Abstract
The two recently established genera ApostrombidiumXu et al., 2009 and VaristrombidiumXu et al., 2009 and the analysis of ontogenetic data in Strombidium constrictum, S. montagnesi, S. wilberti, Omegastrombidium elegans, and Paratontonia gracillima necessitated a revision of the hypothesis about the somatic ciliary pattern evolution in oligotrichid ciliates. As a consequence, the species-rich genus Strombidium was split, establishing two genera for species with a horizontal girdle kinety posterior to the oral primordium: Opisthostrombidium nov. gen. with the extrusome attachment sites along the anterior margin of the girdle kinety and posterior to the oral primordium and Foissneridium nov. gen. with the extrusome attachment sites distinctly apart from the girdle kinety and anterior to the oral primordium. The ontogenetic data revealed that the Ω-shaped girdle kinety pattern evolved convergently from the Pseudotontonia pattern with its horizontal girdle kinety in the tailed genus Paratontonia and from the Novistrombidium pattern with its dextrally spiralled girdle kinety in the tailless genus Omegastrombidium. The somatic ciliary pattern of the latter genus probably gave rise to the patterns of Apostrombidium and Varistrombidium.
Keywords: Ciliophora, New genera, Oligotrichida, Somatic ciliary pattern, Taxonomy
Introduction
Although the somatic ciliature of the Oligotrichida (Ciliophora, Spirotricha) typically comprises only a girdle and a ventral kinety, the diversity of ciliary patterns created by these two rows is considerable. Recently, Xu et al. (2009) published descriptions of two strombidiid genera with unique somatic ciliary patterns: Varistrombidium possessing five somatic kineties and Apostrombidium having two inverted U-shaped kinety fragments in the right and left cell halves. The present paper assigns positions for these two genera within the Strombidiidae and expands on the hypothesized evolution of somatic ciliary patterns suggested by Agatha (2004a), by including the genera and the ontogenetic data on Strombidium constrictum, S. montagnesi, S. wilberti, Omegastrombidium elegans, and Paratontonia gracillima. Thus, this study provides a better understanding of the diversity within the Oligotrichida.
Material and Methods
The original literature was used (references in Agatha 2004a; Agatha 2004c; Agatha et al. 2005; Liu et al. 2009; Skovgaard and Legrand 2005; Song 2005; Wilbert and Song 2005; Xu and Song 2006; Xu et al. 2005, 2006a,b,c, 2007, 2008, 2009) and the following genera were considered: Apostrombidium Xu, Warren and Song, 2009; Cyrtostrombidium Lynn and Gilron, 1993; Laboea Lohmann, 1908; Limnostrombidium Krainer, 1995; Novistrombidium Song and Bradbury, 1998; Omegastrombidium Agatha, 2004; Parallelostrombidium Agatha, 2004; Paratontonia Jankowski, 1978; Pelagostrombidium Krainer, 1991; Pseudotontonia Agatha, 2004; Spirostrombidium Jankowski, 1978; Spirotontonia Agatha, 2004; Strombidium Claparède and Lachmann, 1859; Tontonia Fauré-Fremiet, 1914; and Varistrombidium Xu, Warren and Song, 2009. Note, a description and diagnosis of Varistrombidium were given by Xu, Warren, and Song (2009), referring to an original description by Xu, Yi, Song and others in press, but this latter publication does not exist; hence, Xu, Warren, and Song (2009) were considered the authorities on this genus.
By regarding the somatic kineties in the Oligotrichida as homologs of the dorsal kineties in the hypotrich and stichotrich spirotrichs (Agatha 2004a,b; Agatha and Strüder-Kypke 2007; Foissner et al. 2007), the evolution of the ciliary patterns can be inferred from the orientation of the somatic dikinetids (based on which basal body bears the cilium) and the location of the oral primordium. The orientation of the somatic dikinetids was inferred from a line drawing of the ventral side in Apostrombidium and a micrograph of the posterior ventral cell portion in Varistrombidium; both patterns were verified by D. Xu (Department of Biology, North Carolina Central University, NC, USA) and W. Song (Laboratory of Protozoology, Ocean University of China, Qingdao, China), using the type slides of A. pseudokielum and the protargol slides of V. kielum.
