scholar]
- Weeda G, Rossignol M, Fraser RA, Winkler GS, Vermeulen W, van ‘t Veer LJ, Ma L, Hoeijmakers JH, Egly JM (1997) The XPB subunit of repair/transcription factor TFIIH directly interacts with SUG1, a subunit of the 26S proteasome and putative transcription factor. Nucleic Acids Res 25: 2274–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel NL, Moore NL (2007) Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 21: 2311–2319 [DOI] [PubMed] [Google Scholar]
- Yan S, Sun X, Xiang B, Cang H, Kang X, Chen Y, Li H, Shi G, Yeh ET, Wang B, Wang X, Yi J (2010) Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J 29: 3773–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P (2000) HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101: 173–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.