Abstract
A fraction of simian immunodeficiency virus (SIV)-infected macaques develop rapidly progressive disease in the apparent absence of detectable SIV-specific antibody responses. To characterize the immunopathogenesis of this syndrome, we studied viral load, CD4+ T-lymphocyte numbers as well as cellular and humoral immune responses to SIV and other exogenous antigens in four SIVsm-infected rhesus macaques that progressed to AIDS 9 to 16 weeks postinoculation. Each of these animals exhibited high levels of viremia but showed relatively preserved CD4 T lymphocytes in blood and lymphoid tissues at the time of death. Transient SIV-specific antibody responses and cytotoxic T-lymphocyte responses were observed at 2 to 4 weeks postinoculation. Two of the macaques that were immunized sequentially with tetanus toxoid and hepatitis A virus failed to develop antibody to either antigen. These studies show that the SIV-infected rapid progressor macaques initially mounted an appropriate but transient cellular and humoral immune response. The subsequent immune defect in these animals appeared to be global, affecting both cellular and humoral immunity to SIV as well as immune responses against unrelated antigens. The lack of CD4 depletion and loss of humoral and cellular immune responses suggest that their immune defect may be due to an early loss in T helper function.
Human immunodeficiency virus (HIV) infection results in a highly variable disease course ranging from rapid progression to long-term nonprogression (3, 8, 10, 11, 29, 31, 34, 37, 39). The rate of disease progression is tightly linked with the extent of virus replication. Thus, postseroconversion plasma viral RNA levels are predictive of disease outcome (28, 34). The extent of viremia is influenced by a wide range of host factors. For example, cellular immune responses are temporally associated with downregulation of viremia following primary infection (23), and the strength of these responses is predictive of viral load and disease progression (32, 35). Other potential host factors influencing disease progression include genetic polymorphisms of major histocompatibility complex (MHC) genes and deletions in the CCR5 gene (4, 6). While long-term nonprogressors have been studied extensively (3, 29, 37, 40), less attention has been paid to the study of rapid progression. Individuals who progress to AIDS in a period of 1 to 2 years from the time of infection have been identified among adult and infant populations (10, 11, 30, 51). These individuals demonstrate rapid loss of CD4+ T cells and lack strong cellular and humoral immune responses. However it is not clear why such patients develop AIDS so rapidly, and the relative contributions of host and viral factors remain undefined.
The pathogenesis of SIV infection in macaques appears to be similar to that of HIV infection, covering the full spectrum of long-term nonprogression to rapid progression (2, 9, 15, 16, 17, 20, 27, 36, 54). Even when a common SIV strain is used for experimental infection of macaques, the disease outcome can be highly variable, consistent with a strong influence of host factors (12, 17, 27, 46). As with HIV infection, the level at which plasma viral RNA stabilizes following primary infection with SIV is a highly predictive correlate of the rate of disease progression (17, 50), and CD8+ T cells play a major role in early control of viremia (24, 42). Macaques that progress rapidly following SIV infection are characterized by persistent antigenemia, high and increasing levels of plasma viral RNA, and lack of apparent SIV-specific antibody responses (7, 20, 36, 41, 54). Macaques with this extremely rapid disease course are observed with all cohorts inoculated with pathogenic SIV strains at a frequency of approximately 25 to 30% of the cohort.
The purpose of the present study was to evaluate the immunopathogenesis of this disease syndrome in SIV-infected macaques in an attempt to determine the mechanisms for immune dysfunction in such animals. Since rapid progression occurs sporadically within a cohort and we did not wish to artificially perturb the immune system by manipulations such as CD8 depletion to increase the proportion of such animals, the macaques for this study were derived from three separate studies. We studied the kinetics of viral replication in these animals, CD4 T-cell numbers in blood and tissues, humoral and cell-mediated immune responses for SIV, and immune responses to other exogenous antigens.
MATERIALS AND METHODS
Viruses and macaques.
Four rhesus macaques of Indian origin were inoculated intravenously with 50 monkey infectious doses of SIVsmE660 (H538 and H567 [43]) or 2000 tissue culture infectious doses of SIVsmE543-3 (H445 [2]) or SIVsmH445 (H635). SIVsmE660, SIVsmE543-3, and SIVsmH445 are closely related viruses. EDTA-anticoagulated blood samples were collected sequentially and evaluated for isolation of infectious SIV, plasma viral RNA loads, lymphocyte subsets by flow cytometry, SIV-specific antibody, and cytotoxic T-cell responses. The two rhesus macaques infected with SIVsmE660 expressed the Mamu-A*01 MHC class I molecule. These animals were selected by Mamu-A*01-specific reverse transcriptase-PCR of total RNA isolated by RNeasy purification (Qiagen, Chatsworth, Calif.) from 5 × 106 peripheral blood mononuclear cells as described previously. Verification was achieved by direct sequencing of PCR products (QIAquick PCR purification kit, Qiagen) by automated sequencing on the ABI 377 sequencer.
The ability of herpesvirus papio-transformed B lymphoblastoid cell lines (B-LCL) from these macaques to act as targets in p11C-specific functional cytotoxicity assays was used to confirm the expression of the Mamu-A*01 allele. Monkeys were maintained in accordance with the guidelines of the Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (33). Symptoms in each of the four animals were similar: weight loss, anorexia, and chronic diarrhea that was unresponsive to supportive and antibiotic therapy. Macaques were euthanized when they developed intractable diarrhea, and weight loss of greater than 10% of body weight.
Assays for SIV-specific antibodies.
Serology for antibodies to SIVsm was performed by Western blot analysis as previously described (15, 18) and radioimmunoprecipitation. Briefly, CEM174 cells were infected with SIVsmE660 (14) and, at the peak of reverse transcriptase activity, labeled overnight with l-[35S]methionine and l-[35S]cysteine (Amersham, Arlington Heights, Ill.). The next day, the labeled cells were lysed with 1 ml of radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl, pH 7.5, 5 mM EDTA, pH 8.0, 312.5 mM NaCl, 1.0 g of sodium deoxycholate, 1% Nonidet P40) and centrifuged and the cell lysate supernatant was preabsorbed with 50 μl protein A-agarose beads (Gibco BRL, Gaithersburg, Md.) for 1 h. Then 10 μl of plasma was combined with 50 μl of protein A-agarose beads and incubated with shaking for 1 h at 4°C. The protein A-agarose bead-antibody complex was washed once with phosphate-buffered saline (PBS), combined with equal aliquots of cell lysate, incubated with shaking for 1 h at 4°C, and then washed five times with 1 ml of RIPA buffer. The pellet was resuspended in 50 μl of RIPA buffer and 50 μl of 2× sodium dodecyl sulfate (SDS) gel loading buffer (100 mM Tris-HCl, pH 6.8, 200 mM 2-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol), boiled for 4 min. and then loaded onto an SDS-10% polyacrylamide gel. The dried gel was exposed to a Kodak Bio-Max MR film (Kodak, Rochester, N.Y.) for 5 days.
