Abstract
To study the pharmacokinetic profile of artemether in children and in the context of antiviral drugs for HIV infected patients co-infected with malaria, an LC-MS/MS method was developed and validated to simultaneously determine artemether and its metabolite dihydroartemisinin in human plasma. Using artemisinin as the internal standard, 0.5 mL samples were processed with solid phase extraction (Waters Oasis® HLB column), the elutes were directly injected onto a C18 LC column (Waters, Symmetry®, 150 × 4.6 mm, 5μm). Mass detection utilized ESI+ as the ionization mode and MRM as the quantitation mode. In respect to the low ionization capacity of artemether, ammonium formate was added to the LC mobile phase to facilitate ionization (M+NH4+). The calibration range was 2 – 200 ng/mL. The recovery was 73-81% for artemether and 90-99% for dihydroartemisinin. The validated method was applied to analysis of clinical samples with results in good agreement with an existing method.
Keywords: LC-MS/MS, artemether, dihydroartemisinin, artemisinin, solid phase extraction
1. Introduction
Malaria is a life threatening disease caused by malaria parasites (Plasmodium strains) and transmitted by the female anopheles mosquitoes. Its symptoms include fever, chills, sweats, headache, nausea, and vomiting. Each year the estimated cases of malaria are 350 – 500 million leading to 1.5 – 2.7 million deaths worldwide. Particularly, 9% of all deaths in children less than 5 years of age are attributed to the disease and this proportion is as high as 20% in sub-Saharan Africa [1].
Artemether (ARM) is a semi-synthetic derivative of artemisinin (Qinghaosu). Qinghaosu is a natural product first discovered in China as the major active component in Qinghao (Artemisia annua L. or sweet wormwood) for malaria therapy [2]. Artemether has an improved bioavailability compared to artemisinin and is the compound most widely used clinically. Both ARM and artemisinin are rapidly metabolized to dihydroartemisinin (DHA) that also has potent antimalarial activity. To support our pharmacokinetic studies of ARM in pediatric patients and HIV infected patients co-infected with malaria, a sensitive and specific analytical method for ARM and DHA quantification is needed.
Several LC/MS methods have been reported for the quantification of artemisinin and its derivatives [3-8]. Three of these methods simultaneously determine ARM and DHA in human plasma [3-5], but either Q-TOF as the analyser or APCI as the ionization mode was used. The LLOQ was 5 ng/mL or higher. We currently report an alternative method using electro-spray ionization in positive ion mode (ESI+) as the ionization mode and multiple reactions monitoring (MRM) as the quantitation mode to determine ARM and DHA simultaneously.
2. Experimental
2.1. Reagents and materials
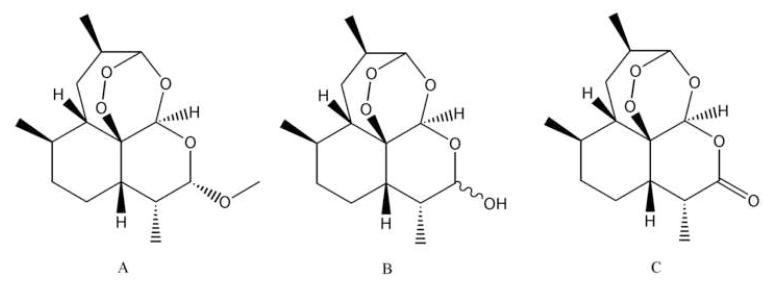
ARM, DHA, and artemisinin (I.S.) reference standards were purchased from A.K. Scientific (Mountain View, CA) (Figure 1). Acetonitrile (MeCN), methyl acetate, methanol, water, ammonium formate (NH4FA), and formic acid were obtained from Fisher Scientific (Fair Lawn, NJ, USA). All chemicals were of HPLC grade. Water was distilled water if not mentioned specifically. Human plasma was purchased from Biological Specialty Co., Colmar, PA, USA).
Figure 1.
Chemical structures of artemether (A), dihydroartemisinin (B) and artemisinin, the I.S. (C).
2.2. Instrumental and analytical conditions
The PE Sciex API 2000 triple quadrupole mass spectrometer with TurboIonSpray sample inlet was purchased from Perkin-Elmer-Sciex (Concord, Ont., Canada; currently owned by Applied Biosystem, Foster City, CA, USA). Perkin-Elmer (Norwalk, CT, USA) supplied the PE Biosystems 200 series autosampler and twin PE Biosystems series 200 micro HPLC pumps. Chromatographic separation was achieved on a C18 analytical column (150 × 4.6 mm, 5 μm; Symmetry®, Waters, USA) equipped with a pre-column filter (MAC-Mod Analytical, Chadds Ford, PA). The LC setting was as follows: solvent A was aqueous 10 mM NH4FA at pH 4.1. Solvent B was MeCN with 0.1% formic acid. Injection volume was 50 μL. LC elution was accomplished with 80% solvent B in isocratic mode at a flow rate of 1 mL/min for 6 min. The eluent split via a “T” connector and ~0.3 mL was directed to the mass spectrometer (MS) source. The divert valve was set to direct LC eluent to MS source at 2.0 min and to waste line at 5.9 min. The retention times for ARM, DHA, and I.S. were 5.0 2.4, and 2.7 min, respectively. Before each injection, the needle was cleaned with one pre-injection wash and after injection with two post-injection washes, each with 700 μL of MeCN-water (85-15, v/v) containing 0.1% formic acid. The MS conditions for ARM, DHA, and the I.S. were optimized by separate infusion of 1 μg/mL of each compound in 10 mM NH4FA aqueous solution-MeCN (1:1) into the MS at a flow rate of 10 μL/min constantly while adjusting the MS parameters to achieve maximal signal. ESI+ was used for ionization and MRM mode was chosen for quantification. Ammonium adduct (M+NH4+) ion pairs were selected from Q1. The precursor-product ion pair was m/z 316 → 267 for ARM, m/z 302 → 267 for DHA, and m/z 300 → 209 for the I.S. artemisinin. The optimized acquisition parameters were as follows: Turbo (Heater) set at 275 °C; Curtain gas (CUR), 25 psi (99.999% nitrogen); Nebulizer Gas (Gas 1), 40 psi (nitrogen); Auxiliary (turbo) Gas (Gas2), 60 psi (nitrogen); Collision-Activated Dissociation (CAD) Gas: 4; IonSpray Voltage (IS),5500 v. The optimized parameters for ARM, DHA, and I.S. are summarized in Table 1. The scan time was set at 250 ms for each transition. Data was processed with Analyst 1.4.2. (Applied Biosystem, Foster City, CA, USA).
