Abstract
Nephrolithiasis is associated with a variety of abnormalities in urinary composition. These abnormal urinary risk factors are due to dietary indiscretions, physiological-metabolic disturbances or both. Stone disease is morbid and costly, and the recurrence rates may be as high as 30-50% after 5 years. Efforts to prevent stone formation are, therefore, essential. Dietary factors play an important role in kidney stone formation. Tailored dietary recommendations based on metabolic evaluation should be offered to patients for the prevention of recurrence of stone formation. Dietary intervention and subsequent evaluations of therapeutic efficacy should be based on results from multiple 24-h urine collections. Urine flow of >1 ml/kg/h almost eliminates the risk of supersaturation for calcium oxalate, calcium phosphate and uric acid, thus protecting from the formation of corresponding kidney stones. In patients with cystenuria, the required urine flow may even be higher and, in cases such as primary xanthinuria, high fluid intake is required. Milk intake in these patients should be within the RDA of calcium and protein. In children, recommendation of a high fluid intake has only limited success. Nevertheless, each patient should be advised about adequate fluid intake to increase urine volume in accordance with body size. Although children with hypocitraturia may benefit from therapeutic agents that raise the urine citrate concentration, all children bearing residual fragments should be counseled on adequate fluid intake along with potassium citrate treatment to prevent stone regrowth or formation.
Keywords: Calcium, diet, kidney stone
INTRODUCTION
The natural history of stone disease is not well defined in children as it is in adults. The pathology is associated with considerable morbidity, with reported recurrence rates of 6.5-44%.[1] Without follow-up and medical intervention, recurrence rates have been reported to be as high as 50% within 5-6 years.[2] Nephrolithiasis is associated with a variety of abnormalities in urinary composition. These abnormal urinary risk factors are due to dietary indiscretions, physiological-metabolic disturbances or both.[3] The correction of abnormal risk factors by dietary modifications and pharmacological intervention has been shown to prevent recurrent stone formation. The epidemiology of urolithiasis differs according to geographical area and historical period. Vesical stones in children continue to occur endemically in many developing countries. “Primitive” vesical stone is fairly widespread in Asia, with calculi composed of ammonium urate and calcium oxalate. Vesical stones, due to malnutrition in the very early years of life, are currently frequent in huge areas of Turkey, Iran, India, China, Indochina and Indonesia, although the incidence is decreasing in proportion with the gradual improvements in social conditions.[4] India, Pakistan and Southern China comprise an important part of the stone belt in Asia. In children, calcium oxalate accounts for 45-65% of the stones, followed by calcium phosphate (14-30%), struvite (13%), cystine (5%), uric acid (45) and mixed or miscellaneous (4%).[5]
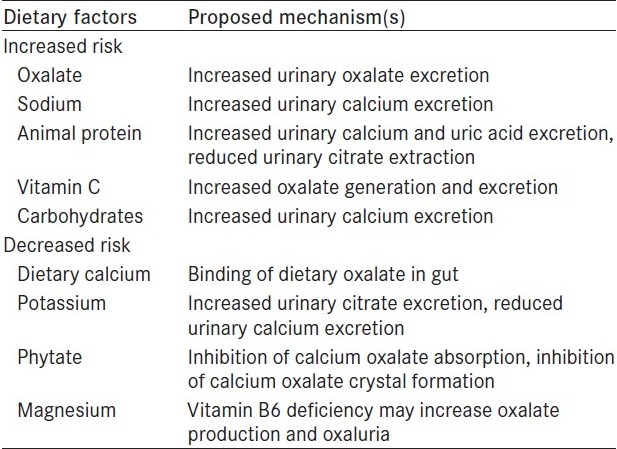
Hypercalciuria is the most common etiology of urolithiasis in children and adults.[6,7] Calcium oxalate and/or phosphate stones account for almost 70% of all renal stones observed in economically developed countries. The prevalence of this type of stones varies considerably on account of environmental factors, especially dietary intake and lifestyle, while radiolucent and infection stones seem to be less influenced by environmental conditions [Table 1]. In the 70s, the pathogenetic role for calcium oxalate stones of a diet rich in proteins, refined carbohydrate and sodium became evident, while the effect of alimentary calcium and oxalate is still debated. However, the concurrence of a genetic predisposition seems to be crucial for calcium stone formation. In fact, the importance of family history for idiopathic calcium stone disease is clearly demonstrated, although little is known about the metabolic alterations underlying this predisposition and their genetic transmission mechanisms.[4]
Table 1.
