Abstract
Tumor necrosis factor alpha (TNF-α) is a key mediator of host immune and inflammatory responses and inhibits herpesvirus replication by cytolytic and noncytolytic mechanisms. TNF-α effects are primarily mediated through the major TNF-α receptor, TNF-R1, which is constitutively expressed in most cell types. Here we show that the Epstein-Barr virus (EBV) immediate-early protein BZLF1 prevents TNF-α activation of target genes and TNF-α-induced cell death. These effects are mediated by down-regulation of the promoter for TNF-R1. Additionally, we demonstrate that expression of TNF-R1 is downregulated during the EBV lytic replication cycle. Thus, EBV has developed a novel mechanism for evading TNF-α antiviral effects during lytic reactivation or primary infection.
Epstein-Barr virus (EBV) is a ubiquitous human γ-herpesvirus that infects the majority of the world population. Primary infection with EBV occurs in the oropharynx via saliva exchange and causes the syndrome infectious mononucleosis (31). Additionally, EBV is associated with several B-cell and epithelial-cell malignancies. Following initial infection, EBV establishes a lifelong infection of the host by latently infecting B cells. However, the virus undergoes periodic spontaneous reactivation, resulting in lytic replication and release of virus into the saliva for transmission to new hosts.
EBV lytic infection is initiated by the expression of two immediate-early (IE) genes, BZLF1 and BRLF1 (32). The BZLF1 gene product (called BZLF1, Zta, ZEBRA, or EB1) is a member of the basic leucine zipper family of DNA binding proteins that binds to AP-1-like sites, termed Z response elements, present in EBV IE and early gene promoters. BZLF1 functions as a transcriptional transactivator and, along with BRLF1, induces the lytic cascade of viral gene expression.
The host response to viral infection includes the elaboration of antiviral cytokines, such as tumor necrosis factor alpha (TNF-α) and interferons. TNF-α is secreted by macrophages, lymphocytes, natural killer (NK) cells, and epithelial cells. TNF-α induces a wide range of genes that play a role in the immune and inflammatory response, including major histocompatibility complex (MHC) molecules, adhesion molecules, chemokines, and cytokines (37). In addition, TNF-α plays a role in host defense by induction of cellular apoptosis and has been demonstrated to act as a potent antiviral cytokine that is directly cytotoxic to cells infected with both RNA and DNA viruses, including herpesviruses (26, 40). In vitro, TNF-α has been shown to inhibit the replication of herpes simplex virus, varicella-zoster virus, and cytomegaloviruses (6, 16, 23). Additionally, administration of neutralizing anti-TNF-α antibodies leads to increased herpes simplex virus type 1 (19) and murine cytomegalovirus titers in vivo (10). However, the most compelling evidence of the importance of TNF-α in antiviral defense is the wide variety of mechanisms evolved by viruses to circumvent the effects of TNF-α (3).
Cellular responses to TNF-α are elicited by two distinct receptors, TNR receptor 1 (TNF-R1) and TNF-R2 (37). These receptors have sequence homology in their extracellular ligand binding domains; however, the intracellular sequences are mostly unique. TNF-R1 contains a death domain (DD), distinguishing this TNF receptor subtype as a member of the death receptor family. The DD promotes the recruitment of the adaptor protein TNF-R1-associated DD protein (TRADD) (14). TRADD then serves to recruit other adaptor proteins, including TNF-R-associated factor 2 (TRAF2), receptor-interacting protein (RIP), and Fas-associated DD protein (FADD) (12, 13). Recruitment of RIP and TRAF2 leads to activation of NF-κB and AP-1, whereas recruitment of FADD can lead to the recruitment and activation of caspase-8 and ultimately the activation of downstream effector caspases, such as caspase 3, and induction of apoptosis (20, 21, 36, 41).
In this report, we demonstrate that BZLF1 prevents cellular responses to TNF-α, including TNF-α-induced gene expression and, most importantly, TNF-α-induced cell death. Although the TNF-R1 is constitutively expressed in essentially all cell types, we show that BZLF1 downregulates TNF-R1 promoter activity, thus eliminating TNF-R1 expression at the RNA and protein levels. Thus, inhibition of TNF-R1 promoter activity represents a novel mechanism for attenuating TNF-α responsiveness in cells. Finally, we demonstrate that TNF-R1 is downregulated during reactivation of the EBV lytic cycle.