Data on the ontogenesis of Strombidium montagnesi and S. wilberti were published by Xu et al. (2006c) and Song (2005), respectively. The position of the oral primordium was studied in S. constrictum, using protargol slides from the type locality kindly provided by D.H. Lynn (Department of Integrative Biology, University of Guelph, Canada), and in Paratontonia gracillima, using protargol-impregnated specimens from the North Pacific kindly provided by the Tohoku University Museum (Sendai, Japan). In Omegastrombidium elegans, however, the position of the oral primordium could only be inferred from micrographs, because of the two slides stated to be deposited in the Natural History Museum in London, one was inadequate to evaluate the target structures and the other was not included into the collection. Hence, the information was taken from Figures 41 and 42 in Song et al. (2000; p. 340).
Results and Discussion
Evolution of the Somatic Ciliary Patterns
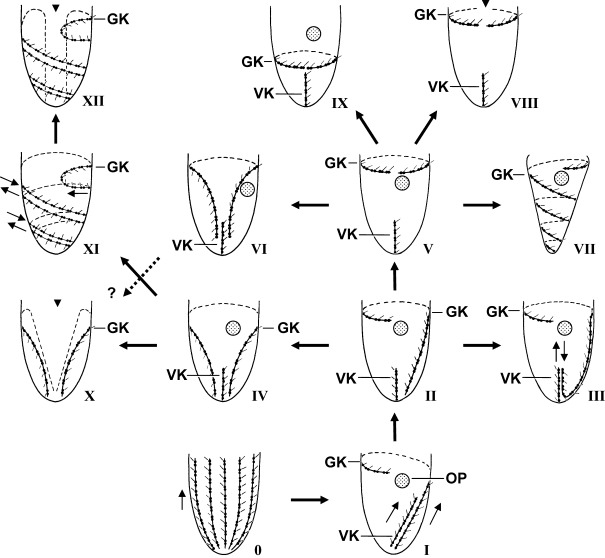
In Agatha (2004a), the hypothetical evolution of the oligotrichid somatic ciliature comprised six patterns: the Types I–V, and VII. Below, five further patterns are recognized, viz., the Types VI, VIII–X, and XII (Fig. 1).
Fig. 1.
Hypothetical evolution of oligotrichid somatic ciliary patterns. Small arrows mark the orientation of the kineties (from posterior to anterior). The dikinetidal somatic kineties of the ancestor (Type 0) were reduced to two and became dextrally spiralled, producing the Type I pattern. Next, the ventral kinety orientated longitudinally in the Type II pattern. The Type III pattern evolved by an elongation of the posterior girdle kinety end, which orientated inversely along the ventral kinety. The Type IV pattern developed by the posterior migration of the right girdle kinety portion, while in the Type V pattern, the left portion migrated anteriorly above the oral primordium. The Type VI pattern was generated by the posterior migration of both girdle kinety ends. In the Type VII pattern, the right girdle kinety end elongated and spiralled sinistrally. The horizontal girdle kinety split dorsally (arrowhead), producing the Type VIII pattern, while the Type IX pattern developed by the posterior migration of the horizontal girdle kinety below the oral primordium. The Type X pattern was generated by a dorsal split (arrowhead) of the girdle kinety and an elongation of the new kinety ends to the posterior pole. The evolution of the Type XII pattern commenced with an elongation and sinistral torsion of both kinety ends, producing the hypothetical Type XI pattern. Next, the first kinety whorl split dorsally (arrowhead), and the new ends elongated to the rear end, breaking through the kinety spirals and producing several kinety fragments. 0 – Type 0 of the ancestor, I–XII – Types I–XII, GK – girdle kinety, OP – oral primordium, VK – ventral kinety.
