Abstract
Aims:
The association of central obesity, hyperinsulinemia, and dyslipidemia with higher grade advanced prostate cancer as determined by Gleason grading is not well understood. We evaluated the effect of central obesity waist hip ratio (WHR ≥ 0.9) and biochemical parameters associated with central obesity on Gleason grading in North Indian patients of prostate cancer presenting at advanced stages.
Materials and Methods:
A cross-sectional study was conducted among 50 nondiabetic patients having clinical stages III and IV prostate cancer. Gleason grading on core biopsy samples by histopathology was done and patients were divided in two groups—group1, Gleason score ≥8; group 2, Gleason score <8. WHR along with serum levels of prostate-specific antigen (PSA), testosterone, insulin, and lipid profile was done in each patient.
Results:
The two groups are similar in Age (67.54 years); range (50-80 years). Group 1 men had statistically higher mean WHR (0.96 vs 0.90; P ≤ 0.001), higher mean triglyceride level (201.34 vs 150.52 mg/dL; P=0.0006), higher mean very low-density lipoprotein (VLDL) (40.27 vs 30.10 mg/dL; P =0.0006), higher mean insulin (19.49 vs 15.04 μIU/mL; P = 0.0024), and lower mean high-density lipoprotein (HDL) levels (32.39 vs 36.82 mg/dL; P = 0.034) than men in group 2. Serum levels of cholesterol, LDL, and testosterone did not show statistically significant differences between the two groups.
Conclusions:
This pilot study involving small number of patients indicates that central obesity, dyslipidemia, and hyperinsulinemia could be associated with high-grade prostate cancer.
Keywords: Central obesity, insulin, lipids, prostate cancer
INTRODUCTION
The International Agency for Cancer Research (IARC) has shown a low incidence of prostate cancer in East Asian countries as compared to western counterparts[1] although its incidence in India is rising as well.[2] Comprehensive search for risk factors that contribute in the initiation and progression of prostate cancer remains a challenge. Emerging literature has implicated that central obesity, hyperinsulinemia , dyslipidemia, westernization, and changes in lifestyle may play potential risk factors for progression of prostate cancer, although not yet clearly established. Central obesity is an excess accumulation of fat in the abdominal area and is associated with development of, glucose intolerance, high blood pressure, atherosclerosis, cardiovascular disease, insulin resistance, altered metabolic profile, metabolic syndrome, and obesity-related lipid disorders.[3] Central obesity is practically assuming epidemic proportions in Indian subcontinent with a study in urban metropolitan city of Chennai reporting a prevalence of 35.1%.[4] Hsing et al. in their study of 238 cases of newly diagnosed prostate cancer and 471 healthy controls found that high levels of waist hip ratio (WHR) (a standard measure of abdominal obesity) related to excess risk for PCa.[5] Whereas there are other studies that showed no association.[6,7] Insulin has also been shown to play a role in the etiology of colon, prostate, pancreas, and breast cancer.[8]
Gleason grading is the most widely used grading method for prostate cancer with higher grades (8-10) predicting poorer outcomes in comparison to low or intermediate grade (2-7).[9] Some studies in western literature have shown an association of high-grade prostate cancer with central obesity, hyperinsulinemia, and dyslipidemia. As there could be different genetic and environmental factors affecting Indian males, we evaluated whether central obesity and obesity-related biochemical alterations in terms of hyperinsulinemia and dyslipidemia are associated with poorer presentation of prostate cancer in terms of high Gleason score.
MATERIALS AND METHODS
A cross-sectional study was conducted to test the association of central obesity, hyperinsulinemia, and dyslipidemia with histological Gleason grade in patients of prostate cancer. Between August 2008 and September 2009, 63 men diagnosed with prostate cancer were evaluated and the following assessments were done.
Anthropometric assessment. WHR was calculated from waist circumference at umbilicus divided by hip circumference at greater trochanter and a cut off of 0.9 was taken to categorize central obesity.
Biochemical assessment. Serum samples of men with suspicion of prostate cancer on the basis of high prostate-specific antigen (PSA) and/or abnormal DRE were withdrawn before biopsy between 8 a.m. and 11 a.m. Serum PSA, testosterone, and insulin levels were estimated using ELISA on the same day. Serum levels of high-density lipoprotein (HDL), triglycerides (TG), cholesterol, low-density lipoprotein (LDL), and very low density lipoprotein (VLDL) were measured by semiautomated instrument.
Staging and grading. Standard staging procedures were followed and for histopathological evaluation of prostate cancer, Gleason grading was done on core biopsy tissue and on the basis of score the patients were divided in two groups—group1, Gleason score ≥ 8; group 2, Gleason score <8.