Dietary factors that may increase or decrease calcium oxalate stones
NUTRITION AND KIDNEY STONES IN CHILDREN
Most children diagnosed with idiopathic hypercalciuria are treated either with dietary means or with pharmacological preparations. The long-term outcome of hypercalciuric children is not completely clear. There are two main types of the disorders – bladder stones in children, a form of disorder that disappeared from Europe in the late 19th and early 20th centuries, and upper urinary tract stones in adults. The former has been decreasing in most countries in the so-called endemic bladder stone belt with gradual improvements in the levels of nutrition. The types of stones formed depend mainly on the composition of urine, which, in turn, reflects the type of diet consumed in the countries concerned. The main factor that leads to the formation of bladder stones in children is a nutritionally poor diet that is low in animal protein, calcium and phosphate, but high in cereal and is acidogenic.[8] This leads to the formation of urine with a relatively high content of ammonium and urate ions and, consequently, to the formation of ammonium acid urate crystals and stones. In countries where there is also a high intake of oxalate from local leaves and vegetables, urinary oxalate is increased and, as a result, the ammonium acid urate stones often contain calcium oxalate as well. The stone problem in the tropics is compounded by low urine volumes resulting in some areas from poor drinking water, which causes chronic diarrhea, and in others from the hot climate and fluid losses through the skin. As nutrition improves in these countries, the formation of bladder stones gives way to upper urinary tract stones consisting of calcium oxalate, often mixed with calcium phosphate or uric acid, such as those that are formed in most Western countries.[8]
Almost all investigators of vesical stones have implicated dietary factors in the causation of stone. Epidemiological studies on dietary intakes from areas endemic for vesicle stones have revealed that these children eat mainly a cereal-based diet lacking in animal proteins, especially milk.[9] Less than 25% of the total protein intake of the diet is of animal origin. The commonly used cereals are whole wheat flour, either alone or mixed with Bengal gram flour, millets and pulses like black gram, red gram and green gram.[10] Studies with experimental diets in Indian children with vesical stone have shown that consumption of whole wheat flour as a staple food leads to the production of urine with a high specific gravity (SG), increased excretions of calcium, phosphorus, magnesium, oxalate and uric acid and supersaturation of urine with uric acid. The uric acid/creatinine ratio is higher in children, which decreases with increasing age. Children consuming wheat as a staple food are at a greater risk of forming a stone because of the increased saturation and precipitation of uric acid.[11]
Early weaning with supplements of pre-masticated rice, vegetables and uncooked fermented fish without other sources of animal protein has been reported from endemic areas of Thailand.[12] The geographical distribution of vesical stones in Indian children revealed that its incidence is highest in areas with mild to moderate protein energy malnutrition (PEM) and low in areas with a severe degree of PEM.
Till date, there are very few studies on pediatric urolithiasis as a majority of the published literature comes from adult populations. Increased protein and salt intake and decreased potassium intake commonly observed in children in the Western society may contribute to the development of hypercalciuria.[13] Long-term compliance with a low-Na/high-K diet might be beneficial to children who are at risk of developing kidney stones. A study evaluated the status of 14 males and 19 females aged 8-17 years studied 4-11 years after the initial diagnosis of idiopathic hypercalciuria not associated with urolithiasis. Based on the first urine specimen, 16 of the 33 (48.4%) patients were hypercalciuric. Their second urinalysis showed persistent hypercalciuria in eight and normocalciuria in eight patients. The urine Na/K ratio (mEq/mEq) decreased in the latter eight patients from 5.08 ± 2.67 to 3.03 ± 2.23 (P < 0.05). Of 23 children (all eight persistently and nine intermittently hypercalciuric plus six normocalciuric), only in one asymptomatic persistently hypercalciuric child was a single small renal calcification noted. Introduction of a low-Na/high-K diet in seven persistently hypercalciuric children resulted in a decrease in the urine Na/K ratio from 7.34 ± 2.15 to 4.14 ± 3.09 (P < 0.01) and the urine Ca/Cr ratio from 0.25 ± 0.04 to 0.13 ± 0.03 (P < 0.01).[14]
A step-wise approach has been proposed in evaluating children with idiopathic urolithiasis in the Western society, in which first only urine calcium is studied. Only if urine calcium is normal should other chemistries be studied. In many hypercalciuric children, a low-Na/high-K diet one is effective while in most others, the addition of potassium citrate is well tolerated, which normalizes calciuria and protects against new stone formation. Children rarely comply with the recommendation of high fluid intake. In this study, treatment of 33 hypercalciuric patients consisted of diet one in 13, potassium citrate in 17, thiazides in two and potassium citrate and thiazide in one. All 33 achieved normocalciuria apart from two who remained mildly hypercalciuric on diet one. The 12 normocalciuric children were treated by diet modification alone. The urine SG at follow-up visits was unchanged in stone formers.[15]
A retrospective study on epidemiology, etiology and dietary and urinary risk factors on 1,440 children from Pakistan in idiopathic stone formers revealed hypercalciuria in 17 (11%), hyperoxaluria in 62 (40%), hyperuricosuria in 41 (27%) and hypocitruria in 97 (63%). Diet involved a low intake of protein in 60 cases (44%), calcium in 45 (33%), potassium in 105 (77%) and high oxalate in 75 (55%). The composition was calcium oxalate in 362 stones (47%), ammonium hydrogen urate in 210 (27%) and struvite in 49 (6.4%).[16]
