Abstract
Introduction:
Paired-kidney exchange (PKE) is used in western countries to increase donor pool. In India, there are not many centers involved in PKE program. We present 10 years of this experience and outcome of the recipients.
Materials and Methods:
Between year 2000 and 2009, 34 transplants with PKE were performed. All donors were live related, and permission from Authorization committee(s) from one or more states was obtained prior to transplantation. Both donor and recipient surgeries were carried out simultaneously in all cases at a single institution. Last 10 donors were offered laparoscopic donor nephrectomy and all other previous donors were operated by open surgery.
Results:
Five donor–recipient pairs were from the state of Rajasthan, one from Madhya Pradesh, and all others from Gujarat. ABO incompatibility between donor and recipient was present in 12 pairs and positive lymphocyte cross-match in 5 pairs.
Conclusion:
Paired-kidney-exchange transplantation expands donor pool and total number of transplantation.
Keywords: Donor, kidney, laparoscopy, nephrectomy, transplantation
INTRODUCTION
The incidence of end-stage-renal disease (ESRD) is increasing worldwide. In India, of the estimated 1,75,000 new patients who developed ESRD annually, less than 10% are able to gain access to renal-replacement therapy.[1] Kidney transplantation is the best option for the treatment of patients with ESRD.[2] However, there is a gross imbalance between organ-donation rate, both deceased and living related, and, the demand for organs.
Several methods are used to improve the organ-donation rate. Improving the deceased donor pool, ABO incompatible transplantation, laparoscopic donor nephrectomy for living donors, use of marginal donors, accepting altruistic donors, and, paired-kidney donation are methods used to increase the number of transplantation. Here, we present our experience of 10 years in paired-kidney exchange (PKE) transplantation at a single institution.
MATERIALS AND METHODS
Between June 2000 and November 2009, a total of 34 crossover kidney transplantation were performed. All the donors and recipients were evaluated by standard biochemical, serological, and radiological evaluation. For donor evaluation, intravenous pyelography and conventional angiography were used till the year 2007, and, since than 64 slice computed tomography was introduced for anatomical and functional study of kidneys. Similarly, evaluation for differential renal function was mandated, since year 2007, in every donor.
When the donor–ecipient pair was from the state other than the State of Gujarat, the permission for PKE donation was obtained by both Authorization Committee of the state of Gujarat and the state other than the Gujarat from where the pair belongs to and has permanent residence. Only after obtaining such permission, the elective transplantation was scheduled. Both pair of donor and recipients were allowed to meet each other and various members of the team of transplantation. Both donor–;recipient pairs were operated simultaneously in a single institution. Before year 2008 all donors were operated by open donor nephrectomy. However, in year 2009 all donors were operated by retroperitoneoscopic donor nephrectomy, performed simultaneously by two surgeons trained in retroperitoneoscopic donor nephrectomy. The recipient transplantation surgery was performed simultaneously in two different operation rooms. A special care was taken in the operation rooms so that appropriate kidney should go to the appropriate recipient.
RESULTS
Initial donor-recipient pairs were from the state of Gujarat only. However, since year 2005, donor-recipient pairs from other states were also included; five pairs were from Rajasthan and one from Madhya Pradesh accepted for PKE transplantation. ABO incompatibility or positive lymphocyte cross match were found in 12 pairs and 5 pairs respectively.
All donor and recipient procedures were performed successfully. Open donor nephrectomy in two different operation rooms were performed simultaneously by two donor surgeons. In year 2009, five pairs were offered laparoscopic donor nephrectomy. Two experienced surgeons performed simultaneously two donor nephrectomies in the same institution and two recipient surgeons performed transplantation. Allograft dysfunction was not seen in any of the recipients. Cyclosporine/tacrolimus-based immunosuppressants were used in all patients. Figure 1 shows graft and patient survival data. Eight patients were lost while graft was still functioning; the commonest cause of death was sepsis and hepatitis C related acute liver failure.
Figure 1.