Table 1.
MS parameters for artemether, dihyroartemisinin, and I.S.
| DP | FP | EP | CE | CEP | CXP | |
|---|---|---|---|---|---|---|
| ARM, 316/267 |
5 | 340 | 5 | 12.5 | 10 | 5 |
| DHA, 302/267 |
4 | 380 | 6 | 13 | 9 | 12 |
| Artemisinin 300/209 |
4 | 370 | 6 | 13 | 10 | 10 |
Note: DP is declustering potential. FP is focusing potential, helps to focus the ions through the skimmer. EP is entrance potential. CE is collision energy. CEP is collision cell entrance potential, CXPis collision cell exit potential.
2.3. Preparation of standard and quality control samples
Primary stock solutions of ARM, DHA, and I.S. were prepared at 1 mg/ml for each in 50% MeCN in water. DHA solution freshly made from solid form required standing at room temperature overnight for epimer equilibration (2α↔2β) [9]. Thesesolutions were diluted with 25% MeCN in water to prepare working stock solutions (10 μg/mL) and working solution. The I.S. working solution (200 ng/mL) was prepared in 5% MeCN in water. The working solutions of ARM-DHA were spiked to blank plasma at 1:25 ratio to obtain calibration standards of 2, 5, 10, 20, 50, 100, 150, and 200 ng/mL. QC samples were spiked at 6, 80, and 170 ng/mL by adding working solutions into blank human plasma at 1:25 ratio. Calibration standards and QC samples were prepared from separately weighted stock solutions. The stock solutions, standards, QC samples, and the I.S. solution were stored at −70 °C freezer between uses.
2.4. Sample preparation
A 0.5 mL aliquot of each standard, QC, and blank plasma was thawed and loaded onto a SPE column (Waters, Oasis® HLB, 1cc, 10mg) that was preconditioned with 1 mL MeOH and 1 mL water subsequently. After each sample was drained through the column completely, the column was washed with water (1 mL × 3) and 10% MeCN (0.5 mL) subsequently. The column was then dried under vacuum (~8 in Hg) for 30 min, followed by addition of MeCN-methyl acetate (9:1, 150 μL) to elute samples. Mild vacuum (2-4 in Hg) was applied to pull out residual solvent. The eluted sample (~100 μL) was transferred into an autosampler vial and 50 μL was injected into the LC-MS/MS system. All plasma samples were processed immediately within 30 minutes once they were thawed at room temperature.
2.5. Method validation procedure
2.5.1. Acceptance criteria
The method validation was conducted according to the ACTG guidelines [10], which were developed based on Food and Drug Administration (FDA) guidelines. The assay is considered acceptable if precision, expressed as relative standard deviation (RSD) or coefficient of variation (%CV), is less than 15% for intra and inter-day variation. RSD for lower limit of quantitation (LLOQ) was set at <20%. Accuracy compared to the nominal value (% deviation) is to be within 15% for intra- and inter-day comparison (% deviation for LLOQ was set at <20%). The calibration curve should have a correlation coefficient R of 0.995 or better. The back-calculated values for standards should be within 15% of target (20% at LLOQ). If points are removed from the standard curve, it is recalculated. At least six (6) nonzero concentration points must be used to derive the standard curve, and >75% of points must be within 15% of the targeted value (LLOQ must be within 20%).
2.5.2. Calibration curve
Calibration curves were obtained by quadratic regression of the peak area ratio of analyte to internal standard (Y-axis) versus the nominal analyte concentrations (X-axis) with a weighting factor of 1/x.
2.5.3. Lower limit of quantitation (LLOQ)
The LLOQ was established using five samples independent of standards to determine accuracy and precision. The accuracy should be within 20% of the nominal concentration and precision should be <20%. The signal intensity of the LLOQ should be ≥ 5-fold blank response.