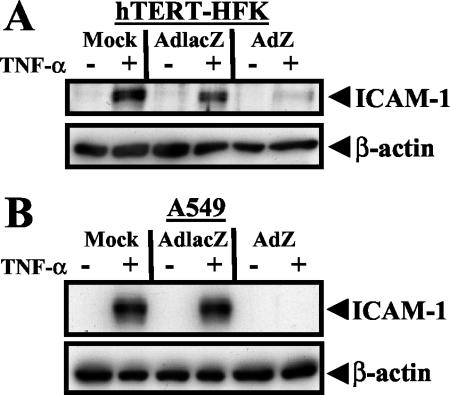
To investigate whether BZLF1 inhibits TNF-α-induced gene expression, telomerase-immortalized human keratinocytes (hTERT-HFK) (5) and A549 cells were mock-infected or infected with adenovirus vectors expressing either the LacZ gene (AdlacZ) or the BZLF1 gene (AdZ) (38), and at 30 h postinfection cells were left untreated or treated with TNF-α (10 ng/ml) for 12 h. Cells were lysed in radioimmunoprecipitation assay lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate supplemented with complete protease inhibitor cocktail [Roche]), and proteins were separated on a polyacrylamide gel. Immunoblot analysis was performed with an antibody directed against intracellular adhesion molecule-1 (ICAM-1) (1:500; Santa Cruz Biotechnology) (15) or β-actin (1:5,000; Sigma). In mock-infected and AdlacZ-infected hTERT-HFK and A549 cells, TNF-α treatment induced expression of the cell adhesion glycoprotein ICAM-1 (Fig. 1). In contrast, TNF-α-induced ICAM-1 expression was significantly inhibited in AdZ-infected cells (Fig. 1A and 1B). These results suggest that BZLF1 interferes with TNF-α-induced cellular gene expression.
FIG. 1.
BZLF1 inhibits TNF-α-induced gene expression. hTERT-HFK (A) and A549 (B) cells were mock infected or infected with adenovirus expressing LacZ or BZLF1 (MOI of 30). At 30 h postinfection, cells were left untreated or treated with 10 ng of recombinant TNF-α per ml for 12 h. Immunoblot analysis was performed with anti-ICAM-1 and anti-β-actin antibodies.
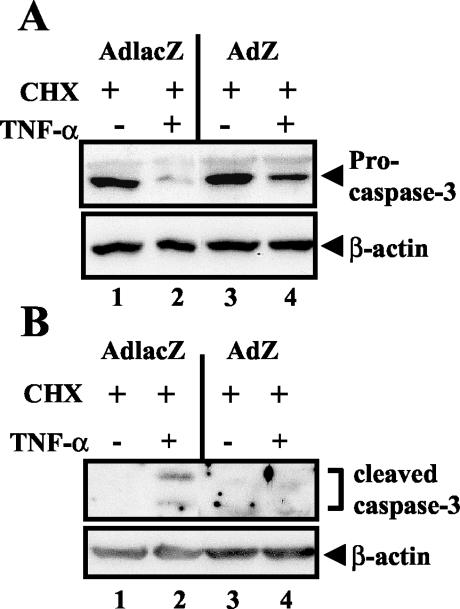
An important downstream effect of TNF-α signaling is the induction of apoptosis, which allows the elimination of virus-infected cells. To investigate the ability of BZLF1 to abolish this critical arm of TNF-R1 signaling, A549 cells were mock infected or infected with either AdlacZ or AdZ and at 36 h postinfection were left untreated or treated with 15 μg of cycloheximide (CHX) per ml alone or CHX in combination with TNF-α (10 ng/ml) for 4 h. Immunoblot analysis was performed with an antibody directed against caspase-3 (1:200 from Santa Cruz Biotechnology) or β-actin (1:5,000 from Sigma). As expected, in both mock infected and AdlacZ-infected cells CHX-TNF-α treatment resulted in decreased amounts of procaspase-3 (the full-length, unactivated form of the enzyme) (Fig. 2A) and the appearance of the cleaved, active forms of the enzyme (Fig. 2B). Treatment with CHX alone had no effect on caspase-3 activation (Fig. 2A and B). In contrast, expression of BZLF1 inhibited the cleavage of procaspase-3 (Fig. 2A) and the appearance of the cleaved, active forms of caspase-3 induced by the combination treatment (Fig. 2B), suggesting that the activation of caspases associated with TNF-α-induced apoptosis is inhibited by BZLF1.