SIV neutralization was measured in CEMx174 cells as previously described (17, 18). Neutralizing antibody titers are the plasma dilution at which 50% of cells were protected from virus-induced killing as measured by neutral red uptake. Virus stocks for neutralization assays were produced in H9 cells (SIVsmH-4 and SIVsmE543-3) and CEMx174 cells (SIVsmE660).
Immunohistochemistry and in situ hybridization.
Formalin-fixed, paraffin-embedded tissues were stained for SIV RNA as previously described (18) with SIV-specific probes. Briefly, the tissue sections were deparaffinized and rehydrated with water, pretreated with 0.2 N hydrochloric acid, proteinase K, prehybridized and hybridized overnight at 51°C with either the antisense or sense riboprobe. The riboprobe consisted of a mixture of probes encompassing 90% of the SIV genome, conjugated with digoxigenin-UTP (Loftstrand Labs Ltd., Gaithersburg, Md.) at a final concentration of 1.75 ng/μl. The hybridized sections were washed in standard posthybridization buffers and RNase A (Sigma, St. Louis, Mo.) and RNase T1 (Roche Molecular Biochemicals, Indianapolis, Ind.). The sections were blocked in 3% normal sheep and horse serum in 0.1 M Tris (pH 7.4) and then incubated with a 1:500 dilution of sheep antidigoxigenin-alkaline phosphatase (Roche Molecular Biochemicals) for 1 h. Sections were then rinsed in Tris buffer and reacted with NBT/BCIP (Vector Laboratories, Ltd., Burlingame, Calif.) for 10 h and visualized with a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, N.Y.).
Formalin-fixed, paraffin-embedded tissue sections were stained with an anti-human CD4 mouse monoclonal antibody clone 1F6 (Novacastra Laboratories). Sections were rehydrated and processed for 6 to 8 min in a Presto pressure cooker (National Presto Industries, Eau Claire, Wis.) in 1 mM EDTA (pH 8.0) or Tris to unmask antigens. The samples were sequentially treated with PBS, aqueous hydrogen peroxide, serum block (3% normal goat serum-1% nonfat milk-0.5% bovine serum albumin), and the specific monoclonal antibody for 1 h. The reaction was visualized with the Vectastain mouse IgG-peroxidase ABC kit (Vector Laboratories) and diaminobenzidine, followed by 10 s of treatment in diaminobenzidine enhancing solution (Vector Laboratories). Samples were then rinsed in distilled water and counterstained with hematoxylin.
Assessment of immune responses to tetanus toxoid and Havrix.
Two cohorts of six macaques each were evaluated for immune responses to tetanus toxoid and hepatitis A virus following establishment of SIV infection. Each cohort included one rapid progressor macaque, H445 (SIVsmE543-3) and H635 (SIVsmH445). The macaques in the first cohort (including H445) were immunized intramuscularly with 5 U in 0.5 ml of tetanus toxoid (Connaught, Swiftwater, Pa.) at 9 weeks after SIV inoculation and antibody responses were measured by a commercial enzyme-linked immunosorbent assay (ELISA) (Quest Diagnostics, San Juan Capistrano, Calif.) in plasma samples collected 1 and 2 weeks later. The macaques were immunized intramuscularly with the pediatric dose (720 ELISA units in 0.5 ml) of the hepatitis A virus vaccine (Havrix, SmithKline Beecham Inc, Philadelphia, Pa.) at 11 and 14 weeks. Antibody responses were measured weekly by ELISA with the Abbott (Abbott Park, Ill.) HAVAB enzyme immune assay. The macaques in the second cohort (including H635) were immunized with tetanus toxoid and hepatitis A virus vaccine simultaneously at 4 weeks post-SIV inoculation and then boosted with hepatitis A virus at 8 weeks post-SIV inoculation.
CTL assay with recombinant vaccinia virus.
Peripheral blood mononuclear cells (107) from the monkeys were cultivated in vitro with paraformaldehyde-fixed, autologous B-LCL infected with either vaccinia virus-SIV env or vaccinia virus-SIV gag. On day 3 of culture, 20 U of recombinant human interleukin-2 per ml was added to the cultures. On day 12 of culture, the lymphocytes were centrifuged over a Ficoll-diatrizoate gradient and assessed as effector cells in a 51Cr release cytotoxicity assay. Target cells were autologous B-LCL (106) cultured overnight with vaccinia virus-SIV gag, SIV env, or vaccinia virus expressing equine herpesvirus (control) at a multiplicity of infection of 10 PFU/cell. Target cells were then washed and labeled with 100 μCi of sodium 51chromate (ICN, Calif.) for 1.5 h. After washing, 104 target cells were added per well in 96-well U-bottomed plates in 100-μl volumes. Effector cells were added in another 100-μl volume at various concentrations to give effector-to-target cell ratios of 20:1, 10:1, 5:1, and 2.5:1. Plates were incubated at 37°C for 4 h. Then 50 μl of supernatant was transferred to counting plates and 200 μl of scintillation fluid was added and analyzed in a 1450 Microbeta liquid scintillation counter. Specific release was calculated as [(experimental release − spontaneous release)/(100% release − spontaneous release)] × 100.
Tetramer staining of whole blood.
One microgram of phycoerythrin-labeled tetrameric Mamu-A*01/p11C in conjunction with fluorescein isothiocyanate-labeled anti-human CD8α (Leu2a; Becton Dickinson), phycoerythrin-Texas red energy coupling dye (ECD)-labeled anti-human CD8αβ (2ST8-5H7; Beckman Coulter), and allophycocyanin-labeled anti-rhesus CD3 (FN18) monoclonal antibodies were used to stain p11C-specific CD8+ T cells. One hundred microliters of whole blood from both vaccinated and control monkeys was directly stained with these reagents, lysed on an Immunoprep Reagent Q-Prep Workstation (Coulter), washed in 3 ml of PBS, and fixed in 0.5 ml of PBS containing 1.5% paraformaldehyde.
