Abstract
Objectives:
The aim of the study was to explore the putative significance of angiotensin-converting enzyme (ACE) insertion/deletion (I/D) polymorphism and its correlation with the lower urinary tract symptoms (LUTS) in benign prostatic hyperplasia (BPH).
Materials and Methods:
ACE I/D polymorphisms were determined by polymerase chain reaction (PCR) in 200 patients with moderate to severe LUTS due to BPH and 200 patients of same age group without the LUTS having normal size prostate. ACE levels were estimated by spectrophotometer method. Logistic regression models were used to determine the genetic effects using SPSS statistical software (version 12.0).
Results and Conclusions:
The distribution of genotypes along with allelic frequency and carriage rate did not significantly differ between study and control groups. This study suggests that I/D polymorphisms within the ACE gene are not associated with the presence of LUTS in BPH patients. Future studies in large cohorts are needed that may reveal the spectrum of cellular mechanism mediated by ACE relevant to pathophysiology of BPH and effect of ethnic differences.
Keywords: angiotensin-converting enzyme polymorphism, polymerase chain reaction-RFLP, benign prostatic hyperplasia
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a highly prevalent disorder that represents the most common cause of lower urinary tract symptoms (LUTS) leading to urinary obstruction in the ageing male population.[1] It is characterized by increased cellular proliferation of stromal and epithelial cells with enhanced sympathetic smooth muscle tone.[2–4] Activation of the renin-angiotensin system (RAS) plays an important role in normal physiology as well as in the progression of cardiac, renal, prostatic, and other diseases.[5–7] BPH frequently coexists with hypertension[8,9] and RAS plays equally important role in both conditions.
Angiotensin-converting enzyme (ACE) degrades vasodilator kinins and generates angiotensin II (Ang II), which is a major effector peptide of the RAS system. The biological actions of Ang II are mediated by at least two different receptor subtypes, Ang II receptor subtype 1 (AT-1) and subtype 2 (AT-2). The physiological role of ACE in the prostate is not well understood, though ACE synthesis by the prostate has been reported[10,11] and the AT-1 receptor subtype is the predominant Ang II prostatic receptor.[6] Ang II is localized to the basal layer of the epithelium, coupled with stromal smooth muscle containing AT-1 receptors, suggesting that Ang II may mediate paracrine functions in the human prostate. Furthermore, a regulatory role has been suggested for Ang II in the prostate stroma.[12] The hyperactivity of local tissue RAS is thought to be involved in the pathophysiology of BPH and overactivated RAS possibly stimulates cellular growth and increases smooth muscle tone, thus affecting both dynamic and somatic component of BPH.[13] Considering the suggested involvement of RAS in BPH, the ACE insertion/deletion (I/D) polymorphism could have implications in the pathophysiology of LUTS due to BPH. Thus, the purpose of the present study was to investigate nature of distribution of ACE genotypes in patients suffering from LUTS due to BPH and comparing the genotypes in patients in the same age group but free from BPH and LUTS.
MATERIALS AND METHODS
Patient and control selection
In present study 200 symptomatic BPH patients from the department of Urology, CSMMU (upgraded KGMC), Lucknow, were enrolled during the period of July 2005 to July 2008. The inclusion criteria were nondiabetic, nonhypertensive patients with BPH with LUTS, age >50 years, prostate size >30 cm3on transrectal ultrasound, AUA symptom score >7, peak flow rate (Qmax) less than 15 ml/s, postvoid residue (PVR) less than 150 ml, and serum prostate specific antigen (PSA) less than 4.0 ng/ml. The digital rectal examination (DRE) was done in all cases and patients having nodular prostate with raised PSA (PSA > 4.0 ng/ml) were excluded. Patients with PSA within the range of 4-10 ng/ml were included in study only if the DRE suggests benign enlargement of prostate and transrectal ultrasound (TRUS) guided tru-cut needle biopsy of prostate confirmed it to be a case of BPH. The patients with history of recent UTI (within 3 months), previous lower tract surgery, known habitual chronic constipation, history of postural hypotension, dizziness, vertigo, orthostasis, or any other signs and symptoms which are suspected to be exacerbated by α-blockers and result in putting the subject at risk of injury were excluded from the study.
A total of 200 controls of age >50 years nondiabetic, nonhypertensive were recruited from patients suffering from kidney stones, upper ureteric stones, renal tumors, small superficial bladder tumor with normal size prostate on DRE, and free from LUTS. They were subjected for transrectal ultrasound which showed normal size of prostate. All the participants in control group were further investigated for the serum PSA and uroflometry. Individuals with PSA value less than 4.0 ng /ml and peak flow rate (Qmax) more than 15 ml/s were included in control group.