2.5.4. Intra- and inter-day precision and accuracy
Intra-day precision and accuracy were determined by analysis of five replicates of each QC sample (n=5) at low (6 ng/mL), medium (80 ng/mL), and high (170 ng/mL) concentration levels extracted with a set of standards in one batch. The same procedure was repeated on five (5) different days with new samples to determine inter-day precision and accuracy (total: n = 25 per concentration level). Precision was reported as RSD or %CV and accuracy as percent of the nominal concentration (% deviation).
2.5.5. Recovery and matrix effect
Three sets of samples were prepared: Set 1, un-extracted analytes, MeCN-methyl acetate (9:1) spiked with ARM-DHA at low (30 ng/mL) and high (850 ng/mL) levels in triplicates (n = 3), the IS was spiked at 100 ng/mL. Set 2, post-extraction spike of analytes into extracted matrix, a 500 μL aliquot of blank plasma was processed with SPE and 80 μL of the extract was spiked with ARM-DHA and IS to make the final concentration the same level as that of the set 1 samples. Set 3, Pre-extraction spike of analytes into matrix then extract, a 500 μL aliquot of each plasma validation samples at the low (6 ng/mL) and high (170 ng/mL) concentration levels was processed in triplicates like real samples. The volume of eluent from the SPE column was about 100 μL. After the processing step, the validation samples were condensed by ~ 5-fold. The recovery of ARM and DHA from plasma following sample preparation was assessed by comparing the peak area of ARM and DHA from set 3 to the peak area of the same concentration of ARM and DHA from set 2. Matrix effects were evaluated by comparing the peak area of ARM-DHA from set 2 to that from set 1. Process efficiency was evaluated by comparing set 3 to set 1. The recovery, matrix effect and process efficiency were calculated with the following formulae:
Matrix effect was also evaluated with an infusion experiment: A mixture of ARM, DHA, and I.S. (1 μg/mL each, 10 μL/min) was infused into the split LC elute (0.3 mL/min) via a “T” connector constantly and the LC elute was directed into the MS source. A 50 μL aliquot of blank plasma extract was injected onto LC column and signals for ARM, DHA, and the I.S. were monitored for 25 min. A 50 μL aliquot of clean ARM-DHA-I.S. solution at 1 μg/mL each was injected as reference. According to ACTG guidelines, 6 different lots of blank plasma were also processed and injected into the LC-MS/MS. Medium QCs were prepared in triplicates with each of 6 plasma lots, processed and injected into LC-MS/MS, their concentrations were compared with nominal concentration (80 ng/mL).
Potential interference of possible concomitant HIV drugs was also evaluated by spiking the medium QC (80 ng/mL) with each of the tested drugs to make a final concentration of 5000 ng/mL (n = 3). The spiked samples were analyzed as usual and compared with medium QC response without other drugs. The following HIV drugs were tested: efavirenz, zidovudine, tenofovir, atazanavir, lopinavir , ritonavir, nelfinavir, nevirapine, indinavir, amprenavir, abacavir, and saquinavir.
2.5.6. Stability
The stability of ARM and DHA in human plasma was evaluated at these conditions: 3 freeze-thaw cycles, storage at −70 °C for 101 days, room temperature (22 °C) for 1 and 3 hours. Each condition was tested with QC samples at low and high concentration levels in triplicates. Fresh samples were used as reference. Stability of processed samples was evaluated after staying in vials in the tray overnight. Stock solutions for ARM and DHA were evaluated at −70 °C for 112 days and 22 °C for 7 days. The I.S. stock solution was tested at −70 °C for 200 days and 22 °C for 3 days.
2.6. Application of the method
The method was further evaluated by analysis of clinical plasma samples collected from three healthy volunteer subjects at various time points after administration of Coartem® (ARM/lumefantrine, 80/480mg) twice daily for 3 days. Blood samples were collected in EDTA-containing tubes prior to the sixth dose (0 hr) and at 0.5, 1, 2, 4, 6, 8, 12 hr after the sixth dose. The tubes were immediately placed on ice and then centrifuged at 2,000g for 10 minutes at 4°C. The resulting plasma was split into aliquots and kept at −70°C until analysis by LC-MS/MS.
3. Results and discussion
3.1. LC-MS/MS optimization
In published literature different C18 columns have been used for separation of ARM and DHA (3-7). Initially, a Zorbax Eclipse XDB-C18 column (50 × 2.1 mm, 5 μm) was selected for this assay as our previous LC columns for the MS were from this source. The peaks for ARM, DHA, and I.S. were tailed. Poor peak shapes caused inconsistency during peak integration and introduced variation to the assay. To improve the peak shape, we tried to adjust the mobile phase by changing the pH (neutral or addition of TFA) and increasing the ammonium formate salt concentration. However, no improvement was achieved. Walter Cabri and coworkers [9] compared nine different brands of C18 columns and found Waters Symmetry® C18 yielded the best peak shapes for DHA and artemisinin (I.S. in this assay). Therefore, we switched to a Symmetry® C18 column (150 × 4.6 mm, 5 μm), which yielded improved peak shapes for ARM and DHA. For mass detection, ESI+ was used for ionization. [M+H]+ for ARM was invisible and [M+H]+ for DHA was also in low abundance. Therefore, ammonium adduct [M+NH4]+ was selected from the mass spectrometer source. The precursor-product ion pairs 316/267 for ARM, 302/267 for DHA and 300/209 for IS were monitored in MRM mode. A representative precursor-daughter ions chromatogram for ARM, DHA, and I.S. is presented in Figure 2. Turbo heat set at 275 °C, because higher temperature caused fragmentation of ARM while DHA was not affected. The higher turbo gas (gas 2), the stronger the signal of DHA, therefore, Gas 2 set at 60 psi. The ESI+ probe position was also adjusted to be closer to the orifice to achieve higher sensitivity. The signal intensity for DHA is stronger than ARM, but the background signal (baseline) is also higher for DHA (~200 cps) than ARM (<50 cps). Compared to MeCN, methanol as mobile phase yielded a dramatically higher signal for both ARM (~10-fold) and DHA (~5-fold), but the noise level was also increased, resulting in no improvement in LLOQ. Hence, MeCN with 0.1% formic acid was used in the mobile phase. We added 0.1% formic acid simply to avoid solvent change when other LC-MS/MS assays were run in the lab.