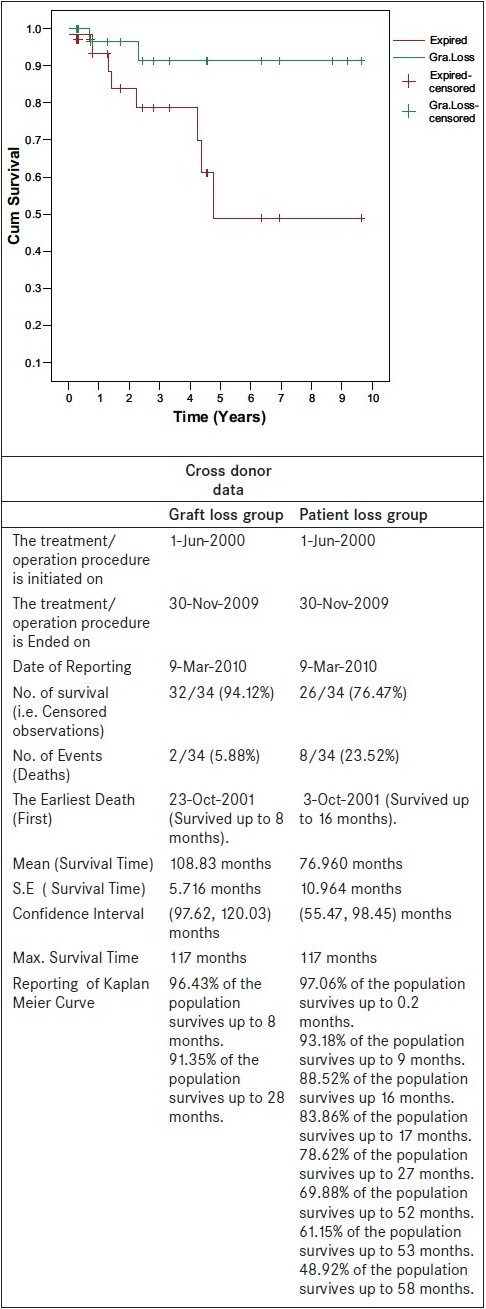
Kaplan curve for patient and graft survival functions
DISCUSSION
PKE transplants are used when a healthy donor is unable to donate to his or her relative. In PKE transplantation a healthy person in one couple donates his or her kidney to the ill person in another couple, in exchange for a kidney from the recipient′s healthy donor [Figure 2ab]. Historically, concept of PKE was given by Felix Rapaport in 1986.[1] However, that concept was not accepted in Europe since most transplant centers were considering unrelated transplantation in any form an illegal process. PKE was performed for the first time in South Korea in 1991 where because of cultural and religious reasons the organ exchange between living donors is easier to accept than the concept of brain death and use of organs from deceased donors.[2] Switzerland and USA performed their first PKE transplantation in year 1999 and 2000 respectively. Subsequently, several other countries in the Europe have legalized the procedure of PKE transplantation.
Figure 2.
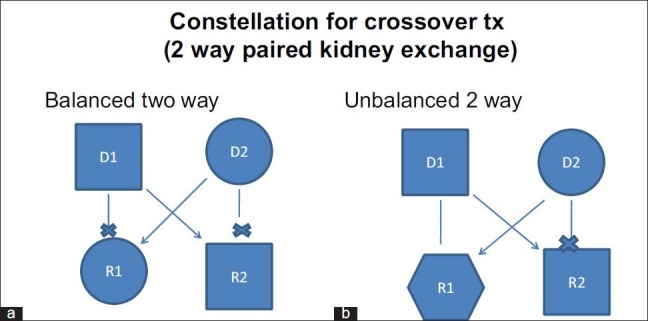
Balanced (a) and unbalanced (b) two way paired kidney donation. Balanced two way: Donor D1 and recipient R1 (related pair) has blood group A and B respectively. Donor D2 and recipient R2 (related pair) has blood group B and A respectively. These ABO imcompatibility does not allow two transplantations. In a balanced PKE donor D1 gives kidney to recipient R2 (Unrelated pair) and donor D2 gives kidney to recipient R1 (Unrelated pair). Unbalanced two way: Donor D1 has blood group O and related recipient R1 has blood group B. So donor D1 can donate to R1. The other pair, donor D2 has blood group B and the related recipient R2 has blood group O. If donor D1 donates to recipient R2 (unrelated) and donor D2 donates to recipient R1 than it is known as unbalanced two way PKE.