Alternatively, peripheral blood leukocytes from rhesus monkeys were isolated and washed in Hanks' balanced salt solution containing 2% fetal calf serum. Peripheral blood leukocytes (5 × 106) in 2 ml of RPMI 1640 medium containing 12% fetal calf serum (R12) were cultured in the presence of 1 μg of the SIV Gag p11C (CTPYDINQM) peptide per ml. On day 3 of culture, 2 ml of 40-units/ml human recombinant interleukin-2 (Hoffman-La Roche) was added. On day 12 of culture, peptide-stimulated peripheral blood leukocytes were centrifuged over a Ficoll gradient and washed. Then 5 × 105 peptide-stimulated peripheral blood leukocytes were resuspended in 100 μl of PBS and stained with 1 μg of phycoerythrin-labeled tetrameric Mamu-A*01/p11C or Mamu-A*01/p68A complexes in conjunction with fluorescein isothiocyanate-labeled anti-human CD8α (Leu2a; Becton Dickinson), ECD-labeled anti-human CD8αβ (2ST8-5H7; Beckman Coulter), and allophycocyanin-labeled anti-rhesus CD3 (FN18) monoclonal antibody. Then the samples were washed in 3 ml of PBS containing 2% fetal bovine serum and fixed in 0.5 ml of PBS containing 1.5% paraformaldehyde. Samples were analyzed by four-color flow cytometry on a Coulter EPICS Elite ESP system. Gated CD3+ CD8αβ+ T cells were examined for staining with tetrameric Mamu-A*01/p11C complex.
Analysis of Gag p11C epitopes in plasma virus.
Analyses of 500-bp regions of viral gag, env, and pol sequences were performed essentially as described previously (1) evaluating 18 to 20 clones per animal. Virus from frozen plasma samples was isolated by centrifugation at 25,000 × g for 1 h and lysed with 48% guanidine thiocyanate, 1.4% dithiothreitol, 1% N-laurolylsarcosine, and 1% sodium citrate. RNA was precipitated with isopropanol and solubilized. First-strand cDNA was synthesized with reverse transcriptase with the SIV gag primer 5′ TGTTTGTTCTGCTCTTAAGCTTTTGTAG-3′. Initial PCR amplification used the primers gag-fwd (5′-ACCTAGTGGTGGAAACAGGAACAG-3′) and gag-rev (5′-TGTTTGTTCTGCTCTTAAGCTTTTGTAG-3′). Secondary nested PCR amplification was performed with the primers gag-fwd (5′-AGCACCATCTAGTGGCAGAGGA-3′) and gag-rev (5′-GAAATGGCTCTTTTGGCCCTT-3′). The amplified fragments were cloned into pCR4-TOPO (Invitrogen, Carlsbad, Calif.) or pAMPI (Stratagene, La Jolla, Calif.), and individual transformed colonies were subjected to T3 or T7 dideoxy sequencing.
RESULTS
Four rhesus macaques were selected from three separate animal studies (12, 43) based upon the persistent presence of SIV p27 antigen in their plasma samples and progression to AIDS in a period of less than 6 months following SIV inoculation. Two animals (H538 and H567) were Mamu-A*01-positive rhesus macaques inoculated intravenously with SIVsmE660 as control animals during the evaluation of a modified vaccinia virus Ankara (MVA) vector expressing SIVsm gag-pol (43). Both of these animals were immunized with nonrecombinant MVA and were SIV naïve at the time of challenge. One animal (H445) was part of a cohort of six Mamu-A*01-negative rhesus macaques inoculated intravenously with SIVsmE543-3 to evaluate the intrinsic susceptibility of their peripheral blood mononuclear cells to SIV as an indicator of subsequent in vivo viral replication (12). A final animal (H635) was part of a cohort of six Mamu-A*01-negative rhesus macaques inoculated intravenously with virus isolated from monkey H445 (unpublished data).
Each of these macaques exhibited a rapidly progressive disease characterized by weight loss (>10% of body weight), and chronic, persistent diarrhea that did not respond to supportive or antibiotic therapy. The macaques were euthanized between 9 and 16 weeks post-SIV inoculation (see Table 1). Pathological changes were similar in the four animals and were characterized by lack of lymphoid hyperplasia or secondary germinal centers and the presence of multinucleated giant cells in many tissues, including the lung, lymph nodes, intestines, and brain (2, 15, 18). The intestinal mucosa and submucosa were diffusely edematous, and villous blunting and fusion and crypt abscesses were observed (data not shown). Granulomatous encephalitis and pneumonia with associated SIV expression was observed in all four macaques. No obvious opportunistic agents were identified in any of these animals.
TABLE 1.
Summary of SIV-infected rapid progressor macaque characteristics
| Macaque | Virus | Mamu-A*01 allele | Survival (wk) |
|---|---|---|---|
| H538 | SIVsmE660 | + | 10 |
| H567 | SIVsmE660 | + | 9 |
| H445 | SIVsmE543-3 | − | 16 |
| H635 | SIVsmH445 | − | 9 |
Each of the four animals was evaluated for sequential plasma viral load, peripheral blood CD4+ T-cell numbers, SIV expression in tissues by in situ hybridization, and CD4+ T-cell numbers in tissues by immunohistochemistry. Cellular immune responses and antibody responses (immunoprecipitation and neutralizing assay) of H538, H567, and H445 were analyzed. Antibody responses to exogenous recall and primary antigens (tetanus toxoid and Havrix) were evaluated in H445 and H635, in parallel with other members of their cohorts.
High plasma viremia.