Figure 2.
MS/MS product ion spectra of the precursor ions [M+NH4]+ of artemether (A), dihydroartemisinin (B), and artemisinin, the I.S. (C).
3.2. Sample preparation
In respect to the high sensitivity requirement for this assay, a protein precipitation method was excluded because of dilution of the sample after sample preparation. Most published methods utilized liquid-liquid (L-L) extraction followed by reconstitution to a small volume (3-7). Several conditions were evaluated: 2,2,4-trimethylpentane-acetate (7:3), n-hexane-ethyl acetate (8:2), methyl t-butyl ether, and diethyl ether. Methyl t-butyl ether was adopted initially because better recovery for ARM and DHA was obtained: Briefly, 0.1 mL plasma sample was mixed with 0.9 mL methyl t-butyl ether, vortexed 1 min then mixed on a tube rotator for 30 min, froze the extraction tubes in dry-ice-ethanol bath then decanted the organic phase and dried under a stream of N2 at 40 °C. The residue was reconstituted with 100 μL 50% MeCN. However, the overall recovery was always below 80%, especially for ARM (40-60%). The problem identified was in the step of solvent evaporation, as higher recovery (~84%) was obtained when a batch of samples was accidentally left on bench overnight to dry. The melting point is 86-88 °C for ARM and 156-157 °C for the I.S. [11], loss of sample might occur if purging N2 too long or too drastically. It is also possible that natural dry afforded better solubilization during reconstitution. Therefore, an overnight evaporation method was adopted. With this sample preparation method, the LLOQ in the API 2000 was ≥10 ng/mL, even if a high sample volume (50 μL) was injected. This did not meet the set target (1-2 ng/mL). To circumvent the instrument limit, the plasma sample volume was increased to 0.5 mL, and sample preparation method switched to solid phase extraction (SPE) using C18 column (Alltech, 30mg, 1cc). Briefly, 0.5 mL plasma was loaded to SPE C18 column preconditioned with 1 mL MeOH and 1 mL water, washed with water (1 mL× 3) and 20% acetone (0.5 mL) followed by eluting with 0.5 mL methyl acetate, dried overnight in hood and reconstituted with 100 μL 50% MeCN. The LLOQ was 1 ng/mL. However, variable results for parallel samples presented considerable challenge for validation. It was found that variation was originated from instrument (presumably due to instability of M+NH4+ adducts) and reconstitution. To minimize variation, we decided to eliminate reconstitution step. We searched for a SPE column that required a small volume of elution solvent. Naik and coworkers used HLB SPE columns (Waters, Oasis®) to process artesunate and DHA [8]. The sorbent in HLB columns has a higher surface area than silica-based sorbents and thus less sorbent (one-third) is required for the same amount of sample, enabling us to use less elution solvent. By using the HLB column (Waters, Oasis®, 10 mg, 1cc), the samples were eluted with only 150 μL solvent and the collectable solution was ~100 μL (~50 μL retained in the column), leading to concentrated analytes by ~5-fold. Coincidently, Lindegardh and coworkers recommended SPE for analysis of patient samples to avoid malaria related hemolytic degradation of artimisinin derivatives during sample preparation [12]. They used HLB 96-well μ–elution plate to process artemisinin derivatives [12, 13]. The final sample preparation method was as follows: a 0.5 mL aliquot of plasma was loaded to HLB column preconditioned with 1 mL MeOH and 1 mL water, washed with water (1 mL× 3) and 10% MeCN (0.5 mL), dried under vacuum (~8 in Hg) for 30 min, and eluted with 0.15 mL MeCN-methyl acetate (9:1).
3.3. Method validation
The detection limit of the instrument was ~ 5 ng/mL for ARM and 2.5 ng/mL for DHA when injection volume was 50 μL. ARM and DHA both have a short half life in human. Cmax is typically below300 ng/mL, and concentration was dropped under10 ng/mL rapidly [4, 5]. Hence, a lower LLOQ is better for this assay. To obtain an adequate LLOQ with the API 2000, the plasma sample was concentrated by ~5-fold after sample preparation. The LLOQs for ARM and DHA in this assay were both set at 2 ng/mL in human plasma.