Children with urolithiasis tend to have a lower 24-h urine volume than do normal children.[17] A study on 32 children with urolithiasis showed that urine flow of >1 ml/kg/h almost eliminated the risk of supersaturation for calcium oxalate, calcium phosphate and uric acid, thus protecting from the formation of corresponding kidney stones.[18] In a child weighing 40 kg, this translates to 960 ml urine/24 h. In patients with cystenuria, the required urine flow may even be higher, and in cases such as primary xanthinuria, high fluid intake is required. Milk intake in these patients should be within the recommended dietary allowance (RDA) of calcium and protein. In children, recommendation of high fluid intake has only limited success.[19] Nevertheless, each patient should be advised about adequate fluid intake to increase urine volume in accordance with body size. Although children with hypocitraturia may benefit from therapeutic agents that raise the urine citrate concentration, all children bearing residual fragments should be counseled on adequate fluid intake along with potassium citrate treatment to prevent stone regrowth or formation during long-term follow-up.[20] Calyceal microlithiasis is reported as a predictor of development of urolithiasis.[21]
DEVELOPMEMTS IN STONE PREVENTION
Nutritional factors implicated in stone disease are linked with the preventive intervention. Dietary measures are the first level of intervention in primary prevention as well as in secondary prevention of recurrences. Most data on the relation between diet and stone disease come from observational and physiological studies rather than randomized trials on adults. There is no consensus yet on the specifics of dietary modification, but several important concepts should be kept in mind when designing a therapeutic diet.
Important concepts
It is important to individualize recommendations based on stone type and urinary composition (for initial evaluation, two 24-h urine collections obtained 6 weeks after a stone episode are important).
The impact of dietary risk factors varies with age, sex and body mass index (BMI).
Patients must provide follow-up 24-h urine collections to evaluate the impact of dietary recommendations.
If urine composition does not change in response to dietary change, then alternative approaches should be tried. It is important to distinguish stone passage from new stone formation. If a patient passes a pre-existing stone after the implementation of dietary change, it does not indicate treatment failure.
DIETARY RISK FACTORS FOR CALCIUM STONE DISEASE
Modern lifestyle, dietary habits and obesity emerge to be the promoters of idiopathic stone disease. Cross-sectional studies on adults have shown significant correlations between these factors and kidney stones, with direct implications on our preventive concepts: normalization of BMI, adequate physical activity, balanced nutrition and sufficient circadian fluid intake. Modern diets containing a lot of animal protein, refined carbohydrates and salt act on the metabolism like an acid load. To overcome these disadvantageous effects, a sufficient supply of potassium and alkali is required. It is important to know that calcium should not be restricted. Usually, the body does not absorb more calcium than is needed; however, certain conditions can cause much calcium to be absorbed or too much to be passed into the kidneys. Several large prospective observational studies in men and women consistently support a reduced risk of stone formation with increasing dietary calcium intake compared with individuals in the lowest quintile of dietary calcium intake.[22] Those in the highest quintile had more than 30% lower risk of forming a stone. A 5-year randomized controlled trial compared stone recurrence in individuals with a history of calcium oxalate nephrolithiasis and idiopathic hypercalciuria. Risk of developing a recurrent stone on higher calcium diet (1,200 mg/d) along with low animal protein and sodium was 51% lower than for the low-calcium diet (400 mg/d). Hence, there is an overwhelming evidence that dietary calcium should not be restricted. As such, a diet increases the risk of stone formation and may be harmful to bone health by inducing negative calcium balance.[22,23]
One potential mechanism to explain this apparent paradox is that higher calcium intake will bind dietary oxalate in the gut, thereby reducing oxalate absorption and urinary excretion. It is also possible that dairy products (major source of dietary calcium) may contain factors that inhibit stone formation. Only in absorptive hypercalciuria does calcium restriction remain beneficial in combination with thiazide and citrate therapy.[24] The risk factor can vary with age. A study has shown no association between dietary calcium and stone formation in men aged 60 years or older.[25]
Calcium intake of the stone patients should be matched to their 24-h urinary calcium excretion. Table 2 gives the calcium content of food items and drinks. The highest values of calcium are found in dairy products. Extreme calcium restriction results in considerable calcium deficit and in a reduction in enteric calcium availability. In children, calcium restriction can affect growth. Calcium intake for children should be according to the RDA for age. A study on the relationship of an animal-rich diet with kidney stone formation has shown that as the fixed acid content of the diet increases, urinary calcium excretion also increases. The inability to compensate for animal protein-induced calciuric response may be a risk factor for the development of osteoporosis.[26] Table 3 gives the dietary recommendation for the prevention of calcium oxalate stones.