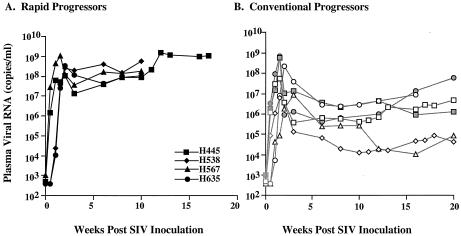
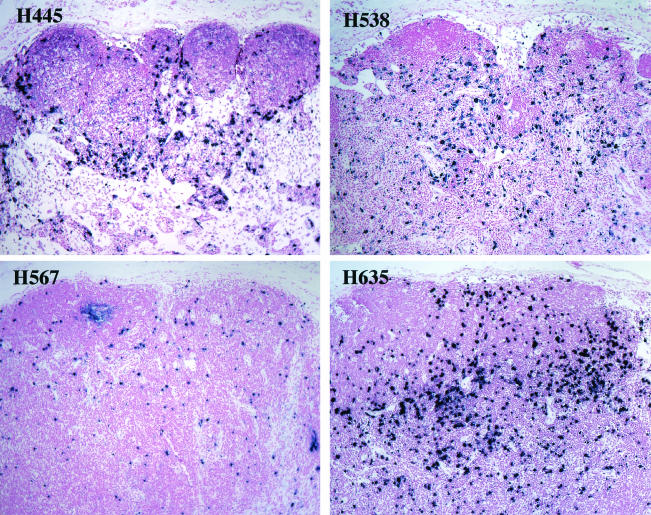
All four animals developed high levels of plasma viral RNA during primary viremia that continued to increase throughout the course of infection (Fig. 1). Plasma viral RNA levels ranged from 108 to 109 copies per ml at the time of euthanasia; these levels were 100- to 1,000-fold higher than plateau levels in the plasma of other animals in their cohorts that developed SIV-specific antibody (see Fig. 1B). Plasma viral RNA levels in conventional progressors generally declined by 2 to 3 logs following primary infection. Consistent with high plasma viremia, large numbers of virus-expressing cells were observed in lymphoid tissues of these four animals as detected by in situ hybridization of mesenteric lymph nodes collected at the time of death (Fig. 2).
FIG. 1.
Sequential plasma viremia in rapid progressor macaques. (A) Plasma viremia is shown graphically for the four study animals from the time of inoculation to euthanasia. (B) For comparison, plasma viremia during the same time period is shown for six conventional progressor macaques; two were selected from each cohort of the rapid progressor macaques.
FIG. 2.
SIV-specific in situ hybridization (bottom) in mesenteric lymph nodes collected at death from H445, H538, H567, and H635. Severe disruption of the lymphoid architecture is evident, with absence of secondary germinal centers and variable degrees of paracortical depletion. Large numbers of virus-expressing cells were observed in the lymph nodes of all four animals. Magnification, ×10.
Maintenance of peripheral CD4+ T lymphocytes.
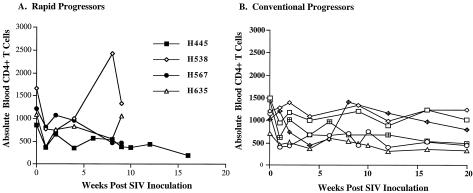
Lymphocyte subsets in the peripheral blood were evaluated prospectively following infection. As shown in Fig. 3A, absolute CD4+ T cells at the time of euthanasia varied considerably (327, 466, 1,000 and 1,332 cells per μl of blood). CD4+ T cells declined to some degree in all animals compared to preinoculation values, but were within normal limits in two of the animals (H538 and H635) and moderately depleted in another (H567) (see Fig. 3A). A similar range in CD4 counts was observed among four other historical rapid progressors, with an overall range in the eight animals of 220 to 1,249, with a mean of 765 cells/μl. Thus, despite the rapidity of disease progression and the high levels of viremia, none of these animals exhibited the low CD4 T-cell counts usually seen in AIDS (16, 25). The alterations in CD4+ T cells were similar to those of conventional progressor macaques observed during the same time period following infection (see Fig. 3B). In contrast to the relative preservation of CD4 cells terminally in the rapid progressors, significant CD4+ T-cell depletion terminally was observed in the conventional progressors from these cohorts (n = 12; mean ± standard deviation, 116 ± 69).
FIG. 3.
(A) Sequential changes in absolute peripheral CD4+ T cells during the course of SIV infection in four rapid progressor macaques is shown graphically. (B) For comparison, CD4+ T cells are shown during the first 20 weeks after inoculation in the same six conventional progressor macaques in Fig. 1B.
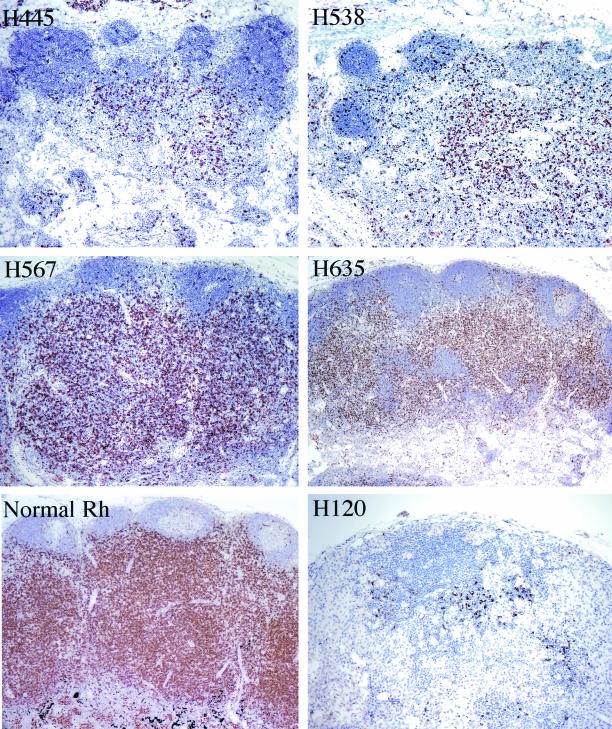
Immunohistochemistry was used to evaluate the numbers of CD4+ T cells in peripheral and mesenteric lymph nodes, spleen and gastrointestinal tract. As shown for the mesenteric lymph nodes in Fig. 4, the numbers of CD4+ T cells varied among the animals. Numerous CD4 cells were observed in lymphoid tissues of H567 and H635 and moderate to severe depletion was observed in lymphoid tissues of H445 and H538 (Fig. 4). The level of depletion was similar in various lymphoid tissues examined (data not shown). However the peripheral CD4 count (Fig. 3) did not directly correlate with the number of CD4 cells seen in lymph nodes by immunohistochemistry. Since these animals were dying rapidly with extremely high levels of viremia and relatively preserved CD4+ T-lymphocyte numbers in the blood and lymphoid tissues, we were interested in the immune responses to viral and other antigens in such animals.