FIG. 2.
BZLF1 inhibits TNF-α-induced caspase 3 activation. A549 cells were mock infected or infected with adenovirus expressing LacZ or BZLF1 (MOI of 30). At 36 h postinfection, cells were left untreated, treated with CHX alone (15 μg/ml), or treated with CHX (15 μg/ml) plus 10 ng of recombinant TNF-α per ml for 4 h. Immunoblot analysis was performed for the full-length proenzyme form of caspase-3 (A) and for the cleaved, active forms of capase-3 (p17 and p12) (B). Immunoblots were reprobed with anti-β-actin antibodies as a loading control.
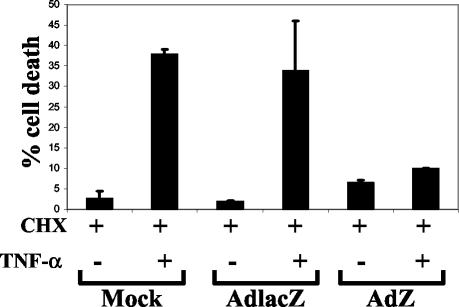
The effectiveness of BZLF1 expression in blocking apoptosis induced by TNF-α was measured by treating A549 cells as described above and determining cell viability by trypan blue exclusion assay. As shown in Fig. 3, treatment of mock-infected and AdlacZ-infected A549 cells with the combination of CHX plus TNF-α for 12 h resulted in averages of 38 and 34% cell death, respectively. BZLF1 expression reduced the amount of cell death induced by the combination treatment to an average of 10%. Additionally, AdZ-infected cells showed increased sensitivity to CHX treatment alone (Fig. 3), suggesting that the amount of cell death induced via TNF-R1 signaling might in fact be further reduced.
FIG. 3.
BZLF1 protects A549 cells from TNF-α-induced cell death. A549 cells were mock infected or infected with adenovirus expressing LacZ or BZLF1 (MOI of 30). At 36 h postinfection, cells were left untreated, treated with CHX alone (15 μg/ml), or treated with CHX (15 μg/ml) plus 10 ng of recombinant TNF-α per ml for 12 h. Apoptosis was assayed as a function of cell viability and measured by trypan blue exclusion assay.
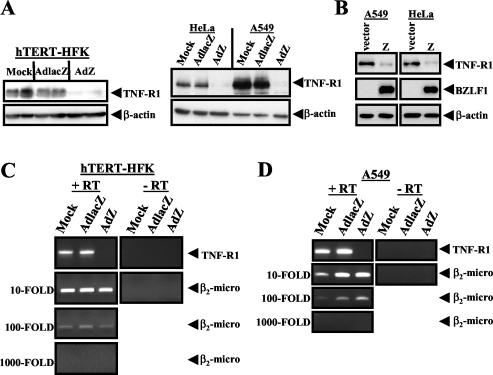
To determine at what point BZLF1 inhibits the TNF-R1 signaling pathway, TNF-R1 expression was quantitated by immunoblot analysis (using anti-TNF-R1 [1:200] from Santa Cruz) in hTERT-HFK, A549, and HeLa cells that were mock infected or infected with either AdlacZ or AdZ. In all cell lines tested, expression of BZLF1 dramatically decreased the protein level of TNF-R1 (Fig. 4A). This decrease was not due to induction of TNF-α by AdZ, as analysis of hTERT-HFK and A549 cell culture supernatants by TNF-α ELISA was negative for all groups (data not shown). Importantly, as previously published, the level of cellular BZLF1 expression in hTERT-HFK, A549, and HeLa cells infected with AdZ at the multiplicity of infection (MOI) used in these experiments is similar to the level of BZLF1 expressed in the lytically infected population of an epithelial cell line (AGS) infected with the intact virus (25, 27). To confirm that the BZLF1-induced decrease of TNF-R1 expression is not an adenovirus-mediated effect, immunoblot analysis was also performed on HeLa cells and A549 cells that had been transfected with a BZLF1 expression plasmid. Cells were seeded into six-well plates 1 day prior to transfection. One microgram of plasmid DNA was combined with 3 μl of FuGENE 6 transfection reagent (Roche) in OptiMEM (Invitrogen) and added directly to cell cultures, and cell extracts were prepared 48 h posttransfection. As shown in Fig. 4B, BZLF1 expression from plasmid DNA also decreased TNF-R1 expression in each of these cell types.