Genotyping
Genomic DNA was extracted from peripheral blood by QIAamp DNA mini kit (QIAGEN, Germany). To determine the ACE genotype, genomic DNA was amplified by PCR using a programmed thermal cycler Master cycler gradient (Eppendorf, USA)[15] initially using a flanking primer pair and subsequently when necessary, with a primer pair that recognizes the specific insertion sequences for confirmation of the specificity of the amplification reactions. The flanking primer pair used was 5'-CTGGAGACCACTCCCATCCTTTCT-3' and 5' GATGTGGCCATCACATTCGTCACGAT-3'. Amplification with this primer pair results in 490 and 190 bp amplification products corresponding to I and D alleles, respectively. Mistyping of ID heterozygotes as D homozygotes may occur due to the preferential amplification of the D allele and inefficiency in the amplification of the I allele.[16] To increase the specificity of DD genotyping, PCR amplifications were also performed with an insertion-specific primer pair 5'-TGGGACCACAGCGCCCGCCACTAC-3' and 5'-TCGCCAGCCCTCCCATGCCCATAA-3' in all samples that were found to be DD after amplification with the flanking primers. In case of presence of I allele, insertion-specific amplification resulted in a 335-bp amplicon and the reaction yielded no products in samples of DD genotype.
Determination of ACE activity
Plasma ACE activity was measured by a spectrophotometric monoreagent assay, which involved the enzymatic hydrolysis of the synthetic substrate, N-[3-(2-furyl) acryloyl]-L-phenylalanyl-glycylglycine (FAPGG) to N-[3-(2-furyl) acryloyl]-L-phenylalanine (FAP) and glycylglycine (GG). The decrease in absorbance due to the hydrolysis of FAPGG corresponded to the activity of ACE. ACE activity was determined by a modification of the macromethod to a micromethod (ELISA plate) on a high-throughput spectrophotometer. Each assay was performed in duplicate and was repeated thrice on different days. Plasma sample (5 μl) was added to 100 μl ACE substrate FAPGG (0.5 mM) and reaction was monitored at 340 nm at 37ºC.[17]
RESULTS
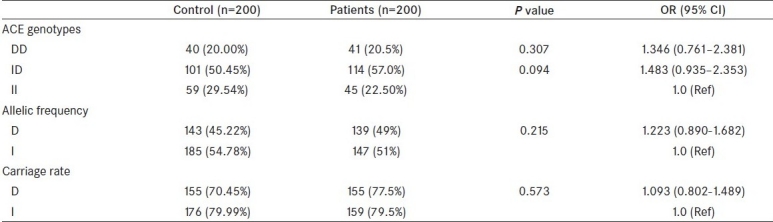
Clinical characteristics of the control and study groups are shown in Table 1. The mean age of the patient population presented with moderate to severe LUTS due to BPH was 58.34 ± 6.04 years and mean age of control was 57.6 ± 3.4 years. The AUA symptom score, mean prostate volume, mean Qmax(peak flow rate), mean PSA, and mean PVR in both groups are shown in Table 1. Upon comparing genotype, allele frequency, and carriage rate of ACE polymorphism, no associations were observed between patients and controls [Table 2]. We also estimated serum level of ACE in control and patient groups and analyzed for probable association of serum level with any of the allele of ACE gene, but our analysis revealed that the level of serum ACE level did not vary significantly (data not shown).
Table 1.
Clinical characteristic of control and patient groups
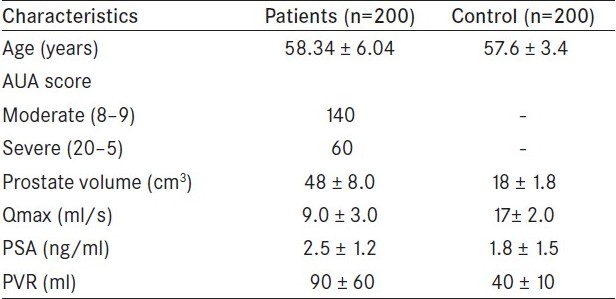
Table 2.