Table 2.
Calcium content of food items in mg/100 g
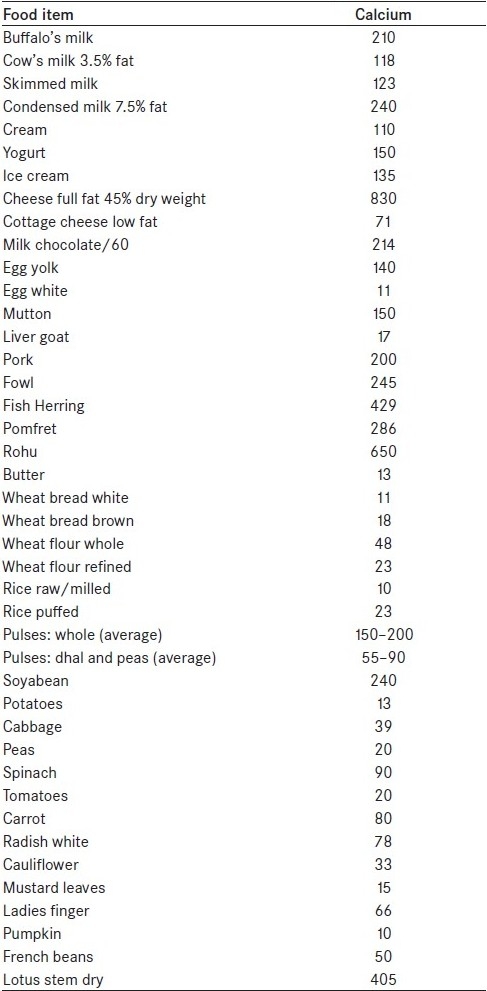
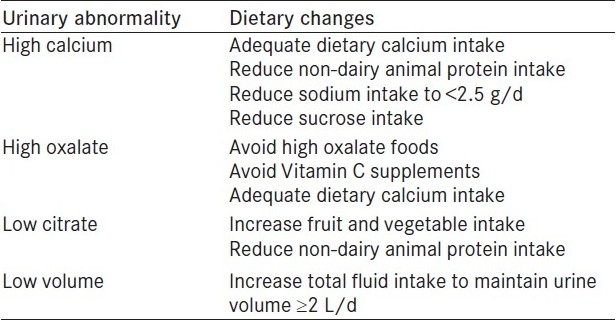
Table 3.
Dietary recommendations for calcium oxalate stone prevention according to the urinary risk factor
Calcium supplementation
For an individual who has had a stone, the impact of calcium supplementation on urine composition should be evaluated before recommending calcium supplementation. The patient on calcium supplements should collect 24-h urine samples on and off supplement and, based on urine composition, recommendations should be reviewed. If supplements are to be taken, they should be taken with meals to avoid hypercalciuria.[24] Natural food products should be preferred over supplements provided the patient is not sensitive to milk products.
Oxalate
Oxalate, the end product of endogenous metabolic pathways, is toxic in high concentrations and is 90-95% excreted in the urine. Additional oxalate originating from intestinal absorption is also excreted by the kidney.[27] The role of dietary oxalate in the pathogenesis of calcium oxalate nephrolithiasis is less clear. The proportion of urinary oxalate derived from dietary oxalate is controversial, estimates ranging from 10% to 50%. Thus, a substantial proportion of urinary oxalate is derived from endogenous production from the metabolism of glycine, glycolate and hydroxyproline. Other dietary factors can influence oxalate in urine.[28,29]
Vitamin C is one such factor. Much of oxalate in food may not be readily absorbed because of low bioavailability and, also, there is significant variation in gastrointestinal tract absorption of oxalate (Oxalobacter formigenes, an intestinal bacterium, degrades oxalate and can decrease its availability for absorption).
Recommendations
Dietary treatment of calcium oxalate lithiasis includes reducing or eliminating nutritional oxalate intake. Intestinal hyperoxiluria is best avoided by restricting cocoa drinks, chocolate, candies, black tea, excessive coffee intake, spinach rhubarb, asparagus, celery, parsley and tomatoes. Calcium oxalate stone formers should limit intake of almonds, peanuts, cashews walnuts, beetroot, cheeko, cocoa, chocolate, tomato, strawberries, eggplant, soy products, tofu, wheat bran and rice bran.