The law in India does not have any mention about the PKE donation.[3] Subclause (3) of clause 9 of Chapter II of The Transplantation of Human Organ Act, 1994 shows following statement: “If any donor authorizes the removal of any of his human organs before his death under sub-section (1) of Section 3 for transplantation into the body of such recipient, not being a near relative as is specified by the donor, by reason of affection or attachment towards the recipient or for any other special reasons, such human organ shall not be removed and transplanted without the prior approval of the Authorization Committee.” In other words, the authorization committee of the state, however, may approve transplantation from an unrelated donor in special circumstances. In a recent review, the government of India has recommended PKE through the Transplantation of Human Organs Act Review Committee of India.[4]
When one family member (close relative) wants to donate the kidney but is unable to do so due either to ABO incompatibility or positive lymphocyte cross match, it leads to situation of performing ABO incompatible transplantation or recipient is deprived of transplantation. ABO incompatible transplantations are very expensive and several cycles of removing various antibodies need to be performed before actual transplantation.[5] Often such transplants requires more than usual immunosuppressants and associated risk of infection and other complications. At our center, to overcome such situation, either the recipients were listed for deceased donor kidney transplant program or offered PKE with legal permissions from various state authorization committees.
At our center we have performed both balanced two way and unbalanced two way transplantation [Figure 2]. Basu et al. from Christian Medical College, Vellore have suggested a model for HLA-matched donor-swap to reduce donor specific HLA antibody mediated rejections and associated reduce allograft survival.[6] The Netherlands has national living donor kidney exchange program since year 2003 and computerized matching and cross matching program with centralized allocation of organs.[7] In United States, at Newton and at Toledo the PKE transplantation programs are regional and involve 19 and 73 transplant centers, respectively.[8] Such programs accept altruistic donors and expand the altruistic donor chain. In India, currently, the law does not permit organ donation by altruistic donor.
In conclusion, the shortage of kidney donors could partly be overcome by PKE living donor kidney transplant program, especially in countries where deceased donor organs are currently limited. For expansion of such program regional or national computarized transplant registry for ABO incompatible donor-recipient pair, positive cross match pairs and also HLA mismatch may be needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chugh KS. Five decades of Indian Nephrology- a personal perspective. Am J Kidney Dis. 2009;54:753–63. doi: 10.1053/j.ajkd.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation and recipients of a first cadaveric transplant. N Eng J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Government of India. Transplantation of Human Organs Act, 1994. Central Act 42 of 1994 Available from: http://www.medindia.net/tho.thobill1.asp .
- 4.The Report of Transplant of Human Organs Act Review Committee. 2005. Directorate General of Health Services, Ministry of Health andFamily Welfare, Government of India.
- 5.Padmanabhan A, Ratner LE, Jhang JS, Duong JK, Markowitz GS, Vasilescu ER, et al. Comparitive outcome analysis of ABO-incompatible and positive crossmatch renal transplantation: a single-center experience. Transplantation. 2009;87:1889–96. doi: 10.1097/TP.0b013e3181a76ae1. [DOI] [PubMed] [Google Scholar]
- 6.Basu G, Daniel D, Rajagopal A, Neelakantan N, John GT. A model for human leukocyte antigen-matched donor-swap transplantation in India. Transplantation. 2008;85:687–92. doi: 10.1097/TP.0b013e318163827e. [DOI] [PubMed] [Google Scholar]
- 7.de Klerk M, Witvliet MD, Hasse-Kromwijk BJJM, Claas FHJ, Wellem Weimar. Hurdles, barriers, and successes of a National living donor kidney exchange program. Transplantation. 2008;86:1749–53. doi: 10.1097/TP.0b013e3181908f60. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari P, de Klerk M. Paired kidney donations to expand the living donor pool. J Nephrol. 2009;22:699–707. [PubMed] [Google Scholar]


