Abstract
The repair of articular cartilage injuries is impeded by the avascular and non-innervated nature of cartilage. Transplantation of autologous chondrocytes has a limited ability to augment the repair process due to the highly differentiated state of chondrocytes and the risks of donor-site morbidity. Mesenchymal stem cells can undergo chondrogenesis in the presence of growth factors for cartilage defect repair. Growth and differentiation factor-5 (GDF5) plays an important role in chondrogenesis. In this study, we examined the effects of GDF5 on chondrogenesis of adipose-derived stem cells (ADSCs) and evaluate the chondrogenic potentials of GDF5 genetically engineered ADSCs using an in vitro pellet culture model. Rat ADSCs were grown as pellet cultures and treated with chondrogenic media (CM). Induction of GDF5 by an adenovirus (Ad-GDF5) was compared with exogenous supplementation of GDF5 (100 ng/ml) and transforming growth factor-β (TGF-β1; 10 ng/ml). The ADSCs underwent chondrogenic differentiation in response to GDF5 exposure as demonstrated by production of proteoglycan, and up-regulation of collagen II and aggrecan at the protein and mRNA level. The chondrogenic potential of a one-time infection with Ad-GDF5 was weaker than exogenous GDF5, but equal to that of TGF-β1. Stimulation with growth factors or CM alone induced transient expression of the mRNA for collagen X, indicating a need for optimization of the CM. Our findings indicate that GDF5 is a potent inducer of chondrogenesis in ADSCs, and that ADSCs genetically engineered to express prochondrogenic growth factors, such as GDF5, may be a promising therapeutic cell source for cartilage tissue engineering.
Keywords: Growth factors, chondrogenesis, GDF5, gene therapy, stem cells, cartilage
Introduction
Articular cartilage injuries can occur as a result of either traumatic mechanical destruction or progressive degeneration. Cartilage has a limited capacity for self-repair, attributable to its avascular, noninnervated nature, low cell density, and lack of normal tissue repair-associated humoral factors (Hardingham et al. 2002; Heng et al. 2004). Consequently, cell transplantation, such as autologous chondrocyte implantation (ACI), and tissue engineering strategies have been widely studied for cartilage repair (Grande et al. 1989; Brittberg et al. 1994; Burkart and Imhoff 2002; Baums et al. 2006; Podskubka et al. 2006). However, there are three major disadvantages of ACI: (1) chondrocytes are highly differentiated cells, so their proliferative capacities are limited and may continue to decrease with the age of the patient (Dozin et al. 2002), (2) chondrocytes undergo a process of de-differentiation during expansion in monolayer culture (Grundmann et al. 1980; Mallein-Gerin et al. 1990; Nixon et al. 1992), and (3) ACI has been associated with significant donor-site morbidity and changes in some mechanical properties of neighboring articular cartilage has been observed following tissue harvest in an animal model (Lee et al. 2000). As a result, alternative cell sources providing adequate cell numbers capable of producing robust levels of cartilagenous extracellular matrix for these cell-based therapeutic strategies could provide significant clinical impact.
Mesenchymal stem cells (MSCs) provide an attractive alternative to chondrocytes, as they have the capacity for differentiation into several tissues, including bone, cartilage, fat, tendon, and muscle. While bone marrow is a major source of these stem cells, MSCs have been isolated from a variety of tissues, including adipose, periosteum, muscle, dermis, pericyte, blood, bone marrow, and trabecular bone (Ringe et al. 2003; Heng et al. 2004; Dudics et al. 2005). Adipose tissue, like bone marrow, derives from the embryonic mesoderm and contains a heterogeneous mix of stromal cells (Zuk et al. 2001; Kofron and Laurencin 2005). Retrieval of adipose tissue involves a minimally invasive procedure that can be easily performed in outpatient clinics. Yields of between 10,000 and 25,000 adherent adipose-derived stem cells (ADSCs) per gram of adipose tissue have been achieved 24 h post-harvest. Adipose tissue therefore appears to be a good source for stem cells, both in terms of ease of procedure and number of cells obtained. Under appropriate culture conditions (Zuk et al. 2002; Rodriguez et al. 2005), ADSCs can be induced to differentiate along adipogenic, chondrogenic, myogenic, or osteogenic lineages. Adenoviral, retroviral and lentiviral vectors can also infect ADSCs with high efficiency, making them potentially very useful for cell-based gene therapy (Strem et al. 2005).
Induction of chondrogenesis requires that MSCs maintain a rounded morphology through pellet (micromass) culture or when seeded in a 3D biomaterial scaffold or hydrogel (Wang et al. 2005). Additionally, multiple cytokines and growth factors have been shown to promote chondrogenesis of MSCs, such as members of the transforming growth factor-β (TGF-β) and bone morphogenetic protein families, insulin-like growth factor-1 (IGF-1), and fibroblast growth factor-2 (Heng et al. 2004). Though, these growth factors or exogenous cytokines augment chondrogenic differentiation in MSCs, significant in vitro culture duration with regular supplementation of fresh growth factor is typically required. This extended ex vivo culture delays the timeline for treating patients, and recent evidence suggests these extended ex vivo culture methodologies may alter the immunogenicity of cultured autologous cells, thereby, resulting in immunorejection upon transplantation (Strem et al. 2005). It is, therefore, desirable to seek strategies that will allow us to minimize the time, the cells spend ex vivo. Gene transfer strategies offer promising utility for directing and controlling the chondrogenic differentiation of stem cells due to their ability to provide sustained transgene expression. This could be achieved by transducing stem cells with recombinant DNA constructs encoding expression of certain proteins or growth factors that promote chondrogenesis. To this point, induction of IGF-1, TGF-β1, TGF-β2, and the transcription factor Sox9 has previously been shown to accelerate chondrogenic differentiation of stem cells (Tsuchiya et al. 2003; Kawamura et al. 2005; Palmer et al. 2005).