FIG. 4.
BZLF1 decreases TNF-R1 protein and RNA levels. (A) hTERT-HFK, HeLa, and A549 cells were mock infected or infected with adenovirus (MOI of 50) expressing either LacZ or BZLF1 for 48 h. Immunoblot analysis was performed with anti-TNF-R1 and anti-β-actin antibodies. (B) A549 and HeLa cells were transfected with a vector control or a BZLF1 expression plasmid. At 48 h posttransfection, cell lysates were examined by immunoblot analysis with anti-TNF-R1, anti-BZLF1, and anti-β-actin antibodies. HTERT-HFK (C) and A549 (D) cells were mock infected or infected with adenovirus (MOI of 50) expressing either LacZ or BZLF1. Semiquantitative reverse transcription-PCR analysis was performed by using serial dilutions of input cDNA with oligonucleotide primers specific for TNF-R1 mRNA sequences or with primers for β2-microglobulin sequences.
To determine if decreased TNF-R1 expression is due to a loss of the TNF-R1 message, we examined the effect of BZLF1 on TNF-R1 RNA expression. Total RNA was isolated from hTERT-HFK and A549 cells by using an RNeasy kit (Qiagen) following the manufacturer's instructions, and cDNA was synthesized with a reverse transcription system (Promega). Semiquantitative PCR was performed by using serial dilutions of input cDNA with the following oligonucleotides: TNFR1, sense, 5′-GTGCCAACAAAGGAACCTACTTGTAC-3′ (spans exon 2 and 3 of genomic sequence); antisense, 5′-GGCCAGGGGCTTAGTAGTAGTTC-3′ (within exon 9 of genomic sequence); β2-microglobulin, sense, 5′-TTCTGGCCTGGAGGGCATCC-3′ (within exon 1 of genomic sequence); antisense, 5′-ATCTTCAAACCTCCATGATG-3′ (within exon 3 of genomic sequence). Semiquantitative reverse transcription-PCR analysis revealed that the level of TNF-R1 RNA was greatly reduced in cells that expressed BZLF1 (Fig. 4C and D). The level of a control transcript, β2-microglobulin, was unaffected by expression of BZLF1 (Fig. 4C and D). Taken together, these results indicate that expression of BZLF1 dramatically decreases cellular expression of TNF-R1 and suggest that the decrease may be a BZLF1-mediated transcriptional effect.