Agatha (2004a) assumed a single origin of the Ω-shaped girdle kinety pattern derived from the Type II pattern. However, ontogenetic data indicate that this only occurred in the Type IV pattern of Omegastrombidium (Fig. 1), while the Ω-shaped pattern of Paratontonia (Type VI pattern) probably evolved from the Type V pattern by the posterior migration of both girdle kinety ends, as indicated by the position of the oral primordium posterior to the girdle kinety (Fig. 1). Accordingly, the Ω-shaped girdle kinety pattern developed not only convergently in the tailed (tontoniid) and tailless (strombidiid) taxa, as suggested by Agatha (2004b), but also from different patterns.
The Type X pattern of Apostrombidium and the Type XII pattern of Varistrombidium probably developed from such a Ω-shaped girdle kinety pattern. It is more parsimonious to assume that these tailless genera originated from the Type IV pattern of the tailless genus Omegastrombidium than from the Type VI pattern of the tailed genus Paratontonia; however, ontogenetic and/or molecular data are required to support this conjecture for Apostrombidium. While the Type X pattern of Apostrombidium probably evolved from the Type IV pattern by a dorsal split of the girdle kinety and an extension of the new kinety ends to the posterior cell pole, the development of the Type XII pattern in Varistrombidium was more complex. First, a hypothetical intermediate Type XI pattern was generated by an elongation and sinistral torsion of the parallel girdle kinety ends, while the ventral kinety disappeared. Next, the dorsal portion of the first whorl broke, and the new kinety ends extended to the posterior cell pole, cutting through the kinety spirals and producing the several ciliary row fragments characteristic of the Type XII pattern. A similar, but convergent dorsal split of the girdle kinety occurred not only in the Type X pattern of Apostrombidium, but also in the Type VIII pattern of Cyrtostrombidium.
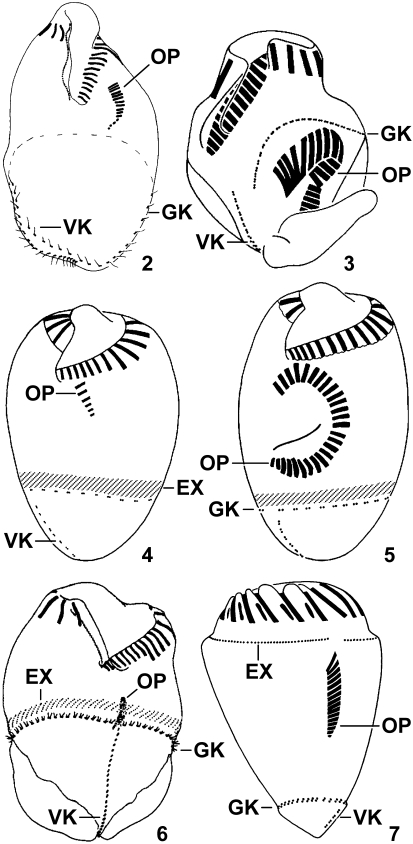
Besides the Type VI pattern of Paratontonia (see above) and the Type VII pattern of Laboea and Spirotontonia, two further somatic ciliary patterns probably developed from the Type V pattern: the Type VIII pattern of Cyrtostrombidium (the pre-equatorial position of the girdle kinety suggests this origin) and the Type IX pattern of Strombidium constrictum, S. montagnesi, and S. wilberti. In contrast to the Type V pattern, e.g., found in the type species Strombidium sulcatum (Fauré-Fremiet 1912, 1953), the formation of the oral primordium commences anteriorly to the girdle kinety in the latter pattern (refer to Fig. 1; own data; Song 2005; Xu et al. 2006c). Since the extrusome attachment sites usually form a stripe along the anterior margin of the girdle kinety in the oligotrichids, the insertion of the extrusomes distinctly separate from the girdle kinety and anterior to the oral primordium in S. constrictum (Fig. 7) suggests the former position and course of the girdle kinety. Thus, it seems likely that the Type IX pattern evolved from the Type V pattern by a migration of the horizontal girdle kinety below the oral primordium, as proposed by Agatha (2004b). However, this arrangement of the girdle kinety developed twice independently: in S. montagnesi and S. wilberti, both the girdle kinety and the associated extrusome attachment sites migrated posteriorly, while in S. constrictum, merely the girdle kinety migrated posteriorly. Accordingly, two new strombidiid genera must be established based on the differences in the position of the girdle kinety and the extrusome attachment sites (see ‘Taxonomic Implications’). Probably, the pelagostrombidiid genus Limnostrombidium also belongs to the Type IX pattern, as the neoformation organelle opens anteriorly to the girdle kinety within the stripe of extrusome attachment sites (Foissner et al. 1999).