Distribution of ACE genotypes, allele frequency, and carriage rate among BPH patients and controls
DISCUSSION
BPH has been defined as an enlargement of the prostate gland from the progressive hyperplasia of stromal and glandular prostatic cells.[18] ACE degrades vasodilator kinins and generates angiotensin II (Ang II), which is the major effector peptide of the RAS system. Further, studies have reported that there are stromal–epithelial cellular interactions involved in fetal prostate development, postnatal prostate growth and maturation, maintenance of differentiation status, hormone responsiveness, and ageing and senescence of the prostate gland in adulthood.[19,20]
Several candidate gene polymorphism association studies in BPH have been conducted, and few of them have shown positive association in well-designed, large-scale genetic epidemiological studies.[21]
RAS is traditionally an endocrine system regulating blood pressure and body fluid homeostasis.[22] It also mediates normal and pathophysiological processes in a variety of tissues including prostate.[23] ACE plays a central role in this system by converting Ang I to the potent vasoconstrictor Ang II.[24] It is well documented that Ang II facilitates sympathetic transmission in many cardiovascular organs by enhancing the release of the chemical transmitter noradrenaline from sympathetic nerve terminals. However, little is known about the interaction of the RAS with sympathetic neurotransmission in the prostate, which may have implication in the pathophysiology of BPH. Fabiani et al.[25] provided direct evidence in support of a functional role for the local RAS in modulating sympathetic transmission in the prostate, which may have important implications for the pathophysiology of BPH.[25] ACE is suggested not only to be involved in static component of cellular proliferation but also in modulation dynamic component of voiding and discomfort symptom. Therefore the aim of the present study was to evaluate the influence of variants in ACE gene, in the pathogenesis of BPH.
One of the important points to be noted in our study is that we included only patients with symptomatic enlargement of prostate particularly with voiding problem. Therefore, our subjects are not only ideal for the study of association of ACE with benign enlargement but specifically with discomfort problem. We observed that patients had the similar ACE I/D genotype and allele distribution when compared to the control population demonstrating no association between I/D polymorphism of the ACE gene and the risk of BPH in Indian population. This could be explained by the fact that since the ACE I/D polymorphism is intronic, there could be other locus in linkage to this gene regulating the ACE gene expression.[26] There are numerous studies reporting positive disease associations of ACE polymorphism with cardiovascular disease, breast cancer, and myocardial infarction.[27,23,28] However in other studies[29–31] and a meta-analysis by Lindpaintner et al.,[32] did not show any association.no association of ACE polymorphism was observed with the category of moderate to severe symptomatic BPH patients. Negative finding in disease association studies underlines the important concept that multiple factors like genetic, environmental, and diet interaction contribute to the development of BPH. ACE polymorphism alone may not be involved with the risk of BPH or at least could not independently influence the pathophysiology of BPH.
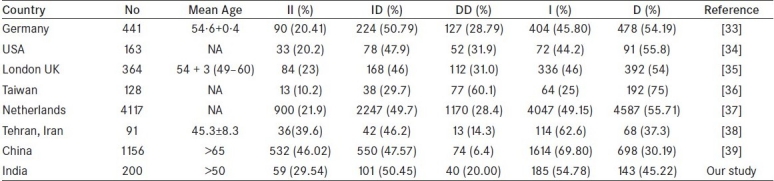
Further, the lack of association in ACE polymorphism with BPH may be due to ethnic differences in Indian population, which is a frequent observation in worldwide gene variants profile of different genes. Therefore, we also compared and analyzed the variation of ACE polymorphism among different ethnic populations including our data [Table 3]. We found that normal population of different ethnic regions exhibit significant differences with comparison to our population with respect to genotype or allele frequency distribution. To the best of our knowledge, this is the first report from India on the ACE polymorphism suggesting that I/D polymorphism within the ACE gene is not associated with risk of BPH patients with LUTS.
Table 3.
Variation of genetic polymorphisms of ACE (I/D) from different countries
Future studies are needed to explore the complete spectrum of interacting factors responsible in mediating the role of ACE in the pathophysiology of BPH.