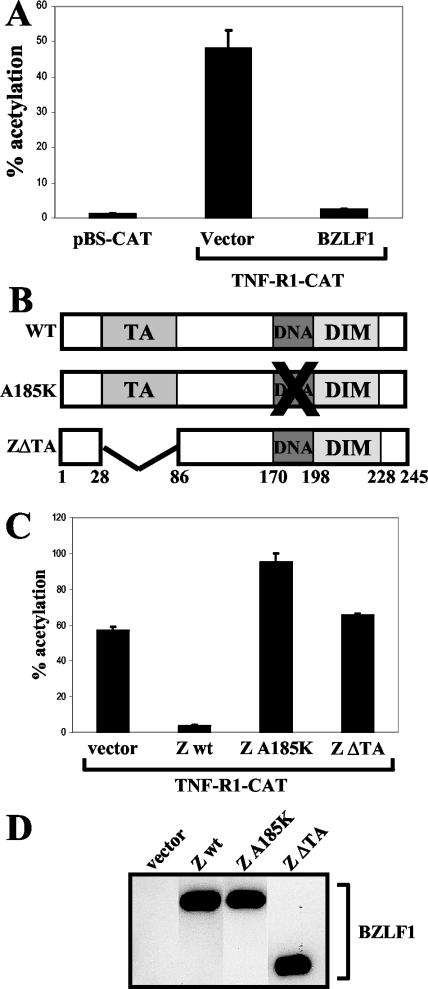
A great deal of research has focused on posttranscriptional and posttranslational regulation of TNF-R1; in contrast, very little is known about the regulation of the TNF-R1 promoter. A 5′ region upstream of the TNF-R1 start codon has been shown to encode promoter activity, and the transcriptional start sites have been determined (18). In order to investigate the effect of BZLF1 on TNF-R1 promoter activity, a TNF-R1-CAT reporter plasmid was constructed by PCR cloning of a 654-bp region from the human genome corresponding to the −619/+35 region of the TNF-R1 promoter (relative to the major transcriptional start site), as previously defined (18). These sequences were inserted upstream of the chloramphenicol acetyltransferase (CAT) reporter gene, and the resulting plasmid was used in cotransfection assays. At 48 h posttransfection, cells were lysed directly in 0.25 M Tris, pH 7.5. Aliquots of cell extracts were incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A. Acetylated forms of chloramphenicol were resolved by thin-layer chromatography, and the percent acetylation of chloramphenicol was quantitated by PhosphorImager screening (Molecular Dynamics). The TNF-R1-CAT construct had significant constitutive activity in HeLa cells relative to the promoterless CAT vector control (Fig. 5A). Furthermore, cotransfection of the TNF-R1-CAT construct with a BZLF1 expression vector dramatically reduced TNF-R1 promoter activity (Fig. 5A). Similar results were obtained with A549 cells (data not shown).
FIG. 5.
BZLF1 inhibits TNF-R1 promoter activity. (A) HeLa cells were transfected with 500 ng of vector control or BZLF1 expression plasmids along with 500 ng of pBS-CAT or TNF-R1-CAT reporter plasmids. At 48 h posttransfection, cells were harvested and extracts analyzed for CAT activity. (B) A schematic depicting the structures of the wild-type and mutant BZLF1 proteins is shown. The transactivation domain (TA), DNA binding domain (DNA), and dimerization domain (DIM) are indicated along with the respective amino acid numbers. (C) HeLa cells were transfected with 500 ng of vector control or various BZLF1 expression plasmids along with 500 ng of the TNF-R1-CAT reporter plasmid. wt, wild type. (D) Immunoblot analysis was performed to confirm equal expression of BZLF1 wild-type and mutant proteins in extracts used for panel C.
We next determined the domains of the BZLF1 protein required to inhibit TNF-R1 promoter activity by cotransfecting the TNF-R1-CAT reporter plasmid with various BZLF1 wild-type and mutant expression plasmids into HeLa cells. Plasmids pSG5-Zwt and pSG5-ZA185K were constructed by subcloning wild-type BZLF1 cDNA or A185K BZLF1 cDNA (containing a point mutation that alters amino acid 185 from alanine to lysine) from the pHD1013 vector (29) into the EcoRI site of pSG5 (Stratagene). Plasmid pSG5-ZΔTA was generated by HindIII cleavage of pSG5-Zwt followed by religation, producing an in-frame deletion of amino acids 24 to 86. As shown in Fig. 5C, a BZLF1 mutant unable to bind DNA due to a single amino acid change at residue 185 in the DNA binding domain completely lost the ability to inhibit TNF-R1 promoter activity. Additionally, removal of amino acids 24 to 86, which deletes the BZLF1 transactivation domain, abolished the ability of BZLF1 to inhibit TNF-R1 promoter activity (Fig. 5C). Immunoblot analysis confirmed similar expression levels of wild-type and mutant BZLF1 proteins, as depicted in one representative blot in Fig. 5D. These results indicate that BZLF1 downregulates TNF-R1 expression by inhibiting TNF-R1 promoter activity.