Figs. 2–7.
Ventral views of oligotrichid dividers (2, after Song et al. 2000; 3, 7, originals; 4, 5, after Xu et al. 2006c; 6, after Song 2005; protargol impregnation; 2, 4, 5, combination of line drawings and micrographs). 2. Omegastrombidium elegans, 43 μm long. 3. Paratontonia gracillima, 37 μm long. 4–6. Opisthostrombidium montagnesi nov. comb. (4, 5) and O. wilberti nov. comb. (6),? μm. 7. Foissneridium constrictum nov. comb., 53 μm long. EX – extrusome attachment sites, GK – girdle kinety, OP – oral primordium, VK – ventral kinety.
Comparison with Molecular Phylogenies
The hypothetical evolution of the oligotrichid somatic ciliary patterns suggested above is tested by a comparison with phylogenies inferred from the small subunit ribosomal RNA (SSrRNA) gene sequences. In the recently published SSrRNA gene trees of the Oligotrichida, most basal nodes were not well supported, having bootstrap values less than 50% in the maximum parsimony and neighbor-joining analyses (Gao et al. 2009a,b; Kim et al. 2010); thus, many polytomies were not resolved. Accordingly, the following comparison cannot be detailed.
Within the monophyletic Oligotrichida, the genus Novistrombidium branches early, followed by the genus Parallelostrombidium and a cluster of Spirostrombidium species. Since the Parallelostrombidium species (GenBank FJ422988) sequenced by Gao et al. (2009a) was described as Novistrombidium orientale by Liu et al. (2009), the gene tree corroborates the present hypothesis, in which the Type II pattern of Novistrombidium gives rise to the Type III pattern of Spirostrombidium (Fig. 1).
The paraphyly of the genus Strombidium in the SSrRNA gene trees might be due to the unresolved polytomies and/or undersampling. On the other hand, it cannot be excluded that future studies will reveal morphological features of the cell and/or the resting cyst, justifying further splits of the species-rich genus Strombidium.
In the gene trees, the genus Pseudotontonia represents an adelphotaxon to a cluster formed by the genera Laboea and Spirotontonia, supporting the close relationship of the Type V and VII patterns (Fig. 1). In contrast to the proposal by Agatha (2004b), the sinistrally spiralled course of the girdle kinety (Type VII pattern) apparently did not evolve convergently in the tontoniids and strombidiids, but was apparently present in the tailed common ancestor of the genus Spirotontonia and the probably secondarily tailless genus Laboea (Gao et al. 2009a).
Besides the somatic ciliary pattern, the absence of a tail, and the position of the oral primordium, the close relationship of the genera Omegastrombidium (Type IV pattern) and Varistrombidium (Type XII pattern) is indicated by the molecular data. In general, the evolution of the oligotrichid somatic ciliary patterns suggested above thus matches the recent gene trees.
Taxonomic Implications
The ontogenetic data provided by Song (2005) and Xu et al. (2006c) and those gathered by me necessitate the establishment of two new genera.
Genus Opisthostrombidium nov. gen.
Diagnosis
Strombidiidae with the oral primordium anterior to the horizontal girdle kinety and the associated extrusome attachment sites. Ventral kinety longitudinal.