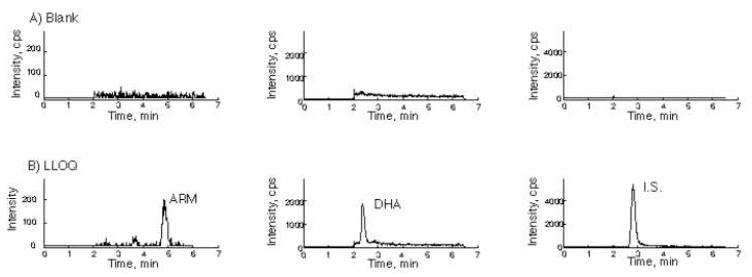
The calibration range was 2-200 ng/mL. When linear regression was used, LLOQ was often underestimated. With quadratic fitting weighted by 1/x, an improved fitting was achieved for both the lower and high ends of the calibration curve. It was also justified with Akaikes information criteroin (AIC) (14). Quadratic fitting yielded smaller AIC values when compared to linear fitting (data not shown). Thus, quadratic regression with 1/x weighting was adopted. The calibration curve had a mean correlation coefficient R of 0.9989 ± 0.0008 for ARM and 0.9994± 0.0006 for DHA (Table 2). No carry over was observed in blank plasma injected after the top calibration point. Representative MRM ion chromatograms of blank human plasma and plasma spiked with I.S. as well as ARM and DHA at LLOQ were shown in Figure 3.
Table 2.
Inter-day average back-calculated standard concentrations (n = 5)
| Theoretical | conc, ng/mL | 2.00 | 5.00 | 10.0 | 20.0 | 50.0 | 100 | 150 | 200 | a | b | c | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ARM | Mean, ng/mL | 1.94 | 5.05 | 10.3 | 20.0 | 49.0 | 100 | 150 | 199 | −8E-06 | 0.013 | −0.0014 | 0.9989 |
| SD | 0.14 | 0.17 | 0.46 | 0.71 | 2.0 | 4.2 | 9.2 | 4.0 | -- | 0.0008 | |||
| Precision (RSD,%) | 7.4 | 3.4 | 4.5 | 3.5 | 4.2 | 4.2 | 6.1 | 2.0 | -- | 0.08 | |||
| Accuracy (% dev) | −2.8 | 1.1 | 2.5 | 0.1 | −2.0 | 0.2 | 0.3 | −0.5 | -- | -- | |||
| DHA | Mean, ng/mL | 2.08 | 4.83 | 10.0 | 19.8 | 49.4 | 101 | 155 | 196 | −5E-05 | 0.087 | −0.0172 | 0.9994 |
| SD | 0.1 | 0.3 | 0.3 | 0.7 | 1.4 | 3.3 | 4.7 | 4.0 | 0.0006 | ||||
| Precision (RSD,%) | 6.4 | 5.3 | 2.5 | 3.5 | 2.9 | 3.3 | 3.1 | 2.1 | 0.1 | ||||
| Accuracy (% dev) | 3.8 | −3.4 | 0.1 | −1.1 | −1.2 | 0.5 | 3.3 | −1.8 |
Quadratic fitting equation: y = ax^2 + bx + c
Figure 3.
Representative chromatograms of blank human plasma (A) and lower limit of quantification (LLOQ) (B). The left panel is for ARM channel, middle panel is for DHA channel, the right panel is the IS channel.
For ARM, the intra-day precisions (n = 5) over 5 days were ranged from 2.0 to 12% at the three concentration levels, and inter-day precisions were ranged from 7.5 to 10%, all of them within 15%. We excluded an outlier for low QCs of ARM in the 1st run day. The intra-day and inter-day precisions for LLOQ were within 20%. Intra-day and inter-day accuracies were also within the acceptance limit (Table 3). For DHA, the intra-day precisions (n = 5) over 5 days were ranged from 1.8 to 8.2% at the three concentration levels, and inter-day precisions were ranged from 5.0 to 5.3%, all of them within 15%. The intra-day precisions (2.1 – 5.8%) and inter-day precision (5.3%) for LLOQ were within 20%. Intra-day accuracies (−3.3 – 5.6%) and inter-day accuracy (0.1%) were also within the acceptance limit (Table 3).
Table 3.
Intra-day and inter-day precision (%RSD) and accuracy (% dev) for analysis of artemether and dihydroartemisinin in human plasma.