Recommendations for children include a high-fluid, low-sodium diet and RDA of calcium and protein.[14] A minimum of half of the body weight in ounces of water (e.g., an 80-pound child would drink 40 oz of water) should be taken daily. Proper hydration helps prevent the urine from becoming concentrated with crystals, which can lead to stone formation. It also reduces the risk for urinary tract infections, which may lessen the risk for struvite stones. In North America, the dietary recommendations for children are healthier diet with less salt and more fruit, vegetable and dairy products. In order to successfully maintain such a dietary modification for long periods of time, children should be followed medically and nutritionally.[30,31]
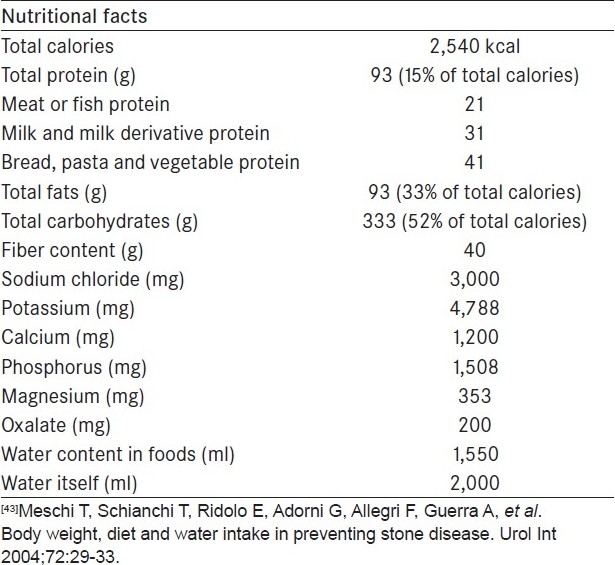
Adult patients with calcium oxalate stones should limit the daily intake of animal protein to 0.8 g/kg of body weight, which is the RDA as determined by the Institute of Medicine, Food and Nutrition Board. Calcium intake should remain within the RDA of 800-1,000 mg/day. In calcium oxalate stone formers with mild to moderate hyperoxaluria, the enteral administration of probiotic agents in the form of lactobacillus species or O. formigenes has shown promising results.[32,33] A possible “sate-of-the-art” diet has been proposed [Table 4].[34]
Table 4.
The anti-stone forming diet[43]
Role of sodium, potassium, phytate and magnesium in stone formation
Higher sodium intakes result in decreased sodium reabsorption, with subsequent reduction in calcium reabsorption in the distal nephron. High sodium intake increases urinary calcium, modestly reducing urinary citrate and promoting the induction of urate-induced calcium oxalate crystallization. Ingestion of an additional 5 g of table salt could raise urinary calcium by 40 mg/day and lower urinary citrate by 50 mg/day. Moreover, a high-sodium diet attenuates the ability of thiazide or indapamide to control hypercalciuria, rendering these drugs relatively ineffective in treating hypercalciuric nephrolithiasis.[3] The Food and Nutrition Board of the Institute of Medical, National Academy of Sciences, states that the optimal daily intake of sodium at ages 4-8 years is 1.2 g and at ages 9-18 years is 1.5 g, and the upper limit values are 1.9 and 2.3 g, respectively. These values are two to three times lower than the current average daily intake of sodium. Observational studies reveal a positive independent association between sodium consumption and new kidney stone formation in women but not in men. In 24-h urine collections, urine sodium excretion appeared to be associated with an increased risk in men, but not consistently in women.[35]
In adults, the sodium intake should not exceed 2 g/day. A high-potassium intake is inversely associated with kidney stones in men and older women but not in younger women. Dietary potassium restriction can increase calcium excretion. Hypokalemia stimulates citrate reabsorption, decreasing the urinary excretion of citrate, an important inhibitor of calcium oxalate formation. Potassium in food also accompanies organic ions such as citrate that are metabolized to bicarbonate. Therefore, consumption of potassium-containing foods such as fruits and vegetables represents an alkali load that increases the urinary excretion of citrate.[24]
The optimal daily potassium intake provided mostly in the form of fruit, vegetable and dairy products is 3.8 g at 4-8 years and 4.5 g at ages 9-18 years. These values are two to three times higher than the current average intake.[19]
Uric acid, cystine and calcium phosphate stones
Uric acid crystal formation depends on two factors: uric acid concentration and urine pH (solubility of uric acid increases substantially as the urine pH increases from 5.0 to 6.5). Stone formation due to increased uric acid excretion should be combated by an increased diuresis by neutralizing an acid urine and by the administration of a xanthine oxidase inhibitor. Primary congenital hyperoxiluria is extremely uncommon and, in such cases, the only recommended prophylaxis against stone formation consists of high doses of vitamin B1 and B6 together with magnesium preparations and hydrochlorothiazide.[29]
Decreasing the consumption of meat, chicken and seafood will decrease the intake of purine and, therefore, production of uric acid. Higher intake of fruits and vegetables should raise urinary pH and reduce the risk of uric acid crystal formation.
Patients with cystine stones should restrict sodium intake and increase fruit and vegetable intake, which will raise urinary pH and increase solubility of cystine. There is little evidence to support dietary restriction of protein, although reducing animal protein will be beneficial in increasing urinary pH. Restriction of methionine-containing foods like peanuts, pistachio, popcorns, broccoli, mushroom, cauliflower, avocado, bean sprouts, potatoes, spinach, green peas, tofu, kidney beans, black beans and tempeh may prevent cystine crystal formation.