Type Species
Strombidium montagnesi Xu, Song and Warren, 2006.
Etymology
Composite of the Greek prefix opistho (behind) and the genus-group name Strombidium, referring to the position of the girdle kinety and the extrusome attachment sites posterior to the oral primordium. Neuter gender.
Comparison with Similar Genera and Species Assignable
In Strombidium montagnesi and S. wilberti, the formation of the oral primordium commences anteriorly to the horizontal girdle kinety and its associated extrusome attachment sites (refers to Fig. 1). The species are thus separated on generic level from Strombidium (the oral primordium forms posteriorly to the horizontal girdle kinety in the type and further species; Agatha 2003; Agatha et al. 2005; Fauré-Fremiet 1912, 1953; Kormos and Kormos 1958; Petz 1994; Song and Wang 1996), becoming Opisthostrombidium montagnesi (Xu, Song and Warren, 2006) nov. comb. and Opisthostrombidium wilberti (Song, 2005) nov. comb.
Genus Foissneridium nov. gen.
Diagnosis
Strombidiidae with the oral primordium posterior to the horizontal stripe of extrusome attachment sites and anterior to the horizontal girdle kinety. Ventral kinety longitudinal.
Type Species
Conocylis constricta Meunier, 1910.
Etymology
Dedicated to Wilhelm Foissner (Department of Organismic Biology, University of Salzburg, Austria) due to his substantial contribution to ciliate taxonomy and evolution and his great support during all the years. Neuter gender.
Comparison with Similar Genera and Species Assignable
Like in the genus Opisthostrombidium nov. gen. (see above), the oral primordium of Strombidium constrictum (basionym Conocylis constricta; Meunier 1910) develops anteriorly to the horizontal girdle kinety (Fig. 7; own data), while posteriorly to this ciliary row in the genus Strombidium. The arrangement of the extrusome attachment sites along the anterior margin of the girdle kinety in Opisthostrombidium nov. gen. is typical of the Oligotrichida. According to the redescription by Lynn et al. (1988) and own data, however, the horizontal stripe of extrusome attachment sites is pre-equatorial in S. constrictum and thus not only distinctly separate from the subterminally located horizontal girdle kinety, but also anterior to the oral primordium. These differences in the position of the extrusome attachment sites and the oral primordium relative to the girdle kinety justify a generic separation of the species from Strombidium and Opisthostrombidium. Since Jankowski (2007) subsequently designated Conocylis helix Meunier, 1910 as type of the genus Conocylis Meunier, 1910, which might also include C. striata Meunier, 1910, a new genus, viz., Foissneridium nov. gen., is established for S. constrictum, which becomes Foissneridium constrictum (Meunier, 1910) nov. comb.
Acknowledgements
This study was supported by the Austrian Science Foundation (FWF, Project P20461-B17). Many thanks are due to Dapeng Xu (Department of Biology, North Carolina Central University, North Carolina, USA) and Weibo Song (Laboratory of Protozoology, Ocean University of China, Qingdao, China) for their help to ascertain the orientation of the somatic dikinetids in the genera Apostrombidium and Varistrombidium. Thanks are also due to Denis Lynn (Department of Integrative Biology, University of Guelph, Canada) and the Tohoku University Museum (Sendai, Japan) for providing protargol slides. Furthermore, I wish to thank David Montagnes for his helpful comments and some linguistic improvements.