| Intra-day |
Inter-day |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nominal, ng/mL | 2 | 6 | 80 | 170 | 2 | 6 | 80 | 170 | |
| ARM | mean, ng/mL | 1.8-2.3 | 5.2-6.4 | 70.5-86.8 | 160-182 | 1.96 | 5.98 | 76.8 | 170 |
| SD | 0.1-0.2 | 0.5-0.6 | 4.0-8.7 | 4.7-14.2 | 0.22 | 0.63 | 7.7 | 13 | |
| RSD,% | 5.2-8.0 | 2.9-12 | 2.0-7.7 | 2.9-7.9 | 11 | 10 | 10 | 7.5 | |
| %dev | −11-13 | −13-7.1 | −12-9.1 | −5.8-6.8 | −2.1 | −0.3 | −4.0 | 0.1 | |
| n | 5 | 5(4) | 5 | 5 | 25 | 24 | 25 | 25 | |
| DHA | mean, ng/mL | 1.9-2.1 | 5.2-5.7 | 72.5-79.9 | 152-165 | 2.00 | 5.42 | 74.9 | 158.2 |
| SD | 0.1-0.1 | 0.1-0.3 | 1.8-4.6 | 4.0-13 | 0.11 | 0.27 | 3.8 | 8.4 | |
| RSD,% | 2.1-5.8 | 1.8-5.7 | 1.9-6.0 | 2.5-8.2 | 5.3 | 5.0 | 5.1 | 5.3 | |
| %dev | −3.3-5.6 | −13- −4.8 | −9.3- −0.1 | −10- −3.2 | 0.1 | −9.7 | −6.4 | −6.9 | |
| n | 5 | 5 | 5 | 5 | 25 | 25 | 25 | 25 | |
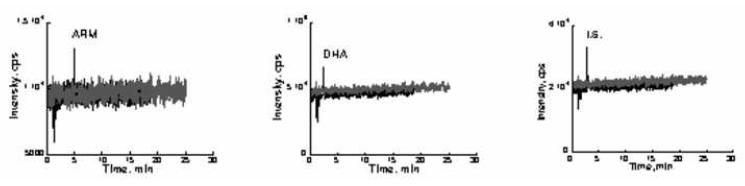
The results for recovery, matrix effect, and process efficiency are shown in Table 4. The recovery of DHA was very high ranging from 90 – 99%. The recovery for IS was 92.2%. Relatively low recovery was obtained for ARM, but it was acceptable as long as the recovery was consistent, reproducible, and repeatable. The matrix effect was evaluated in three ways: when compared blank plasma extract spiked sample to clean sample, the signal deviation was less than 10% (ME ranged from 93.9 to 105), indicating matrix effect was not significant (Table 4). The infusion experiment demonstrated that the signals at the retention times of ARM, DHA, and IS was not changed when blank plasma extract solution was injected into LC-MS/MS system (Figure 4). Six different lots of blank human plasma were also tested: the signals for ARM and DHA were less than 1/10 of LLOQs, and the deviation for medium QC samples spiked in 6 lots of plasma was also less than 10% (Data not shown). From the above three experiments, it was determined that matrix effects for ARM, DHA, and IS were negligible. Process efficiency (PE) reflects both recovery and matrix effect. The process efficiency for both DHA and the internal standard were ≥85%. PE for ARM was ranged 72-81%. (Table 4).
Table 4.
Recovery (RE), matrix effect (ME), and process efficiency (PE).
| conc.(ng/mL) | Peak area (×10e3), n = 3 | RE (%) | ME (%) | PE (%) | |||
|---|---|---|---|---|---|---|---|
| unextracted (in MeCN-MeOAC) |
postextraction spiked |
preextraction spiked |
|||||
| ARM | Low (6.00) | 6.21 ± 0.39 | 6.18 ± 0.26 | 5.00 ± 0.10 | 80.9 | 99.5 | 80.5 |
| High (170) | 184 ± 5 | 183 ± 4 | 133 ± 11 | 72.8 | 99.1 | 72.2 | |
| DHA | Low (6.00) | 26.1 ± 0.4 | 25.8 ± 0.4 | 25.5 ± 2.1 | 99.0 | 98.9 | 97.8 |
| High (170) | 881 ± 19 | 1828 ± 2 | 747 ± 40 | 90.3 | 93.9 | 84.8 | |
| IS | 200 ng/mL | 33.9 ± 2.2 | 35.5 ± 0.3 | 32.7 ± 1.1 | 92.2 | 105 | 96.4 |
Figure 4.
Infusion experiment for matrix effect. Blank plasma extract (50 μL) was injected onto the LC column while a mixture of ARM, DHA, and I.S. (1 μg/mL each in 50% MeCN) was infused into the MS fraction of LC eluate via a “T” connector at a rate of 10 μL/min (gray line). A 50 μL aliquot of 50% MeCN containing 1 μg/mL ARM, DHA, and I.S. was also injected as a control (black line).
Results for stability in human plasma (samples) and 50% MeCN solution (stocks) were shown in Table 5. All three stock solutions in 50% MeCN were stable in freezer (−70 °C) for at least 112 days and at room temperature (22 °C) for at least 3 days. If the stock solutions were left at room temperature for a few weeks, degradation was observed for DHA but ARM was stable (data not shown). ARM and DHA in plasma were stable during storage in freezer (−70 °C) for 25 days. Stability for longer storage time at −70 °C is ongoing. When the plasma samples stood on bench (22 °C) for up to 3 hr, there was no significant degradation observed for both ARM and DHA. However, there were several reports showing that DHA was not stable in plasma at room temperature while ARM was relatively stable [4,15,16]. M. Mordi and coworkers [15] found that at room temperature ARM was stable for at least 7 days but DHA was degraded more than 80% after 1 day. Bin Shi et al [4] reported that ARM in human plasma was stable for at least 8 hours at room temperature but DHA was only stable for 2 hours and degraded by 55 – 66% after 4 hours. Lindegardh and coworkers reported that both ARM and DHA were stable (>93% remaining) for 1 hour at room temperature. But they were stable on ice for 24 hours [13]. Therefore, we suggest that plasma samples be thawed and processed within 30 min. After processing, ARM and DHA were stable even if left on bench for 1 day.
Table 5.