Patients with Type 1 renal tubular acidosis and stone disease may benefit from alkali supplementation (potassium citrate). They may also benefit from diets rich in fruits and vegetables. However, increasing urine pH can increase the risk of calcium phosphate crystal formation.
DIETARY COMPONENTS IN THE INDIAN SUBCONTINENT
Dietary components influence the biochemical parameters such as oxalate, uric acid and calcium sodium. A study from Marathwada has shown that in this region the diet contains groundnuts, tomato, spinach and animal proteins, with greater use of salt and use of bore well water for drinking. These factors may serve as risk factors for stone formation. A high dietary intake of a purine-rich diet causes elevated secretion of uric acid, which leads to calcium oxalate crystal formation and precipitation.[36] The pattern of stone disease has changed from a predominantly lower tract site in the mid 1980s to the upper tract in the mid 1990s. Stone composition, urinary risk factors and dietary analysis suggest that diet, dehydration and poor nutrition are the main causative factors of stone disease.[37]
PREVENTION OF STONE FORMATION
General measures aimed at the prevention of stone disease should avoid or reduce the effect of known risk factors. The first principle of any dietary or drug treatment should be to avoid the precipitation of lithogenic salts or minerals by controlling their relative saturation in solution. The cardinal requirement is thus adequate urinary dilution. This can usually be achieved by adequate fluid intake, evenly distributed over day and night. Most pediatric urologists and nephrologists recommend a urine SG below 1.010, namely hypo or isosthenuric. Only a minority of the pediatric patients maintain such dilute urine. This may indicate either a consultant′s failure in educating patients or, more likely, the common tendency for asymptomatic children not to comply with such recommendations as their memory of colic fades. This might also indicate that high fluid intake is not a crucial component of intervention in these children when other means of treatment are successful in preventing new stone formation.[15] Only a continuous intake rather than acute bursts of drinking will guarantee the required urinary SG of <1,015. Fluid losses due to perspiration, hot working conditions, sunbathing and various sporting activities have to be made up for. A high urinary volume generates a series of positive effects that are useful in the prevention of stone recurrence. In adults, urine volume should be >2 L/day. A study has shown that calcium stone formers of both sexes have a lower 24-h urinary volume than non-stone formers of the same age.[38] Subjects presenting with their first episode of calcium stone disease who were instructed to increase their water intake and, hence, their urinary volume, reduced the risk of recurrence by 50% compared with those who were not instructed to do so and who did not significantly increase the urinary volume over the 5-year duration period.[37] Weight reduction and all forms of physical activity should be encouraged.[39]
In addition to the total amount of fluid consumed, the type of beverage might also play a role. In a study on middle-aged women, coffee, tea and wine were found to be independently protective whereas grapefruit juice promoted incident kidney stone disease.[24]
These findings were also confirmed in men; however, in this cohort, beer consumption reduced and apple juice increased the risk of kidney stone.[40] However, a general recommendation for consumption of alcoholic beverages cannot be given at the present time. Coffee, as opposed to tea consumption, has repeatedly been shown to be protective in a dose-dependent fashion, which likely relates to urine flow.[41]
A comprehensive stone prevention program must include public education on sensible dietary habits and avoidance of excess calorie intake or nutritional imbalance.[42] Such programs should be aimed particularly at high-risk groups. High-risk groups comprise chiefly the obese persons suffering from metabolic disorders, those with inadequate physical activity and individuals with a strong family history.