References
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Eur. J. Protistol. 2003;39:245–266. [Google Scholar]
- Agatha S. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology. 2004;107:153–168. doi: 10.1016/j.zool.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S. A cladistic approach for the classification of oligotrichid ciliates (Ciliophora: Spirotricha) Acta Protozool. 2004;43:201–217. [PMC free article] [PubMed] [Google Scholar]
- Agatha S. New observations on the tontoniid ciliate Spirotontonia grandis (Suzuki and Han, 2000) Agatha, 2004 (Ciliophora, Oligotrichida, Tontoniidae); comparison with the similar Laboea strobila. Eur. J. Protistol. 2004;40:295–301. [Google Scholar]
- Agatha S., Strüder-Kypke M.C. Phylogeny of the order Choreotrichida (Ciliophora, Spirotricha, Oligotrichea) as inferred from morphology, ultrastructure, ontogenesis, and SSrRNA gene sequences. Eur. J. Protistol. 2007;43:37–63. doi: 10.1016/j.ejop.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S., Strüder-Kypke M.C., Beran A., Lynn D.H. Pelagostrobilidium neptuni (Montagnes and Taylor, 1994) and Strombidium biarmatum nov. spec. (Ciliophora, Oligotrichea): phylogenetic position inferred from morphology, ontogenesis, and gene sequence data. Eur. J. Protistol. 2005;41:65–83. doi: 10.1016/j.ejop.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré-Fremiet E. Études cytologiques sur quelques infusoires des marais salants du Croisic. Arch. Anat. Microsc. Morph. Exp. 1912;13:401–479. +Plates 9, 10. [Google Scholar]
- Fauré-Fremiet E. La bipartition énantiotrope chez les ciliés oligotriches. Arch. Anat. Microsc. Morph. Exp. 1953;42:209–225. [Google Scholar]
- Foissner W., Berger H., Schaumburg J. Identification and ecology of Limnetic Plankton Ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Foissner W., Müller H., Agatha S. A comparative fine structural and phylogenetic analysis of resting cysts in oligotrich and hypotrich Spirotrichea (Ciliophora) Eur. J. Protistol. 2007;43:295–314. doi: 10.1016/j.ejop.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Gong J., Lynn D., Lin X., Song W. An updated phylogeny of oligotrich and choreotrich ciliates (Protozoa, Ciliophora, Spirotrichea) with representative taxa collected from Chinese coastal waters. Syst. Biodiv. 2009;7:235–242. [Google Scholar]
- Gao S., Gong J., Lynn D., Lin X., Song W. An updated phylogeny of oligotrich and choreotrich ciliates (Protozoa, Ciliophora, Spirotrichea) with representative taxa collected from Chinese coastal waters – corrigendum. Syst. Biodiv. 2009;7:347. [Google Scholar]
- Jankowski A.W. Phylum Ciliophora Doflein, 1901. Review of taxa. In: Alimov A.F., editor. Protista Part 2: Handbook on Zoology. St. Petersburg; Nauka: 2007. pp. 415–993. (in Russian with English summary) [Google Scholar]
- Kim Y.-O., Kim S.Y., Lee W.-J., Choi J.K. New observations on the choreotrich ciliate Strombidinopsis acuminata Fauré-Fremiet 1924, and comparison with Strombidinopsis jeokjo Jeong et al., 2004. J. Eukaryot. Microbiol. 2010;57:48–55. doi: 10.1111/j.1550-7408.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- Kormos J., Kormos K. Die Zellteilungstypen der Protozoen. Acta Biol. Hung. 1958;8:127–148. (with English and Russian summaries) [Google Scholar]
- Liu W., Xu D., Lin X., Li J., Gong J., Al-Rasheid K.A.S., Song W. Novistrombidium sinicum n. sp. and Novistrombidium orientale n. sp. (Protozoa: Ciliophora): two new oligotrich ciliates from a mangrove wetland, South China. J. Eukaryot. Microbiol. 2009;56:459–465. doi: 10.1111/j.1550-7408.2009.00425.x. [DOI] [PubMed] [Google Scholar]