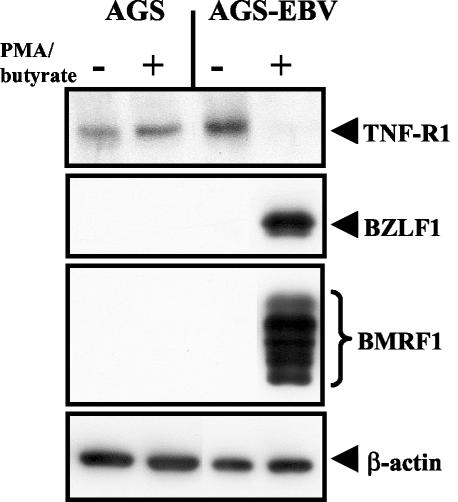
To determine whether TNF-R1 is downregulated during EBV lytic replication, we generated EBV-infected gastric carcinoma cell lines. AGS-EBV cells were generated by infecting gastric carcinoma AGS cells with a recombinant EBV that contains a gene encoding green fluorescent protein and a hygromycin resistance cassette (a gift from Henri J. Delecluse) (7). EBV-positive cells were selected with 100 μg of hygromycin B per ml. EBV is associated with up to 10% of human gastric carcinomas (35), and lytic cycle antigens, including BZLF1, have been detected in tumor cells, indicating that lytic EBV infection occurs in EBV-positive gastric carcinomas (11). EBV-negative and EBV-positive AGS cells were treated with 50 ng of phorbol myristate acetate per ml and 1 mM butyrate for 24 h. As shown in Fig. 6, treatment of AGS-EBV cells resulted in high-level expression of both BZLF1 and the early gene BMRF1 from the endogenous EBV genome, indicating that the virus had entered the lytic replication cycle. TNF-R1 expression was significantly decreased in AGS-EBV cells induced to enter the lytic replication cycle (Fig. 6), while the expression of TNF-R1 in the parental AGS cells was not affected by treatment with the lytic-cycle-inducing agents (Fig. 6). These results suggest that expression of TNF-R1 is downregulated in cells lytically infected with EBV.
FIG. 6.
TNF-R1 expression is downregulated during EBV lytic reactivation. EBV-negative AGS and EBV-positive AGS-EBV cells were treated with the lytic-cycle-inducing agents phorbol myristate acetate (50 ng/ml) and butyrate (1 mM) for 24 h. Immunoblot analysis was performed using anti-TNF-R1, anti-BZLF1, anti-BMRF1, and anti-β-actin antibodies.
In this paper, we report that the EBV IE protein BZLF1 inhibits cellular responses to TNF-α by downregulating expression of TNF-R1. Additionally, we have found that BZLF1 downregulates TNF-R1 expression by inhibiting TNF-R1 promoter activity. To our knowledge, this is the first report describing regulation of TNF-R1 by a viral protein at the promoter level.
The fact that other DNA viruses encode functions that directly antagonize TNF-R1 activity suggests an important role for this receptor in antiviral defenses. Recently, both HCMV (2) and murine cytomegalovirus (28) have been shown to target TNF-R1. Additionally, poxvirus, adenovirus, and human papillomavirus (8, 30, 34) also target TNF-R1 signaling. Our finding that BZLF1 downregulates the TNF-R1 promoter represents a novel mechanism used by a virus to inhibit the action of this receptor.
TNF-α induces a wide array of molecules, such as cytokines, MHC, and adhesion molecules, which mediate the inflammatory response and regulate immune cell function. Thus, the ability of BZLF1 to inhibit TNF-α-induced gene expression, including ICAM-1, may be an important mechanism to limit immune responses to viral infection.
Importantly, we also found that BZLF1 inhibits the induction of cell death by TNF-α. Because apoptosis induced by death receptor family members, such as TNF-R1, plays a key role in the host response to viral infection, our data suggest that inhibition of TNF-R1-induced apoptosis by BZLF1 may protect virus-infected cells, allowing the virus to complete its replication cycle.
TNF-R1 is constitutively expressed on most cell types (22, 33) and thought to be regulated primarily via posttranscriptional (39) and posttranslational mechanisms. In particular, shedding of a soluble form of the TNF-R1 protein plays a key role in attenuating TNF-α effects (9). To date, however, few studies have focused on regulation of the TNF-R1 promoter. Mutational analysis of BZLF1 revealed that inhibition of TNF-R1 promoter activity requires both the transactivation and the DNA binding domains of BZLF1. These results suggest that BZLF1 may bind to and activate the promoter of a gene that encodes a repressor of the TNF-R1 promoter. Less likely, BZLF1 may bind directly to the TNF-R1 promoter and prevent expression.