Normalization of body weight and cardiovascular risk factors and sufficient physical activity balances nutrition and sufficient circadian fluid intake would be the appropriate measure to avoid new calculus formation in about 85% of all stone formers.[24]
Dietary phytate (inositol hexaphosphate), found in foods high in fiber (cereals, legumes and vegetables), may play a role in preventing the formation of calcium stones. Phytates inhibit urinary crystallization of calcium salts. Observational data from younger women show that dietary phytate is inversely related with incidence of kidney stone formation, but no such association has been observed in men. Magnesium may reduce oxalate absorption in the gastrointestinal tract and can form soluble complexes with oxalate in the urine, potentially decreasing calcium oxalate supersaturation. In observational studies, higher dietary magnesium is associated with a 30% lower risk of stone formation in men but not in women.[34] Experimental studies have shown that urine phytate-depleted individuals have increased crystallization risk.[36] Fish-oil supplementation has shown advantageous effects on the lithogenic serum and urine parameters.[43]
SUMMARY
Stone disease is morbid and costly, and recurrence rates may be as high as 30-50% after 5 years. Consequently, efforts to prevent stone formation are essential. Dietary factors play an important role in kidney stone formation. Tailored dietary recommendations based on metabolic evaluation should be offered to patients for the prevention of recurrence of stone formation. Dietary intervention and subsequent evaluations of therapeutic efficacy should be based on results from multiple 24-h urine collections. The fundamental principle in the treatment of kidney stone is to supply adequate fluids like water, barley water, sharbat, aerated water and fruit juice in order to ensure urine of desired SG. The effect of excess animal protein (purine) is most obvious for the uric acid stone former. Protein from plant sources (beans, legumes and others) can be substituted as a dietary alternative without negative consequences. The role of excess protein in promoting calcium stone formation is less obvious, but is equally important. High dietary protein is associated with increased urinary calcium. Citrate is a known inhibitor of calcium oxalate crystal formation and, also increases urinary pH, which can prevent uric acid stones. Hence, benefits of protein restriction for stone formers are enormous.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hoppe B, Jahnen A, Bach D, Hesse A. A urinary calcium oxalate saturation in healthy infants and children. J Urol. 1997;158:557–9. [PubMed] [Google Scholar]
- 2.Kroovand RL. Pediatric Urolithiasis. Urol Clin North Am. 1997;24:173–85. doi: 10.1016/s0094-0143(05)70362-1. [DOI] [PubMed] [Google Scholar]
- 3.Pak Charles YC. Medical management of urinary stone disease. Nephron Clin Pract. 2004;98:c49–53. doi: 10.1159/000080252. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri A. Epidemiology of urolithiasis. Arch Ital Urol Androl. 1996;68:203–49. [PubMed] [Google Scholar]
- 5.Milliner DS, Murphy ME. Urolithiasis in pediatric patients. Mayo Clin Proc. 1993;68:241–8. doi: 10.1016/s0025-6196(12)60043-3. [DOI] [PubMed] [Google Scholar]
- 6.Kingwatannakul P, Alon US. Hypercalciuria and urolithiasis in childhood. In: Trachman H, Gauthier B, editors. Pediatric Nephrology. Amsterdam: Harwood Academic Press; 1999. pp. 253–68. [Google Scholar]
- 7.Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M, et al. Spectrum of stone composition: Structural analysis of 1050 upper urinary tract calculi from northern India. Int J Urol. 2005;12:12–6. doi: 10.1111/j.1442-2042.2004.00990.x. [DOI] [PubMed] [Google Scholar]
- 8.Robertson WG. Renal stones in the tropics. Semin Nephrol. 2003;23:77–87. doi: 10.1053/snep.2003.50007. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DA. The nutritional significance of primary bladder stones. Br J Urol. 1962;34:160–77. doi: 10.1111/j.1464-410x.1962.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 10.Teotia M, Teotia SP. Kidney and bladder stones in India. Postgrad Med J. 1977;53:41–8. [PubMed] [Google Scholar]
- 11.Teotia M, Krishna S, Teotia SP. Kidney and vesical stones in children Chapter 106. In: Teotia SP, Teotia M, editors. Nutritional and Metabolic Bone and Stone Disease An Asian Perspective. New Delhi: CBS Publishers and Distributors; 2008. pp. 795–807. [Google Scholar]
- 12.Halstead SB, Valyasevi A, Umpaivit P. Dietary habits and disease prevalence. Am J Clin Nutr. 1967;20:1352–68. doi: 10.1093/ajcn/20.12.1352. [DOI] [PubMed] [Google Scholar]
- 13.Pak CY. Genetic guidelines in medical evaluation. In: Resnik MI, Pak CY, editors. Urolithiasis. 1990. pp. 153–72. Saunders, Phildelphia: A medical and surgical reference. [Google Scholar]
- 14.Alon US, Berenbom A. Idiopathic hypercalciuria of childhood: 4- to 11-year outcome. Pediatr Nephrol. 2000;14:1011–5. doi: 10.1007/s004670050064. [DOI] [PubMed] [Google Scholar]
- 15.Alon US, Zimmerman H, Alon M. Evaluation and treatment of pediatric idiopathic urolithiasis-revisited. Pediatr Nephrol. 2004;19:516–20. doi: 10.1007/s00467-004-1422-3. [DOI] [PubMed] [Google Scholar]