- Lynn D.H., Montagnes D.J.S., Small E.B. Taxonomic descriptions of some conspicuous species in the family Strombidiidae (Ciliophora: Oligotrichida) from the Isles of Shoals, Gulf of Maine. J. Mar. Biol. Assoc. UK. 1988;68:259–276. [Google Scholar]
- Meunier A. Bulens; Bruxelles: 1910. Campagne arctique de 1907. Microplankton des mers de Barents et de Kara. i–xviii + 1–355 + Plates 1–36. [Google Scholar]
- Petz W. Morphology and morphogenesis of Strombidium kryalis nov. spec. (Ciliophora, Strombidiida) from Antarctic sea ice. Arch. Protistenk. 1994;144:185–195. [Google Scholar]
- Skovgaard A., Legrand C. Observation of live specimens of Pseudotontonia cornuta (Ciliophora: Oligotrichida) reveals new distinctive characters. J. Mar. Biol. Assoc. UK. 2005;85:783–786. [Google Scholar]
- Song W. Taxonomic description of two new marine oligotrichous ciliates (Protozoa, Ciliophora) J. Nat. Hist. (London) 2005;39:241–252. [Google Scholar]
- Song W., Wang M. Morphogenesis of Strombidium sulcatum during asexual binary division. Prog. Nat. Sci. 1996;6:741–746. [Google Scholar]
- Song W., Wang M., Warren A. Redescriptions of three marine ciliates, Strombidium elegans Florentin, 1901, Strombidium sulcatum Claparède & Lachmann, 1859 and Heterostrombidium paracalkinsi Lei, Xu & Song, 1999 (Ciliophora, Oligotrichida) Eur. J. Protistol. 2000;36:327–342. [Google Scholar]
- Wilbert N., Song W. New contributions to the marine benthic ciliates from the Antarctic area, including description of seven new species (Protozoa, Ciliophora) J. Nat. Hist. (London) 2005;39:935–973. [Google Scholar]
- Xu D., Song W. Hapantotypification and morphological redescription of the marine planktonic ciliate, Spirostrombidium cinctum (Kahl, 1932) Petz, Song et Wilbert, 1995 (Ciliophora: Oligotrichida) Acta Protozool. 2006;45:17–25. [Google Scholar]
- Xu D., Song W., Hu X. Notes on two marine ciliates from the Yellow Sea, China: Placus salinus and Strombidium apolatum (Protozoa, Ciliophora) J. Ocean Univ. China. 2005;4:137–144. [Google Scholar]
- Xu D., Song W., Lin X., Warren A. On two marine oligotrich ciliates, Spirostrombidium agathae n. sp. and S. schizostomum (Kahl, 1932) n. comb. from China, with a key to the identification of seven well-characterized Spirostrombidium spp. (Ciliophora: Oligotrichida) Acta Protozool. 2006;45:433–442. [Google Scholar]
- Xu D., Song W., Sun P., Chen X. Morphology and infraciliature of the oligotrich ciliate Strombidium rapulum (Yagiu, 1933) Kahl, 1934 (Protozoa, Ciliophora, Oligotrichida) from the intestine of sea urchin Hemicentrotus pulcherrimus Agassiz. Zootaxa. 2006;1113:33–40. [Google Scholar]
- Xu D., Song W., Warren A. Morphology and infraciliature of two new species of marine oligotrich ciliates (Ciliophora: Oligotrichida) from China. J. Nat. Hist. (London) 2006;40:1287–1299. [Google Scholar]
- Xu D., Song W., Warren A., Roberts D., Hu X. Redescriptions of two marine planktonic ciliates from China, Parastrombidium faurei (Kahl, 1932) Maeda, 1986 and Strombidium capitatum (Leegaard, 1915) Kahl, 1932 (Ciliophora, Oligotrichea) Eur. J. Protistol. 2007;43:27–35. doi: 10.1016/j.ejop.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Xu D., Sun P., Song W., Warren A. Studies on a new endocommensal ciliate, Strombidium foissneri nov. sp. (Ciliophora, Oligotrichida), from the intestine of the sea urchin Hemicentrotus pulcherrimus (Camarodonta, Echinoida) Denisia. 2008;23:273–278. [Google Scholar]
- Xu D., Warren A., Song W. Oligotrichs. In: Song W., Warren A., Hu X., editors. Free-living Ciliates in the Bohai and Yellow Seas, China. Science Press; Beijing: 2009. pp. 307–351. [Google Scholar]