Acknowledgments
Grant support received from Ministry of Health and Family Welfare (MOH), Government of India, New Delhi.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–71. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- 3.McNeal J. Pathology of benign prostatic hyperplasia: insight into etiology. Urologic Clinics of North America. 1990;17:477–86. [PubMed] [Google Scholar]
- 4.Madsen FA, Bruskewitz RC. Benign prostatic hyperplasia: pathophysiology and pharmacological treatment. Curr Opinion in Nephrol Hypertension. 1995;4:455–9. doi: 10.1097/00041552-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Dinh D, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci. 2001;100:481–92. [PubMed] [Google Scholar]
- 6.Dinh DT, Frauman AG, Sourial M, Casley DJ, Johnston CI, Fabiani ME. Identification, distribution and expression of angiotensin II receptors in the normal human prostate and benign prostatic hyperplasia. Endocrinol. 2001;142:1349–56. doi: 10.1210/endo.142.3.8020. [DOI] [PubMed] [Google Scholar]
- 7.Baudin B. Angiotensin II receptor polymorphisms in hypertension.Pharmacogenomics considerations. Pharmacogenomics. 2002;3:65–73. doi: 10.1517/14622416.3.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Nandeesha H, Koner BC, Dorairajan LN, Sen SK. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta. 2000;6370:89–93. doi: 10.1016/j.cca.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Maruenda J, Bhatnagar V, Lowenthal DT. Hypertension in the elderly with coexisting benign prostatic hyperplasia. Urol. 1999;53:7–12. doi: 10.1016/s0090-4295(98)00533-0. [DOI] [PubMed] [Google Scholar]
- 10.Krassnigg F, Niederhauser H, Fink E, Frick J, Schill WB. Angiotensin converting enzyme in human seminal plasma is synthesized by the testis, epididymis and prostate. Int J Androl. 1989;12:22–8. doi: 10.1111/j.1365-2605.1989.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 11.Nassis L, Frauman AG, Ohishi M, Zhuo J, Casley DJ, Johnston CI, et al. Localization of angiotensin- converting enzyme in the human prostate: pathological expression in benign prostatic hyperplasia. J Pathol. 2001;195:571–9. doi: 10.1002/path.999. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Freeman MR. Transactivation of ErbB1 and ErbB2 receptors by angiotensin II in normal human prostate stromal cells. Prostate. 2003;54:1–7. doi: 10.1002/pros.10160. [DOI] [PubMed] [Google Scholar]
- 13.Dinh DT, Frauman AG, Somers GR, Ohishi M, Zhou J, Casley DJ, et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol. 2002;196:213–19. doi: 10.1002/path.1021. [DOI] [PubMed] [Google Scholar]
- 14.Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 Guidelines on Assessment, Therapy and Follow-Up of Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Obstruction (BPH Guidelines) Euro Urol. 2004;46:547–54. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A, et al. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996;50:657–64. doi: 10.1038/ki.1996.362. [DOI] [PubMed] [Google Scholar]
- 16.Shanmugam V, Sell KW, Saha BK. Mistyping of ACE heterozygotes. PCR Methods Appl. 1993;3:120–1. doi: 10.1101/gr.3.2.120. [DOI] [PubMed] [Google Scholar]
- 17.Bala M, Gupta S, Pasha MA. Angiotensin-converting enzyme assay optimization: influence of various buffers and their concentrations. Clin Biochem. 2000;33:687–9. doi: 10.1016/s0009-9120(00)00180-6. [DOI] [PubMed] [Google Scholar]
- 18.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–9. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 19.Chung LW, Gleave ME, Hsieh JT, Hong SJ, Zhau HE. Reciprocal mesenchyme-epithelial interaction affecting prostate tumour growth and hormonal responsiveness. Cancer Surv. 1991;11:91–121. [PubMed] [Google Scholar]
- 20.Chung LWK, Zhau HE. Stromal-epithelial interaction.In Prostate Cancer: Biology, Genetics and the New Therapeutics. In: Chung LWK, Isaacs WB, Simons JW, editors. Totowa, NJ. Humana Press; 2001. pp. 341–62. [Google Scholar]
- 21.Konwar R, Chattopadhyay N, Bid HK. Genetic polymorphism and pathogenesis of benign prostatic hyperplasia. BJU Int. 2008;102:536–44. doi: 10.1111/j.1464-410X.2008.07667.x. [DOI] [PubMed] [Google Scholar]
- 22.Oparil S, Haber E. The renin-angiotensin system (first of two parts) N Engl J Med. 1974;291:389–401. doi: 10.1056/NEJM197408222910805. [DOI] [PubMed] [Google Scholar]