FIG. 4.
Immunohistochemical detection of CD4+ T cells in mesenteric lymph nodes of four rapid progressor macaques at the time of autopsy, showing variable degrees of CD4 depletion in lymphoid tissues. Lymph nodes of H445 and H538 (top) demonstrated moderate to severe CD4+ T-cell depletion, whereas depletion was much less pronounced in nodes from H567 and H635 (middle). This contrasts with the preservation of CD4 cells in the paracortical region of a lymph node from a normal uninfected rhesus macaque (bottom left) and the severe depletion in a lymph node of a conventional progressor macaque, H120, in end-stage disease (bottom right).
Transient SIV-specific antibody responses.
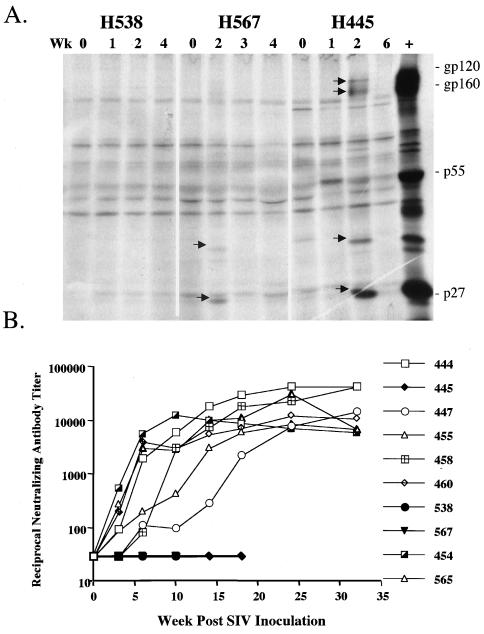
SIV-specific antibody was not detectable by Western blot analysis or whole virus ELISA of plasma samples at 4 weeks postinoculation, at a time when all other animals had seroconverted (data not shown). Since radioimmunoprecipitation may be more sensitive for detecting SIV-specific antibody responses, early sequential plasma samples from three of the macaques were evaluated for their ability to immunoprecipitate SIV proteins from SIVsmE660-infected cell lysates. As shown in Fig. 5A, weak and transient SIV-specific antibody responses were observed in two of the three macaques at 2 weeks postinoculation. One macaque (H567) responded solely to Gag antigens and the other responded to both envelope and Gag antigens (H445). The rapid progressor macaques did not mount a detectable neutralizing antibody response to their challenge virus (SIVsmE543-3 for H445 and SIVsmE660 for H538 and H567) or a laboratory-adapted, neutralization-sensitive strain, SIVsmH4 (Fig. 5B). This contrasted with robust neutralizing antibody responses to SIVsmH4 by 6 weeks postinoculation in all the conventional progressors (Fig. 5B). Neutralization of the challenge strain was only observed by 18 to 24 weeks following infection in the conventional progressor macaques (data not shown) and thus would not be expected to be seen by the time of death of the rapid progressor macaques (9 to 16 weeks).
FIG. 5.
Analysis of SIV-specific antibody responses of rapid progressor macaques H538, H567, and H445. (A) Radioimmunoprecipitation analysis of SIV-specific antibody in plasma of macaques H538, H567, and H445 during the primary phase of infection. Lanes are numbered according to the week post-SIV inoculation of the plasma samples. The locations of SIV gp120, gp160, p55, and p27 are indicated. (B) Sequential reciprocal neutralizing antibody titers in SIV-inoculated macaques assayed with the laboratory-adapted, homologous SIVsmH4 strain. Macaques H538, H567, and H445 did not develop detectable neutralizing antibody.
Transient SIV-specific cellular immune responses.
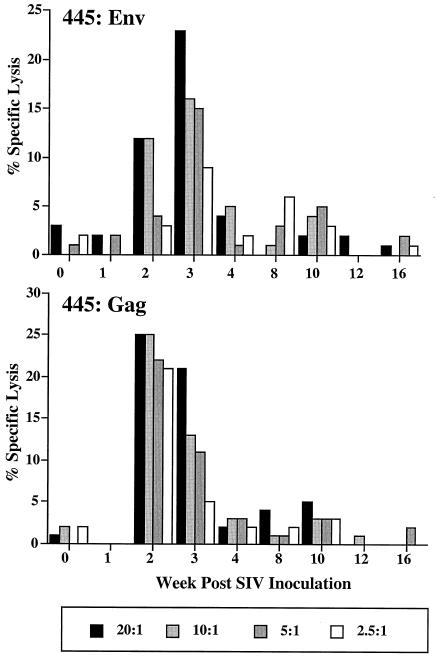
SIV-specific cytotoxic T-cell responses were assayed prospectively in three of the macaques. Functional assays utilizing vaccinia virus to express SIVmac envelope and Gag in autologous target cells were used to evaluate CTL in H445, a Mamu-A*01-negative macaque. As shown in Fig. 6A, Gag- and Env-specific CTL responses were detected at 2 and 3 weeks postinoculation but rapidly waned to background levels by 4 weeks postinoculation. In contrast, other macaques in the cohort of SIVsmE543-3-inoculated macaques developed robust and persistent SIV-specific CTL responses by 2 weeks postinoculation (data not shown).
FIG. 6.
Env- and Gag-specific CTL responses in peripheral blood of macaque H445. Specific lysis of vaccinia virus-expressed Env (top) and Gag (bottom) target cells at different effector-to-target cell ratios are shown graphically throughout the course of infection of H445.
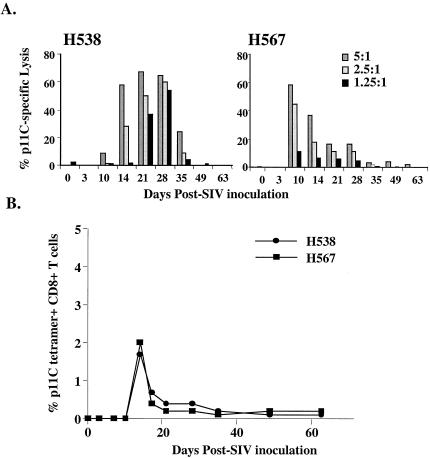
The CTL responses of the two Mamu-A*01-positive macaques were assayed with an epitope-specific functional CTL assay and tetramer technology. As shown in Fig. 7A, p11C-specific CTL were detected in a functional assay at 2 to 5 weeks postinoculation and then rapidly declined to below background levels. This functional activity coincided with the presence of CD8+ T cells that bound the p11C tetramer in the peripheral blood (Fig. 7B). The kinetics of the appearance of this response was similar to that of two other naïve Mamu-A*01-positive SIV-infected macaques in the cohort of SIVsmE660-infected macaques that had a more usual clinical course but was lower in magnitude (data not shown) (43).