Exclusion criteria. Patients suffering from diabetes, chronic liver, kidney, heart disease, those taking lipid-lowering drugs or 5-alpha reductase inhibitors were excluded from the study leaving only 50 patients who were included.
Software used for Statistics is Strata 10.1 (College Station, TX, USA), two-sample t test used, if data met normally assumption otherwise, Mann-Whitney U test is used. Normally all data tested by using Shapiro Wilk′s test. Data were presented as mean ± SD, median (IQR), and P value. P value <0.05 was considered as significant.
RESULTS
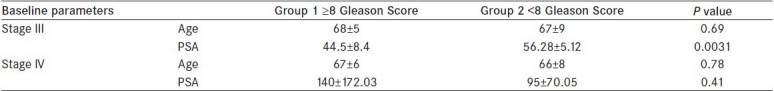
The mean age of 50 patients was 67.54 years (range 50-80), mean PSA (113.6 ng/mL) range (25-630 ng/mL). All the patients were in either stage III (n = 21) or stage IV (n = 29) disease. Group 1 (Gleason score ≥ 8) had (n = 30) patients in comparison to (n = 20) in group 2.
Group 1 patients had statistically higher mean [Table 2] WHR (0.96 vs 0.90; P ≤ 0.001), higher mean TG level (201.34 vs 150.52 mg/dL; P = 0.0006), higher mean VLDL (40.27 vs 30.10 mg/dL; P = 0.0006), higher mean insulin (19.49 vs 15.04 μIU/mL; P = 0.0024), and lower HDL levels (32.39 ± 36.82 mg/dL; P = 0.034) than patients in group 2. Serum levels of cholesterol, LDL, and testosterone did not show statistically significant differences between the two groups. Baseline parameters (age, PSA, and stage) are shown in Table 1.
Table 2.
The values of various parameters
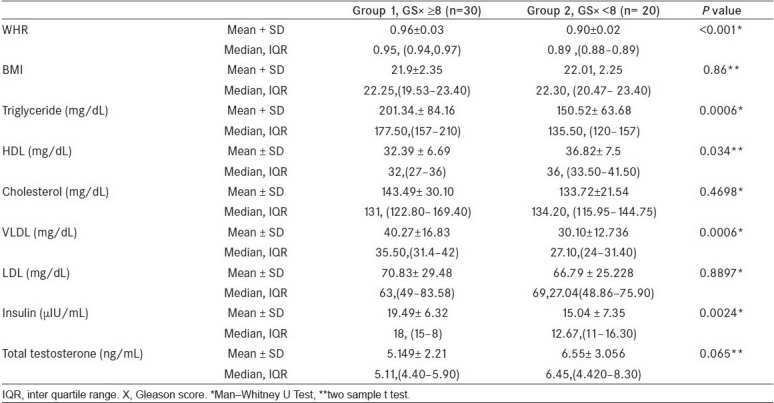
Table 1.
Baseline parameters (Age, PSA and Stage)
DISCUSSION
Obesity has long been associated with increased risk of prostate cancer, although studies have been inconsistent.[10–12] Body mass index (BMI) may not fully reflect the disease-related dimensions of obesity since it does not differentiate muscle mass from fat mass.[13] Central obesity, which is best diagnosed by measuring the WHR correlates much stronger with hormonal and metabolic alterations in comparison to BMI. In our study, high Gleason score was statistically associated with higher WHR raising the suspicion that central obesity may predispose to high-grade prostate cancer (P<0.001). Except for a few prospective studies,[12,13] there is less support for the hypothesis that central adiposity (measured as waist circumference or WHR) is a risk factor for prostate cancer.[10] There are data to suggest that waist circumference and/or WHR may predict health problems independently of BMI, as in diabetes mellitus[14] and cardiovascular disease.[15] Furthermore, there is accumulating evidence for a role of waist circumference[16,17] and WHR[18,19] in predicting mortality independently of BMI in men. In 1997, by analyzing about 135,000 men, Andersson et al. found that the risk of death from prostate cancer was statistically significant above the reference category in all BMI categories.[20] In a Swedish study, WHR was associated with increased risk of prostate cancer, particularly before age 65 and independent of BMI.[21] A case-control study in China by Hsing et al. showed that higher WHR was related to increased risk of prostate cancer with men in higher quartile (WHR > 0.92) having an almost threefold elevated risk (OR , 2.71, 95% CI = 1.66-4.41, P = 0.001) compared with men in lowest quartile (WHR<0.86).[5] Rohrmann et al. in their study on men of less than 55 years found that risk of high-grade disease increased with increasing BMI (P = 0.02) indicating that BMI in younger men correlates with lean body mass rather than fatty mass, which increases with age.[22] Literature suggests that obesity lowers the risk of indolent, less aggressive tumors, whereas it increases the risk of aggressive cancer.[10,23] This was also supported by other studies although only to some extent.[24,25] Most of these studies have used BMI as a marker of general obesity. Keeping in mind that central obesity is more common in Indian subcontinent[26] and is associated with metabolic alterations in the body, we tried to evaluate its effect on Gleason score.