Stability of artemether, dihydroartemisinin, and the I.S.
| conc(ng/mL) | %remained | CV% | ||
|---|---|---|---|---|
| 3 fr-th | ARM | 6.00 | 105 | 7.2 |
| 170 |
104 | 3.2 | ||
| DHA | 6.00 | 94.4 | 2.0 | |
| 170 |
101 | 1.5 | ||
|
| ||||
| 22 °C, 3hr | ARM | 6.00 | 99.4 | 2.6 |
| 170 |
97.1 | 1.2 | ||
| DHA | 6.00 | 97.6 | 4.5 | |
| 170 | 96.3 | 4.0 | ||
|
| ||||
| 22 °C, 20 hr (processed) |
ARM | 6.00 | 99.9 | 3.5 |
| 170 |
104.0 | 14 | ||
| DHA | 6.00 | 94.5 | 13 | |
| 170 | 99.3 | 14 | ||
|
| ||||
| −70°C, 101 d | ARM | 6.00 | 108 | 7.7 |
| 170 |
94.3 | 7.3 | ||
| DHA | 6.00 | 103 | 7.4 | |
| 170 | 98.0 | 8.1 | ||
|
| ||||
| −70°C, 112 d Stock |
ARM | 97.8 | 2.3 | |
| DHA | 109 | 1.0 | ||
|
| ||||
| 22 °C,7d Stock |
ARM | 106 | 4.7 | |
| DHA | 98.7 | 4.1 | ||
|
| ||||
| −70°C, 200 d stock |
IS | 102 | 2.2 | |
|
| ||||
| 22 °C 3 d stock |
IS | 101 | 3.2 | |
We tested selectivity of the method for ARM and DHA over other potential concomitant HIV drugs. The peak area ratios of the HIV drug-spiked samples were compared with that of non-spiked control sample. The data shows two of them may affect this assay: Lopinavir caused under-estimation of DHA (−21%) while Efavirenz resulted in over-estimatation of both ARM (25%) and DHA (30%). No significant signal change was observed in the presence of the other 10 drugs evaluated including nevirapine. Therefore, those drugs, if used by patients, would not affect quantification of ARM and DHA. This result confirmed that the method would be valid for one current clinical study, where nevirapine will be dosed together with ARM. (Table 6).
Table 6.
Potential concomitant medication interferences. Medium validation sample (80 ng/mL) was spiked with potential concomitant drugs at 5000 ng/mL and processed as usual.
| Drugs |
mean ratio (ARM/IS) |
STD | %CV | %dev due to matrix effect |
|---|---|---|---|---|
| n = 3 | ||||
| Control (80) | 1.75 | 0.03 | 1.8 | |
| EFV | 2.19 | 0.05 | 2.2 | 25 |
| ZDV | 1.70 | 0.04 | 2.4 | −2.7 |
| TFV | 1.89 | 0.13 | 6.7 | 8.1 |
| ATV | 1.67 | 0.08 | 4.7 | −4.5 |
| LPV | 2.00 | 0.13 | 6.5 | 14 |
| RTV | 1.84 | 0.08 | 4.5 | 4.9 |
| NFV | 1.89 | 0.08 | 4.1 | 8.1 |
| NVP | 1.95 | 0.17 | 8.7 | 11 |
| IDV | 1.91 | 0.01 | 0.4 | 9.2 |
| APV | 1.90 | 0.11 | 5.9 | 8.7 |
| ABV | 1.90 | 0.03 | 1.7 | 8.3 |
| SQV | 1.97 | 0.04 | 2.0 | 13 |
| Drugs |
mean ratio (DHA/IS) |
STD | %CV | %dev due to matrix effect |
|---|---|---|---|---|
| n = 3 | ||||
| Control (80) | 8.96 | 0.18 | 2.0 | |
| EFV | 11.7 | 0.29 | 2.5 | 30 |
| ZDV | 8.74 | 0.29 | 3.4 | −2.4 |
| TFV | 8.69 | 0.59 | 6.8 | −3.0 |
| ATV | 8.77 | 0.18 | 2.1 | −2.1 |
| LPV | 7.11 | 0.08 | 1.1 | −21 |
| RTV | 8.14 | 0.44 | 5.4 | −9.2 |
| NFV | 8.32 | 0.34 | 4.1 | −7.1 |
| NVP | 9.35 | 0.17 | 1.8 | 4.3 |
| IDV | 8.45 | 0.02 | 0.2 | −5.7 |
| APV | 8.53 | 0.35 | 4.1 | −4.8 |
| ABV | 8.59 | 0.32 | 3.8 | −4.2 |
| SQV | 8.52 | 0.29 | 3.4 | −4.9 |
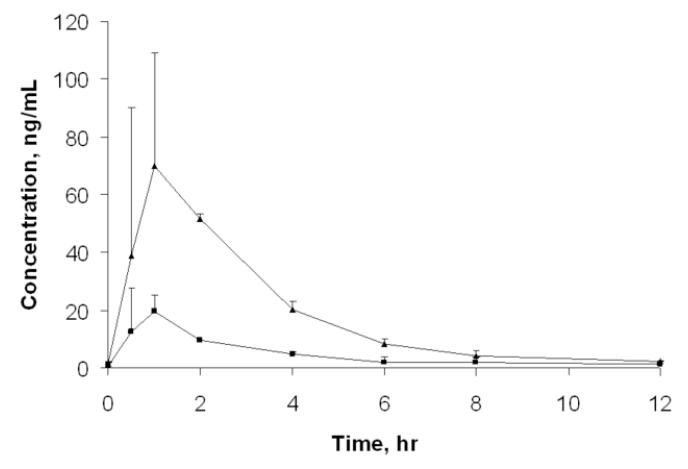
Finally, the method was applied to existing clinical samples from a completed study [17]. Samples from three subjects were tested as a pilot test. Plasma samples taken at the time of 0, 0.5, 1, 2, 4, 6, 8, and 12 hr after three days of ARM (co-formulated with lumefantrine: Coartem® and given at a regimen of 80/480 mg twice daily) were analyzed. The results are shown in Figure 5.