FIG. 7.
Gag peptide, p11C-specific CTL activity in the blood of MVA-immunized rhesus monkeys H538 and H567 after infection with SIVsmE660. (A) Sequential p11C-specific lysis in peripheral blood mononuclear cells at effector-to-target cell ratios of 5:1 to 1.25:1 with CTL activity detectable between days 14 and 28 following infection is shown graphically. (B) Sequential percent tetramer-positive CD8+ T cells in whole blood of macaques H538 and H567 shown sequentially throughout SIV infection.
Loss of CTL activity in these rapid progressor animals could be due to viral escape from CTL recognition. We evaluated the frequency of viral sequence mutations within Mamu-A*01-restricted Gag p11C epitope at the time of euthanasia of the two Mamu-A*01+ animals and compared these sequences with the inoculum. The viral sequence of the dominant Gag p11C-M epitope was conserved (data not shown), suggesting that the loss of CTL activity in these animals was not due to viral escape in this epitope. Thus, the loss of CTL activity appears mechanistically distinct from the progressive loss of activity observed in conventional progressors where viral immune escape is an accumulative and ongoing process (1).
Rapid progressor macaques are unable to respond to unrelated antigens.
Finally since these macaques showed a failure to maintain both humoral and cellular antiviral immune responses, we were interested in their ability to respond to other exogenous antigens. The ability of two rapid progressor macaques to respond immunologically to tetanus toxoid and hepatitis A virus was assessed (Fig. 8). One cohort of SIVsm-inoculated, Mamu-A*01-negative macaques were immunized with tetanus toxoid at 9 weeks after SIV infection and with hepatitis A virus antigen (Havrix) at 11 and 14 weeks. The second cohort was immunized simultaneously with tetanus toxoid and Havrix (primary) at 4 weeks and boosted with Havrix at 8 weeks postinoculation. All of the animals had been immunized with tetanus toxoid within the last 5 years, but antibody titers were low at the time of immunization and therefore this antigen represented a recall antigen.
FIG. 8.
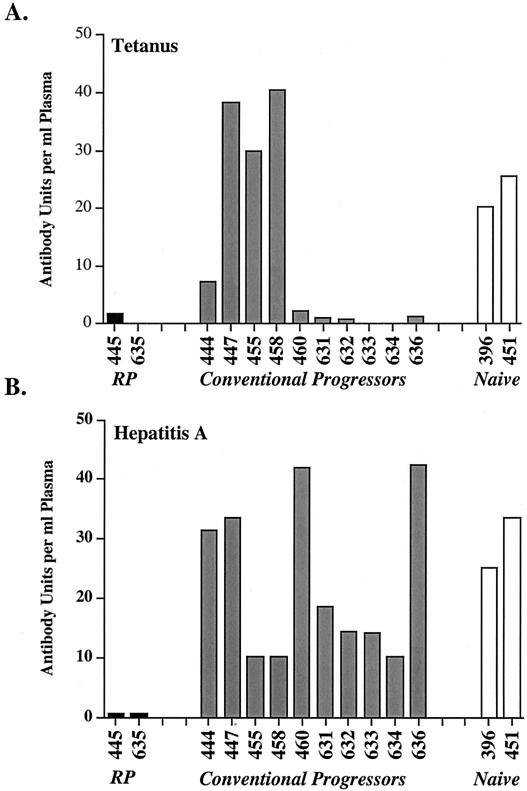
Response of SIV-infected rhesus macaques to immunization with tetanus toxoid and hepatitis A virus. The antibody responses to tetanus toxoid (A) and hepatitis A virus antigen (B) are shown graphically with the rapid progressor macaques H445 and H635 shown on the left, the responses of conventional progressors from the same cohorts in the middle, and SIV-naïve macaques on the right.
Three SIV-infected animals (447, 455, and 458) and the two SIV-naïve animals (396 and 451) responded vigorously to tetanus toxoid. Weak or transient responses were observed in the remaining animals, including the two macaques with rapidly progressive disease, H635 and H445 (Fig. 8A). Each of the animals that responded poorly to tetanus toxoid had high levels of primary plasma viremia. Ten of the SIV-infected macaques developed a primary response to hepatitis A virus immunization after the first immunization that was boosted to high levels by the second immunization (Fig. 8B). In contrast, neither rapid progressor macaque, H445 or H635, mounted detectable primary or secondary antibody responses to hepatitis A virus. The poor response to both tetanus toxoid and hepatitis A virus suggests a global immune defect in rapid progressor macaques.
DISCUSSION
This prospective study of both cellular and humoral immune responses in SIV-infected rapid progressor macaques revealed a number of novel observations. First, macaques that progress rapidly to AIDS following SIV infection develop both virus-specific humoral and cell-mediated immune responses but subsequently lose these responses very rapidly. Second, these animals rapidly lost their ability to respond to other recall and primary antigens, indicative of profound immunodeficiency. Yet despite severe immunosuppression, these animals maintained nearly normal circulating and tissue CD4+ T lymphocytes, consistent with loss in lymphocyte function as the underlying cause of the immune dysfunction.
Previous reports have described rapid progressors as macaques that fail to mount SIV-specific antibody responses (15, 54). It has been assumed that rapid progressor macaques develop rapid disease because they fail to react immunologically to the virus. However, our present study clearly demonstrated transient immune responses by 2 weeks of infection. The kinetics of appearance and loss of CTL reactivity were similar whether assays were done with Gag p11C peptide-pulsed targets or targets infected with vaccinia virus-expressed SIV Gag and Env. Thus, loss of CTL reactivity appears to be a global loss to multiple epitopes. Analysis of the Gag p11C epitope in the virus infecting two of these animals revealed that the loss of reactivity to this epitope was not due to mutations of the virus away from CTL recognition, as has been observed in conventional progressor macaques infected with SIVsmE660 (1). The failure to maintain SIV-specific immune responses in rapid progressor macaques suggests a global loss or dysfunction in critical lymphocyte subsets required to maintain a humoral and cellular immune response.