Central obesity is associated with insulin resistance, hyperinsulinemia, and dyslipidemia. In our study, high Gleason score was statistically associated with higher insulin levels (P = 0.027). Insulin is known to be a direct mitogen for in vitro prostate growth and is necessary for the growth of prostate cancer cells in culture.[27] A case-control study in China[28] found a statistically significant positive association between fasting insulin and prostate cancer risk indicating that insulin could be a mediator that promotes prostate cancer development. Lehrer et al. showed higher insulin levels (P = 0.033) to be present in patients with high-risk prostate cancer, which was defined as Gleason score >7, tumor in seminal vesicle on biopsy, PSA >15, or stage T2c or T3.[29] It is known that prostate cancer cells migrate to adipocytes within red bone marrow where metastases are very common[30] and an experiment showed that attractiveness of human bone marrow to prostate cancer cells decreased when bone marrow stroma was depleted of lipid cells.[31] They are also known to take up lipid directly as a source of energy for the process of tumor maintenance, proliferation, and migration.[32] Platz et al. demonstrated lesser chances of advanced and metastatic prostate cancer (RR=0.51 CI 95% and RR 0.39 95% CI, respectively) and not decreased risk of overall prostate cancer in patients taking statins, which are lipid-lowering drugs. However no association was found between statin intake and Gleason grading.[33] Hammarsten et al. reported that prostate cancer patients with a higher grade tumor were more dyslipidemic, as shown by a higher TG level (P = 0.019) and a lower HDL-cholesterol level (P = 0.005) and higher plasma insulin level (P = 0.019) than those with a lower grade tumor.[34] However no association was found in the levels of raised TG and low HDL on comparing high-grade to low-grade tumors on American prostate cancer patients.[35] Platz et al. reported that men with low cholesterol <200 mg/dL) had a lower risk of Gleason 8 to 10 prostate cancer [OR, 0.41; 95% CI, 0.22-0.77] than men with high cholesterol (≥ 200 mg/dL).[36] Our study has shown a significant relationship with high Gleason score and TG, VLDL, and low HDL levels; however no relation was found with cholesterol and LDL levels.
Testosterone is a key prostate growth factor although prostate cancer presents at an age when testosterone levels are declining. It is known that aromatase present in adipose tissue leads to decreased testosterone levels due to peripheral conversion of testosterone to estradiol. Studies have suggested that testosterone may exert a differentiating effect on prostate cancer and decreased serum testosterone and increased estradiol levels may be associated with more advanced and poorly differentiated prostate cancer.[37,38] Lower testosterone levels may also be due to insulin resistance leading to decreased testosterone production by Leydig cells,[39] or due to negative feedback effect of inhibitory proteins present in patients with prostate cancer on hypothalamic-pituitary -gonadal axis.[40] In our study, there was a trend of decreased testosterone levels in patients with high Gleason score, which however was not statistically significant (P = 0.065). To date, few studies have investigated the relationship between central obesity hyperinsulinemia and dyslipidemia with higher grade prostate cancer majority of which are in western literature. The genetic and environmental factors affecting men of Indian population cannot be ignored and there is always a possibility that factors that affect the western males might not be the same in India. Small sample size was a drawback in our study, also all our patients had advanced or metastatic prostate cancer on account of center bias as our center is located in a poor province. Similarly the lipid levels seen in our patients might not reflect that seen in the general population.