Interestingly, our lab reported previously that expression of BZLF1 also downregulates a component of the cellular receptor for gamma interferon (IFN-γ), IFN-γ-Rα, and likewise inhibits cellular responses to IFN-γ (27). Thus, downregulation of TNF-R1, together with downregulation of IFN-γ-Rα, by BZLF1 may prolong the life of a lytically infected cell and enhance virus production.
In summary, this report describes a novel mechanism to regulate expression of TNF-R1 and inhibit the cellular responses to TNF-α. These data, together with previous reports that BZLF1 induces immunosuppressive cytokines such as interleukin 10 and transforming growth factor beta (4, 24), inhibits cellular responses to IFN-γ (27), and disperses nuclear promyelocytic leukemia bodies (1) and the recent report that BZLF1 inhibits upregulation of MHC class I expression by the viral protein LMP1 (17), provide compelling evidence that BZLF1 plays a major role in viral subversion of host immune responses.
Acknowledgments
This work was supported by grants 2-R01-CA58853 and P01-CA19014 from the National Institutes of Health.
We thank the UNC Gene Therapy Center for preparation of the adenovirus vectors.
REFERENCES
- 1.Adamson, A. L., and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie, J., A. Sahlender, and J. H. Sinclair. 2003. Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-α) signaling by targeting the 55-kilodalton TNF-α receptor. J. Virol. 77:7007-7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 4.Cayrol, C., and E. K. Flemington. 1995. Identification of cellular target genes of the Epstein-Barr virus transactivator Zta: activation of transforming growth factor βigh3 (TGF-βigh3) and TGF-β1. J. Virol. 69:4206-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feduchi, E., M. A. Alonso, and L. Carrasco. 1989. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J. Virol. 63:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippova, M., H. Song, J. L. Connolly, T. S. Dermody, and P. J. Duerksen-Hughes. 2002. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells for TNF-induced apoptosis. J. Biol. Chem. 277:21730-21739. [DOI] [PubMed] [Google Scholar]
- 9.Galon, J., I. Aksentijevich, M. F. McDermott, J. J. O'Shea, and D. L. Kastner. 2000. TNFRSF1A mutations and autoinflammatory syndromes. Curr. Opin. Immunol. 12:479-486. [DOI] [PubMed] [Google Scholar]
- 10.Heise, M. T., and H. W. Virgin IV. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshikawa, Y., Y. Satoh, M. Murakami, M. Maeta, N. Kaibara, H. Ito, T. Kurata, and T. Sairenji. 2002. Evidence of lytic infection of Epstein-Barr virus (EBV) in EBV-positive gastric carcinoma. J. Med. Virol. 66:351-359. [DOI] [PubMed] [Google Scholar]
- 12.Hsu, H., J. Huang, H. B. Shu, V. Baichwal, and D. V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF-receptor 1 signaling complex. Immunity 4:387-396. [DOI] [PubMed] [Google Scholar]
- 13.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, H., J. Xiong, and D. V. Goeddel 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 15.Ishido, S., J.-K. Choi, B.-S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 16.Ito, M., T. Nakano, T. Kamiya, K. Kitamura, T. Ihara, H. Kamiya, and M. Sakurai. 1991. Effects of tumor necrosis factor on replication of varicella-zoster virus. Antivir. Res. 15:183-192. [DOI] [PubMed] [Google Scholar]
- 17.Keating, S., S. Prince, M. Jones, and M. Rowe. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 76:8179-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemper, O., and D. Wallach. 1993. Cloning and partial characterization of the promoter for the human p55 tumor necrosis factor (TNF) receptor. Gene 134:209-216. [DOI] [PubMed] [Google Scholar]
- 19.Kodukula, P., T. Liu, N. V. Rooijen, M. J. Jager, and R. L. Hendricks. 1999. Macrophage control of herpes simplex virus 1 replication in the peripheral nervous system. J. Immunol. 162:2895-2905. [PubMed] [Google Scholar]