- 23.Matsusaka T, Hymes J, Ichikawa I. Angiotensin in progressive renal diseases: theory and practice. J Am Soc Nephrol. 1996;7:2025–43. doi: 10.1681/ASN.V7102025. [DOI] [PubMed] [Google Scholar]
- 24.Matsusaka T, Ichikawa I. Biological functions of angiotensin and its receptors. Annu Rev Physiol. 1997;59:395–412. doi: 10.1146/annurev.physiol.59.1.395. [DOI] [PubMed] [Google Scholar]
- 25.Fabiani ME, Sourial M, Thomas WG, Johnston CI, Frauman AG. Angiotensin II enhances noradrenaline release from sympathetic nerves of the rat prostate via a novel angiotensin receptor: implications for the pathophysiology of benign prostatic hyperplasia. J Endocrinol. 2001;171:97–108. doi: 10.1677/joe.0.1710097. [DOI] [PubMed] [Google Scholar]
- 26.Davis GK, Robert DH. Molecular genetics of the renin-angiotensin system: implications for angiotensin II receptor blockade. Pharmacol. 1997;75:43–50. doi: 10.1016/s0163-7258(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 27.Woon-Puay Koh, Jian-Min Yuan, Can-Lan Sun, David van den Berg, Adeline Seow, Hin-Peng Lee, et al. Angiotensin I-Converting Enzyme (ACE) Gene Polymorphism and Breast Cancer Risk among Chinese Women in Singapore. Cancer Res. 2003;63:573–8. [PubMed] [Google Scholar]
- 28.Schieffer B, Drexler H. ACE gene polymorphism and coronary artery disease.A question of persuasion or statistical confusion? Arterioscler Thromb Vasc Biol. 2000;20:281–2. doi: 10.1161/01.atv.20.2.281. [DOI] [PubMed] [Google Scholar]
- 29.Bhaskar S, Reddy DN, Mahurkar S, Rao GV, Singh L, Chandak GR. Lack of significant association of an insertion/deletion polymorphism in the angiotensin converting enzyme (ACE) gene with tropical calcific pancreatitis. BMC Gastroenterol. 2006 Dec;12(6):42. doi: 10.1186/1471-230X-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Kelten C, et al. Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J Exp Med. 2006;210:109–16. doi: 10.1620/tjem.210.109. [DOI] [PubMed] [Google Scholar]
- 31.Serrano NC, Díaz LA, Páez MC, Mesa CM, Cifuentes R, Monterrosa A, et al. Angiotensin-converting enzyme I/D polymorphism and preeclampsia risk: evidence of small-study bias. PLoS Med. 2006;3:520. doi: 10.1371/journal.pmed.0030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindpaintner K, Pfeffer MA, Kreutz R, Stampfer MJ, Grodstein F, LaMotte F, et al. A prospective evaluation of an angiotensin converting enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–11. doi: 10.1056/NEJM199503163321103. [DOI] [PubMed] [Google Scholar]
- 33.Jeron A, Hengstenberg C, Engel S, Lo wel H, Riegger GA, Schunkert H, et al. The D-allele of the ACE polymorphism is related to increased QT dispersion in 609 patients after myocardial infarction. European Heart Journal. 2001;22:663–8. doi: 10.1053/euhj.2000.2297. [DOI] [PubMed] [Google Scholar]
- 34.Oruc N, Lamb J, Kutlu OC, Barmada MM, Money ME, Slivka A, et al. The Functional Angiotensin Converting Enzyme Gene I/D Polymorphism Does not Alter Susceptibility to Chronic Pancreatitis JOP. 2004;5:457–63. [PubMed] [Google Scholar]
- 35.Hugh E, Keeling PJ, Goldman JH, Humphries SE, Talmud PJ. Lack of Association between the Insertion/Deletion polymorphism of the Angiotensin-Converting Enzyme gene and idiopathic Dilated Cardiomyopathy. JACC. 1995;25:1627–31. doi: 10.1016/0735-1097(95)00109-h. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh YY, Lee CC, Chang CC, Wang YK, Yeh LY, Lin CS. Angiotensin I-Converting Enzyme Insertion-Related Genotypes and Allele are Associated with Higher Susceptibility of Endometriosis and Leiomyoma. Molecular Reproduction And Development. 2007;74:808–14. doi: 10.1002/mrd.20474. [DOI] [PubMed] [Google Scholar]
- 37.Angela M, Alejandro AV, Fakhredin AS, Coebergh JW, Hofman A, Njajou O, et al. Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism and Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2143–6. doi: 10.1158/1055-9965.EPI-05-0045. [DOI] [PubMed] [Google Scholar]
- 38.Abdol RN, Taghi G, Manouchehr N, Mahine Z, Ali A. Angiotensin Converting Enzyme Gene Polymorphism in Iranian Patients with Type 2 Diabetes. Iran J Immunol. 2006;3:1. [Google Scholar]
- 39.Kwok T, Ohlsson C, Vandenput L, Tang N, Zhang YF, Tomlinson B, et al. ACE inhibitor use was associated with lower serum dehydroepiandrosterone concentrations in older men. Clin Chim Acta; 2010 doi: 10.1016/j.cca.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