CONCLUSION
Our study indicates that central obesity, dyslipidemia, and hyperinsulinemia independent of diabetes may be involved in higher grade prostate cancer and raises the potential that control of central obesity in these men or targeted treatment strategies may provide a means of reducing poor outcome in this high-risk group. Future studies with a larger sample size are needed to establish its association.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Whelan SL, Ferlay J, Raymond L, Teppo L, Thomas DB. Cancer Incidence in Five Continents. 2002 Lyon: International Agency for Research on Cancer International Association of Cancer Registries. [Google Scholar]
- 2.Satyanarayana L, Asthana Smita. Life time risk for development of ten major cancers in India and its trends over the years 1982 to 2000. J Med Sci. 2008;62:35–44. [PubMed] [Google Scholar]
- 3.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:843–57. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 4.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63:259–67. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 5.Hsing AW, Deng J, Sesterhenn IA, Mostofi FK, Stanczyk FZ, Benichou J, et al. Body size and prostate cancer: A Population based case control study in China. Cancer Epidemiol Biomarkers Prev. 2000;9:1335. [PubMed] [Google Scholar]
- 6.Hubbard JS, Rohrmann S, Landis PK, Metter EJ, Muller DC, Andres R, et al. Association of prostate cancer risk with insulin, glucose, and anthropometry in the baltimore longitudinal study of aging. Urology. 2004;63:253–8. doi: 10.1016/j.urology.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 7.Demark-Wahnefried W, Conaway MR, Robertson CN, Mathias BJ, Anderson EE, Paulson DF. Anthropometric risk factors for prostate cancer. Nutr Cancer. 1997;28:302–07. doi: 10.1080/01635589709514591. [DOI] [PubMed] [Google Scholar]
- 8.Boyd DB. Insulin and cancer, Integrative Cancer Therapies. 2003;2:315–29. doi: 10.1177/1534735403259152. [DOI] [PubMed] [Google Scholar]
- 9.Gleason DF. The Veteran's Administration Cooperative Urologic Research Group: histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic Pathology The Prostate. Philadelphia: Lea and Febiger; 1977. pp. 171–98. [Google Scholar]
- 10.MacInnis RJ. English DR Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63. [PubMed] [Google Scholar]
- 12.Lee IM, Sesso HD, Paffenbarger RS., Jr A prospective cohort study of physical activity and body size in relation to prostate cancer risk (United States. Cancer Causes Control. 2001;12:187–93. doi: 10.1023/a:1008952528771. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC. Anthropometric measures and body composition. In: Willett WC, editor. Nutritional Epidemiology. New York: Oxford University Press; 1998. pp. 244–72. [Google Scholar]
- 14.Gastaldelli A. Abdominal fat: does it predict the development of type 2 diabetes? Am J Clin Nutr. 2008;87:1118–9. doi: 10.1093/ajcn/87.5.1118. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf SS, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 16.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–5. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 17.Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JTM, et al. Waist circumference and mortality. Am J Epidemiol. 2008;167:1465–75. doi: 10.1093/aje/kwn079. [DOI] [PubMed] [Google Scholar]
- 18.Lahmann PH, Lissner L, Gullberg B, Berglund G. A prospective study of adiposity and all-cause mortality: the Malmö Diet and Cancer Study. Obes Res. 2002;10:361–9. doi: 10.1038/oby.2002.50. [DOI] [PubMed] [Google Scholar]
- 19.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–60. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 20.Andersson SO, Wolk A, Bergström R, Adami HO, Engholm G, Englund A, et al. Body size and prostate cancer: a 20 year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–9. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 21.Wallström P, Bjartell A, Gullberg B, Olsson H, Wirfält E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009;100:1799–805. doi: 10.1038/sj.bjc.6605077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55:140–6. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Giovannucci E, Platz EA. Are findings from studies of obesity and prostate cancer really in conflict? Cancer Causes Control. 2006;17:5–9. doi: 10.1007/s10552-005-0378-3. [DOI] [PubMed] [Google Scholar]
- 24.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 25.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 26.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis? Int J Epidemiol. 2007;36:220–5. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund TE, Miller GJ. A serum-free defined medium capable of supporting growth of four established human prostatic carcinoma cell lines. Prostate. 1994;24:221–8. doi: 10.1002/pros.2990240502. [DOI] [PubMed] [Google Scholar]
- 28.Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, et al. Prostate Cancer Risk and Serum Levels of Insulin and Leptin: a Population-Based Study. J Natl Cancer Inst. 2001;93:783–9. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Serum insulin level, disease stage, prostate specific antigen (PSA) and Gleason score in prostate cancer. Br J Cancer. 2002;87:726–8. doi: 10.1038/sj.bjc.6600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke N, Brown M. The Influence of Lipid Metabolism on Prostate Cancer Development and Progression: Is it Time for a Closer Look? European Urology. 2007;52:46–53. doi: 10.1016/j.eururo.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br J Cancer. 2006;94:842–53. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke WN, Claire A. Hart and Mick D Brown: Molecular mechanisms of metastasis in prostate cancer. Asian J Andrology. 2009;11:57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. JNCI. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 34.Hammarsten J, Högstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 35.Beebe-Dimmer JL, Nock NL, Neslund-Dudas C, Rundle A, Bock CH, Tang D, et al. Racial differences in Risk of Prostate Cancer Associated With Metabolic Syndrome. Urology. 2009;74:185–90. doi: 10.1016/j.urology.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;11:2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–5. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 38.Schatzl G, Madersbacher S, Thurridl T, Waldmüller J, Kramer G, Haitel A, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–8. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 39.Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90:2636–41. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 40.Zhang PL, Rosen S, Veeramachaneni R, Kao J, DeWolf WC, Bubley G. Association between Prostate cancer and serum testosterone. The Prostate. 2002;53:179–82. doi: 10.1002/pros.10140. [DOI] [PubMed] [Google Scholar]