- 20.Lee, S. Y., A. Reichlin, A. Santana, K. A. Sokol, M. C. Nussenzweig, and Y. Choi. 1997. TRAF2 is essential for JNK but not NF-κB and regulates lymphocyte proliferation and survival. Immunity 7:703-713. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 22.Loetscher, H., Y.-C. E. Pan, H.-W. Lahm, R. Gentz, M. Brockhaus, H. Tabuchi, and W. Lesslauer. 1990. Molecular cloning and expression of the human 55kd tumor necrosis factor receptor. Cell 61:351-359. [DOI] [PubMed] [Google Scholar]
- 23.Lucin, P., S. Jonjic, M. Messerle, B. Polic, H. Hengel, and U. H. Kosinowski. 1994. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumor necrosis factor. J. Gen. Virol. 75:101-110. [DOI] [PubMed] [Google Scholar]
- 24.Mahot, S., A. Sergeant, E. Drouet, and H. Gruffat. 2003. A novel function of the transcription factor EB1/Zta: induction of transcription of the hIL-10 gene. J. Gen. Virol. 84:965-974. [DOI] [PubMed] [Google Scholar]
- 25.Mauser, A., S. Saito, E. Appella, C. W. Anderson, W. T. Seaman, and S. C. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J. Virol. 76:12503-12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestan, J., W. Digel, S. Mittnacht, H. Hillen, D. Blohm, A. Moller, H. Jacobsen, and H. Kirchner. 1986. Antiviral effects of recombinant tumor necrosis factor in vitro. Nature 323:816-819. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, T. E., A. Mauser, A. Wong, J. P.-Y. Ting, and S. C. Kenney. 2001. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 28.Popkin, D. L., and H. W. Virgin IV. 2003. Murine cytomegalovirus infection inhibits tumor necrosis factor alpha responses in primary macrophages. J. Virol. 77:10125-10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinlivan, E., E. Holley-Guthrie, M. Norris, D. Gutsch, S. Bachenheimer, and S. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reading, P. C., A. Khanna, and G. L. Smith. 2002. Vaccinia virus CrmE encodes a soluble and cell surface tumor necrosis factor receptor that contributes to virus virulence. Virology 292:285-298. [DOI] [PubMed] [Google Scholar]
- 31.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 32.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 33.Schall, T. J., M. Lewis, K. J. Koller, A. Lee, G. C. Rice, G. H. W. Wong, T. Gatanaga, G. A. Granger, R. Lentz, H. Raab, W. J. Kohr, and D. V. Goeddel. 1990. Molecular cloning and expression of a receptor for human tumor necrosis factor receptor. Cell 61:361-370. [DOI] [PubMed] [Google Scholar]
- 34.Shisler, J. L., C. Yang, B. Walter, C. F. Ware, and L. R. Gooding. 1997. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J. Virol. 6:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada, K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ting, A. T., F. X. Pimentel-Muinos, and B. Seed. 1996. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1 initiated apoptosis. EMBO J. 22:6189-6196. [PMC free article] [PubMed] [Google Scholar]
- 37.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 38.Westphal, E.-M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 39.Winzen, R., S. Kafert, B. Preiβ, H. A. Mylius-Spencker, K. Resch, and H. Holtmann. 1996. Interaction between the mRNA of the 55-kDa tumor necrosis factor receptor and cellular proteins. Possible involvement in post-transcriptional regulation of receptor expression. J. Biol. Chem. 271:13461-13467. [DOI] [PubMed] [Google Scholar]
- 40.Wong, G. H. W., and D. V. Goeddel. 1986. Tumor necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature 323:819-822. [DOI] [PubMed] [Google Scholar]
- 41.Yeh, W. C., J. L. Pompa, M. E. McCurrach, H. B. Shu, A. J. Elia, A. Shahinian, M. Ng, A. Wakeham, W. Khoo, K. Mitchell, W. S. El-Deiry, S. W. Lowe, D. V. Goeddel, and T. W. Mak. 1998. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279:1954-1958. [DOI] [PubMed] [Google Scholar]