In addition to the failure of SIV-specific immune responses, the rapid progressor macaques in this study lost the ability to generate both primary and secondary immune responses to other antigens, tetanus toxoid and hepatitis A virus antigen. An impaired response to the memory recall antigen tetanus toxoid was observed in a number of the animals that did not progress rapidly to AIDS and is presumably a bystander effect analogous to that observed during primary viremia in other viral infections (45, 53). However, the response to hepatitis A virus (a primary antigen) was selectively impaired in the rapid progressor macaques. The loss of primary immune responses suggests a more profound and permanent immune defect than in these other viral infections or than observed in macaques that progress more slowly to AIDS.
Finally, rapid progressor macaques developed severe immunosuppression without profound depletion of CD4+ T cells. This is distinct from the severe CD4 depletion that is observed in macaques that progress slowly to AIDS in the face of persistent immune responses (16) or in HIV-1 infection in humans. In both of these scenarios, there are essentially no clinical consequences of infection until the CD4 cell count is <250. In contrast, only subtle declines in CD4 T cells in the peripheral blood and tissues were observed despite evidence of severe early immune defects in these rapid progressor macaques. These data suggest that their immune dysfunction was not a direct result of the depletion of CD4+ T cells. This study evaluated only total CD4+ T-cell populations and thus specific naïve, memory, and effector subsets of CD4+ T cells could be selectively affected without observing substantial CD4+ T-cell depletion in the blood or lymph nodes. Indeed, studies of SIVmac-infected conventional progressor macaques have revealed a selective depletion of activated memory CD4+ T-cell subsets (49).
The degree of immunosuppression in animals with adequate numbers of circulating and tissue CD4+ T lymphocytes suggests that the CD4+ T cells of such animals may lack functional activity, specific depletion of selective subsets of CD4+ T cells, or profound immune defects in other critical immune cells such as dendritic cells. It is presently unclear whether loss of function of CD4+ T cells is the critical factor in the failure of CTL and humoral responses and the subsequent development of rapidly progressive disease. However, there is considerable historical precedence for this being the case from studies of other viral systems as well as HIV-1. AIDS patients exhibit early CD4 dysfunction prior to severe CD4 depletion, as measured by loss of antigen-specific proliferative capacity of CD4 cells in vitro (44). Levels of p24 proliferative responses positively correlate with Gag-specific CTL frequencies and negatively correlate with plasma viral load, suggesting a causal relationship between these parameters (21, 22, 32, 34, 40). Such patients also exhibit a reduced ability to respond to non-HIV antigens such as tetanus toxoid, and these responses are partially restored by antiviral therapy (38, 48). A similar loss in proliferative capacity of CD4+ T cells has been observed early in disease course in SIV-infected macaques (26), and proliferative responses inversely correlate with plasma viremia. A central role for CD4 cells in the development and maintenance of effective CTL has been observed in other viral systems, including lymphocytic choriomeningitis virus and Friend leukemia virus in mice (13, 45, 52, 53) and HIV infection (20, 21).
In conclusion, although the rapidity of immunodeficiency in rapid progressors distinguishes it from AIDS in humans, rare individuals with HIV infection with a similar syndrome have been reported (11, 30, 31), and this syndrome may occur more frequently in HIV-infected infants (10). These animals are unique in that they develop severe immunodeficiency in the absence of profound loss of CD4+ T cells, suggesting a loss in CD4 function.
Acknowledgments
We thank Malcolm Martin for continued support of this research, Robert Goeken, Sonya Whitted, and Chris Erb for technical support, Dan Barouch for critical reading of the manuscript, and Russell Byrum for performing the animal studies.
This work was supported in part by AI 20729 (N.L.L., S.S., and A.S.) and HD 37356 (S.W., J.G., and K.K.).
REFERENCES
- 1.Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyer S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV/Delta). Vet. Pathol. 25:456-467. [DOI] [PubMed] [Google Scholar]
- 3.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 4.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 5.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 6.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 7.Dehghani, H., B. A. Puffer, R. W. Doms, and V. M. Hirsch. 2003. Unique pattern of convergent envelope evolution in simian immunodeficiency virus-infected rapid progressor macaques: association with CD4-independent usage of CCR5. J. Virol. 77:6405-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Embertson, J., J. L. Zupancic, J. Ribas, and A. Haase. 1994. Massive covert infection of helper T lymphocytes by HIV during the incubation period of AIDS. Science 259:359-362. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. T., L. A. Knapp, P. Jing, J. L. Mitchen, M. Dykhuizen, D. C. Montefiori, C. D. Pauza, and D. I. Watkins. 1999. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol. Lett. 66:53-59. [DOI] [PubMed] [Google Scholar]
- 10.Ganeshan, S., R. E. Dickover, B. T. Korber, Y. J. Bryson, and S. M. Wolinsky. 1997. Human immunodeficiency virus type 1 genetic evolution in children with different rates of development of disease. J. Virol. 71:663-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland, F. C., C. F. Garland, E. D. Gorham, and S. K. Brodine. 1996. Western blot banding patterns of HIV rapid progressors in the U. S. Navy Seropositive Cohort: implications for vaccine development. Navy Retroviral Working Group. Ann. Epidemiol. 6:341-347. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, S., C. R. Brown, H. Dehghani, J. D. Lifson, and V. M. Hirsch. 2000. Intrinsic susceptibility of rhesus macaque peripheral CD4+ T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J. Virol. 74:9388-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenkrug, K. J., D. M. Brooks, and U. Dittmaer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay, C. M., D. J. Ruhl, N. O. Basgoz, C. C. Wilson, J. M. Billingsley, M. P. DePasquale, R. T. D'Aquila, S. M. Wolinsky, J. M. Crawford, D. C. Montefiori, and B. D. Walker. 1999. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J. Virol. 73:5509-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, V. M., P. M. Zack, A. P. Vogel, and P. R. Johnson. 