Figure 5.
Plasma concentration – time profile of ARM and DHA. Triangle with solid line, DHA; Square with solide line, ARM. Each data point represents mean concentrations from three healthy volunteer subjects except for at 0.5 and 2 hrs which are based on the mean concentrations for two subjects. The variation bars for data points except for at 0.5 and 2 hrs are standard deviations representing inter-subject variation.
4. Conclusion
An LC-MS/MS method was developed for determination of ARM and DHA to support pharmacokinetic studies of ARM-based antimalarial treatment in pediatric patients and in the context of the anti-HIV-1 drug nevirapine for patients co-infected with malaria. To reach a low LLOQ (2 ng/mL) with an API 2000, we used 0.5 mL plasma samples that were concentrated ~5-fold after sample preparation, and a relative large volume (50 μL) was injected in the LC-MS/MS system. The sample preparation with Oasis® HLB SPE column minimized use of organic solvent and direct injection avoided sample loss during reconstitution. Quadratic fitting of the calibration curve weighted by 1/x was adopted and justified according to Akaikes information criteroin (AIC) (14). The method was validated and applied to clinical samples. The result was in good agreement with another method reported by Lindegardh and coworkers. (comparison data not shown) [13].
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group (grant number 1U01AI068632). We are thankful to Drs. Sunil Parikh and Niklas Lindegardh for their scientific input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Guinovart C, Navia MM, Tanner M, Alonso PL. Curr. Mol. Med. 2006;6:137. doi: 10.2174/156652406776055131. [DOI] [PubMed] [Google Scholar]
- [2].Webster HK, Lehnert EK. Trans. R. Soc. Trop. Med. Hyg. 1994;88:S27. doi: 10.1016/0035-9203(94)90467-7. [DOI] [PubMed] [Google Scholar]
- [3].Peys E, Vandenkerckhove J, Van Hemel J, Sas B. Chromatographia. 2005;61:637. [Google Scholar]
- [4].Shi B, YU Y, Li Z, Zhang L, Zhong Y, Su S, Liang S. Chromatographia. 2006;64:523. [Google Scholar]
- [5].Souppart C, Gauducheau N, Sandrenan N, Richard F. J. Chromatgr B. 2002;774:195. doi: 10.1016/s1570-0232(02)00207-6. [DOI] [PubMed] [Google Scholar]
- [6].Xing J, Yan H, Zhang S, Ren G, Gao Y. Rapid Commun. Mass Spectrom. 2006;20:1463. doi: 10.1002/rcm.2467. [DOI] [PubMed] [Google Scholar]
- [7].Ortelli D, Rudaz S, Cognard E, Veuthey JL. Chromatographia. 2000;52:445. [Google Scholar]
- [8].Naik H, Murry DJ, Kirsch LE, Fleckenstein L. J. Chromatgr B. 2005;816:233. doi: 10.1016/j.jchromb.2004.11.042. [DOI] [PubMed] [Google Scholar]
- [9].Cabri W, Ciogli A, D’Acquarica I, Di Mattia M, Galletti B, Gasoarrini F, Giorgi F, Lalli S, Pierini M, Simine P. J. Chromatgr. B. 2008;875:180. doi: 10.1016/j.jchromb.2008.06.037. [DOI] [PubMed] [Google Scholar]
- [10].ACTG guidelines for method development and validation based on (and including) FDA guidelines dated by 2001. Version 2 2005.
- [11].Budavari S, editor. The Merck Index. 12th edition 1996. p. 137. [Google Scholar]
- [12].Lindegardh N, Hanpithakpong W, Kamanikom B, Singhasivanon P, Socheat D, Yi P, Dondorp AM, McGready R, Nosten F, White NJ, Day NPJ. J. Chromatgr. B. 2008;876:54. doi: 10.1016/j.jchromb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [13].Hanpithakpong W, Kamanikom B, Singhasivanon P, White NJ, Day NPJ, Lindegardh N. Bioanalysis. 2009 doi: 10.4155/bio.09.6. (in press) [DOI] [PubMed] [Google Scholar]
- [14].Kirkup L, Mulholland M. J. Chromatgr. A. 2004;1029:1. doi: 10.1016/j.chroma.2003.12.013. [DOI] [PubMed] [Google Scholar]
- [15].Mordi MN, Mansor SM, Navaratnam V, Wernsdorfer WH. Br. J. Clin. Pharmacol. 1997;43:363. doi: 10.1046/j.1365-2125.1997.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beekman AC, Woerdenbag HJ, Van Uden W, Pras N, Konings AWT, Wikström HV. J. Pharm. Pharmacol. 1997;49:1254. doi: 10.1111/j.2042-7158.1997.tb06080.x. [DOI] [PubMed] [Google Scholar]
- [17].German P, Parikh S, Lawrence J, Lindegardh N, Rosenthal P, Havlir D, Charlebois E, Dorsey G, Aweeka FT. JAIDS. 2009 doi: 10.1097/QAI.0b013e3181acb4ff. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.