- 16.Rizvi SA, Naqvi SA, Hussain Z, Hashmi A, Hussain M, Zafar MN, et al. Pediatric urolithiasis: Developing nation perspectives. J Urol. 2002;168:1522–5. doi: 10.1016/S0022-5347(05)64509-0. [DOI] [PubMed] [Google Scholar]
- 17.Miller LA, Stapleton FB. Urinary volume in children with urolithiasis. J Urol. 1989;141:240–7. doi: 10.1016/s0022-5347(17)41052-4. [DOI] [PubMed] [Google Scholar]
- 18.Lande MB, Varade W, Erkan E, Niederbracht Y, Schwartz GJ. Role of urinary supersaturation in the evaluation of children with urolithiasis. Pediat Nephrol. 2005;20:491–4. doi: 10.1007/s00467-004-1779-3. [DOI] [PubMed] [Google Scholar]
- 19.Alon US. Medical treatment of pediatric urolithiasis. Pediatr Nephrol. 2009;24:2129–35. doi: 10.1007/s00467-007-0740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarica K, Erturhan S, Yurtseven C, Yagci F. Effect of potassium citrate therapy on stone recurrence and regrowth after extracorporeal shockwave lithotripsy in children. J Endourol. 2006;20:875–9. doi: 10.1089/end.2006.20.875. [DOI] [PubMed] [Google Scholar]
- 21.La Manna A, Polito C, Cioce F, De Maria G, Capacchione A, Rocco CE, et al. Calyceal microlithiasis in children: Report on 196 cases. Pediatr Nephrol. 1998;12:214–7. doi: 10.1007/s004670050440. [DOI] [PubMed] [Google Scholar]
- 22.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for prevention of recurrent stones in idiopathic hypercalciurea. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 23.Curhan GC. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney Stones. N Engl J Med. 1993;328:833–8. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 24.Straub M, Hautmann RE. Developments in stone prevention. Curr Opin Urol. 2005;15:19–26. doi: 10.1097/01.mou.0000160627.36236.6b. [DOI] [PubMed] [Google Scholar]
- 25.Taylor EN, Stampfer MJ, Curghan GC. Dietary factors and the risk of incident of kidney stones in men: New insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225–32. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 26.Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–6. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 27.Williams HE. Oxalsaure: Absorption, eckretion and metabolismus. In: Feldmann U, Newes D, editors. Urolithiasis: Straube Erlangen; 1976. pp. S87–91. Internationales Symposion, Davos, (Hrsg) [Google Scholar]
- 28.Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. 2004;32:311–6. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 29.Rose GA, Arthur LJ, Kasidas GP, Scott IV, editors. Skeinkoff, Darmstadt: Pathogenese und Klinik der Harnsteine IX. 1982:S8–14. [Google Scholar]
- 30.Eaton SB, Konner M. Paleolithic nutrition. N Engl J Med. 1985;312:283–9. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 31.Kurokawa K. Kidney, salt and hypertension: How and why. Kidney Int. 1996;55:S46–51. [PubMed] [Google Scholar]
- 32.Curhan GC, Willett WC, Knight EL. Dietary factors and the risk of incident kidney stones in younger women: Nurses Health Study II. Arch Intern Med. 2004;164:885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 33.Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001;60:1097–105. doi: 10.1046/j.1523-1755.2001.0600031097.x. [DOI] [PubMed] [Google Scholar]
- 34.Lieske JS. Use of probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005;68:1244–9. doi: 10.1111/j.1523-1755.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 35.Deshmukh SR, Khan ZH. Evaluation of urinary abnormalities in nephrolithiasis patients from Marathwada region. Indian J Clin Biochem. 2006;21:177–80. doi: 10.1007/BF02913091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grases F, March JG, Prieto RM, Simonet BM, Costa-Bauzá A, García-Raja A, et al. Urinary phytate in calcium oxalate stone formers and healthy people- dietary effects on phytate excretion. Scand J Urol Nephrol. 2000;43:162–4. doi: 10.1080/003655900750016526. [DOI] [PubMed] [Google Scholar]
- 37.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A, et al. Urinary volume, water and recurrence in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–43. [PubMed] [Google Scholar]
- 38.Taylor EN, Stampfer MJ, Curghan GC. Obesity,weight gain, and the risk of kidney stone. JAMA. 2004;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 39.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol. 1996;143:240–7. doi: 10.1093/oxfordjournals.aje.a008734. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb DS, Fischer ME, Keich Y, Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: A report from Vietnam Era Twin (VET) registry. Kidney Int. 2005;67:1053–61. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 41.Scholz D, Schwille PO, Siegel A. Ernahrungsgewohnheiten von Patienten mit Urolithiasis. (Hrsg) In: Vahlensieck W, Gasser G, editors. Steinkopff Darmstadt: Pathogenese und Klinik der Harnsteine; 1981. pp. S83–7. [Google Scholar]
- 42.Baggio B, Budakovic A, Nassuato MA, Vezzoli G, Manzato E, Luisetto G, et al. Plasma phospholipids arichdonic acid content and calcium metabolism in idiopathic calclium nephrolithiasis. Kidney Int. 2000;58:1278–4. doi: 10.1046/j.1523-1755.2000.00283.x. [DOI] [PubMed] [Google Scholar]
- 43.Meschi T, Schianchi T, Ridolo E, Adorni G, Allegri F, Guerra A, et al. Body weight, diet and water intake in preventing stone disease. Urol Int. 2004;72:29–33. doi: 10.1159/000076588. [DOI] [PubMed] [Google Scholar]