1991. Simian immunodeficiency virus infection of macaques: endstage disease is characterized by widespread distribution of proviral DNA in tissues. J. Infect. Dis. 163:976-988. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32:183-203. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch, V. M., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned pathogenic neutralization resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch, V. M., G. Dapolito, A. Hahn, J. D. Lifson, D. Montefiori, C. R. Brown, and R. Goeken. 1998. Viral genetic evolution in macaques infected with molecularly cloned simian immunodeficiency virus correlates with the extent of persistent viremia. J. Virol. 8:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holterman, L., H. Niphuis, P. J. ten Haaft, J. Goudsmit, G. Baskin, and J. L. Heeney. 1999. Specific passage of simian immunodeficiency virus from end-stage disease results in accelerated progression to AIDS in rhesus macaques. J. Gen. Virol. 80:3089-3097. [DOI] [PubMed] [Google Scholar]
- 21.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T-lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 25.Letvin, N. L., and N. King. 1990. Immunopathologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Acquir. Immune Defic. Syndr. 3:1023-1040. [PubMed] [Google Scholar]
- 26.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lifson, J. D., M. Nowak, S. Goldstein, J. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. Lloyd, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early virus replication is a critical determinant of the natural history of AIDS virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 29.Michael, N. L., A. E. Brown, R. F. Voigt, S. S. Frankel, J. R. Mascola, K. S. Brothers, M. Louder, D. L. Birx, and S. A. Cassol. 1997. Rapid disease progression without seroconversion following primary human immunodeficiency virus type 1 infection-evidence for highly susceptible human hosts. J. Infect. Dis. 175:1352-1359. [DOI] [PubMed] [Google Scholar]
- 30.Migueles, S. A., and M. Connors. 2002. The role of CD4+ and CD8(+) T cells in controlling HIV infection. Curr. Infect. Dis. Rep. 4:461-467. [DOI] [PubMed] [Google Scholar]
- 31.Montagnier, L., C. Brenner, S. Chamaret, D. Guetard, A. Blanchard, J. de Saint Martin, J. D. Poveda, G. Pialoux, and M. L. Gougeon. 1997. Human immunodeficiency virus infection and AIDS in a person with negative serology. J. Infect. Dis. 175:955-959. [DOI] [PubMed] [Google Scholar]
- 32.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. 1996. Guide for the care and use of laboratory animals. National Research Council, Washington, D.C.
- 34.O'Brien, T. R., W. A. Blattner, D. Waters, E. Eyster, M. W. Hilgartner, A. R. Cohen, N. Luban, A. Hatzakis, L. M. Aledort, P. S. Rosenberg, W. J. Miley, B. L. Kroner, and J. J. Goedert. 1996. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA 276:105-110. [PubMed] [Google Scholar]
- 35.Ogg, G. S., S. Kostense, M. R. Klein, S. Jurriaans, D. Hamann, A. J. McMichael, and F. Miedema. 1999. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J. Virol. 73:9153-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 75:4448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantaleo, G., S. Menzo, M. Vaccarezza, C. Graziosi, O. J. Cohen, J. F. Demarest, D. Montefiori, J. M. Orenstein, C. Fox, L. K. Schrager, et al. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 332:209-216. [DOI] [PubMed] [Google Scholar]
- 38.Peruzzi, M., C. Azzari, L. Galli, A. Verrucci, and M. de Martino. 2002. Highly active antiretroviral therapy restores in vitro mitogen and antigen-specific T-lymphocyte responses in HIV-1 perinatally infected children despite virologic failure. Clin. Exp. Immunol. 128:365-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piatak, M., M. S. Saag, L. C. Yang, S. J. Clark, K. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 40.Pontesilli, O., P. Carotenuto, S. R. Kerkhof-Garde, M. T. Roos, I. P. Keet, R. A. Coutinho, J. Goudsmit, and F. Miedema. 1999. Lymphoproliferative response to HIV type 1 p24 in long-term survivors of HIV type 1 infection is predictive of persistent AIDS-free infection. AIDS Res. Hum. Retrovir. 15:973-981. [DOI] [PubMed] [Google Scholar]
- 41.Ryzhova, E., J. C. Whitbeck, G. Canziani, S. V. Westmoreland, G. H. Cohen, R. J. Eisenberg, A. Lackner, and F. Gonzalez-Scarano. 2002. Rapid progression to simian AIDS can be accompanied by selection of CD4-independent gp120 variants with impaired ability to bind CD4. J. Virol. 76:7903-7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 43.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shearer, G. M., and M. Clerici. 1991. Early T-helper cell defects in HIV infection. AIDS 5:245-253. [DOI] [PubMed] [Google Scholar]
- 45.Slifka, M. K., D. Homann, A. Tishon, R. Pagarigan, and M. B. Oldstone. 2003. Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection. J. Clin. Investig. 111:805-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staprans, S. I., P. J. Dailey, A. Rosenthal, C. Horton, R. M. Grant, N. Lerche, and M. B. Feinberg. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J. Virol. 73:4829-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load by real time quantification of product generation in reverse transcriptase PCR. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 48.Valdez H., K. Y. Smith, A. Landay, E. Connick, D. R. Kurtzkes, H. Kessler, L. Fox, J. Spritzer, J. Roe, M. B. Lederman, H. M. Lederman, T. Evans, M. Heath-Chiozzi, M. M. Lederman, and the ACTG375 Team. 2000. Response to immunization with recall and neoantigens after prolonged administration of a HIV-1 protease inhibitor-containing regimen. AIDS 14:11-21. [DOI] [PubMed] [Google Scholar]
- 49.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson, A., J. Ranchalis, B. Travis, J. McClure, W. Sutton, P. R. Johnson, S. L. Hu, and N. L. Haigwood. 1997. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J. Virol. 71:284-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolinsky, S. M., B. T. Korber, A. U. Neumann, M. Daniels, K. J. Kunstman, A. J. Whetsell, M. R. Furtado, Y. Cao, D. D. Ho, J. T. Safrit, and R. A. Koup. 1996. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science 272:537-542. [DOI] [PubMed] [Google Scholar]
- 52.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarozinski, C. C., J. M. McNally, B. J. Lohman, K. A. Daniels, and R. M. Welsh. 2000. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J. Virol. 74:3650-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, J., L. N. Martin, E. A. Watson, R. C. Montelaro, M. West, L. Epstein, and M. Murphey-Corb. 1988. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J. Infect. Dis. 158:1277-1286. [DOI] [PubMed] [Google Scholar]