Growth and differentiation factor-5 (GDF5, also called cartilage-derived morphogenetic protein-1) is a member of the TGF-β superfamily of signaling molecules that is best known for its role in early chondrogenesis and joint formation (Buxton et al. 2001). Up-regulations of chondrocyte-specific expression of collagen II and accumulation of extracellular matrix glycosaminoglycan (GAG), indicate that GDF5 could promote the differentiation of MSCs into chondrocytes (Erlacher et al. 1998; Bobacz et al. 2002; Hatakeyama et al. 2004). In addition, GDF5 initiates chondrogenic differentiation of bone marrow-derived stem cells (BMSCs; Bai et al. 2004; Hatakeyama et al. 2004), yet the role of GDF5 in chondrogenic differentiation of ADSCs has yet to be investigated.
In this study, we constructed an adenovirus vector containing the cDNA encoding GDF5 (Ad-GDF5), and determined the chondrogenic differentiation of ADSCs infected with Ad-GDF5 under high-density pellet culture conditions. Furthermore, we compared the capability of Ad-GDF5 to affect the chondrogenesis of ADSCs with that of exogenously applied GDF5 and TGF-β1.
Material and methods
Construction of Ad-GDF5
Ad-GDF5 was generated using the AdEasy adenoviral vector system (Stratagene, La Jolla, CA, USA) according to the manufacturer’s instructions. Briefly, the 1.7 kb human GDF5 cDNA sequence was cloned into the pShuttle-cytomegalovirus (CMV) vector of the AdEasy system. The resultant pShuttle-CMV-GDF5 was used to generate the adenoviral GDF5 vector through homologous recombination with the adenoviral backbone vector, pAdEasy-1 in BJ5183 bacterial cells. The DNA was then linearized with Pac I and the adenoviral recombinants were used to produce adenoviruses in AD293A packaging cells, resulting in an Ad-GDF5 vector. Virus stocks were amplified in AD293A cells and purified by cesium chloride ultracentrifugation at 35,000 rpm overnight, and tittered by agarose-overlayed plaque assays. Ad-Luciferase (Ad-Luc) was a gift from Dr Greg Helm at the University of Virginia.
Western blot of GDF5
Supernatants from Ad-GDF5 and Ad-Luc infected and non-infected AD293A cells cultured for 2 days post-infection were mixed with laemmli sample buffer containing 5% β-mercaptoehtanol (Bio-Rad, Hercules, CA, USA) and heated at 100°C for 5 min. Protein samples were then separated by electrophoresis on 10% Criterion SDS-PAGE gel (Bio-Rad) under reducing conditions on a minigel apparatus (Bio-Rad) at 150 V for 60 min. Western blot transfer of the proteins from the gel to a nitrocellulose membrane was carried out under semidry conditions at 15 V for 20 min (Bio-Rad). The membrane was blocked by incubation with Tris-buffered saline (Tween-20) containing 5% milk powder for 2 h at room temperature, and incubated overnight at 4°C with a primary mouse anti-GDF5 monoclonal antibody diluted 1:3000 in blocking buffer [5% milk powder, 3% bovine serum albumin (Boehringer Mannheim Corp., IN, USA) in phosphate-buffered saline (PBS)]. After three 15 min washes in 0.1% Triton (Sigma, St Louis, MO, USA)-PBS solution, membranes were blotted with a 1:1000 dilution of secondary goat anti-mouse IgG (Sigma) for 60 min at room temperature. The membranes were then washed again and the proteins were detected using enhanced chemiluminescence with Western blotting detection reagent (Amersham, Arlington Heights, IL, USA).
Isolation of ADSCs
ADSCs were prepared from the inguinal fat pads of five 8-week-old Fischer 344 rats shortly after euthanasia in accordance to an Institutional Animal Care and Use Committee (IACUC) approved protocol. The fat pads were excised, finely minced with scissors and washed three times with PBS containing 100 units/ml penicillin G and 100 μg/ml of streptomycin. The tissue was collected by centrifugation at 500g for 5 min after each wash. Collagenase (0.01%; Crescent Chemical Co., Inc., NY, USA) was added to the samples and the mixture was agitated at 37°C for 30 min. The aqueous portion was carefully removed and centrifuged at 500g for 10 min. The cell pellet was resuspended in Dulbecco’s modified eagle medium (DMEM) with erythrocyte lysis buffer (160 mM NH4Cl), agitated at room temperature for 10 min, and re-centrifuged to obtain a pellet. The cells were then resuspended in DMEM/F-12 (Gibco BRL, Carlsbad, NY, USA) containing 10% fetal bovine serum (Gibco Invitrogen Corporation, Carlsbad, CA, USA), and 1% penicillin/streptomycin, then plated in a 100 mm tissue culture dish and maintained at 37°C in a humidified incubator with 5% CO2. Culture media was changed every other day. Cells were maintained at subconfluent levels and passaged sequentially approximately every 3 days using trypsin/EDTA (Gibco BRL). After cell counting using trypan blue, the cells were plated at a concentration of 105 cells per 100 mm2.
Infection of ADSCs with Ad-GDF5 and determination of the optimal MOI
Passage 3 rat ADSCs were cultured in 24-well plates at a density of 2 × 105 cells/well. Solutions of Ad-GDF5 virus particles at an MOI of 0, 25, 50, 100, 150, and 200 were premixed with 1 ml DMEM containing 1% FBS, 1% penicillin/streptomycin and 1% ascorbic-2-phosphate, and added to the cell culture well. The media was then changed every 3 days. The levels of GDF5 in the cell culture supernatant were measured after 3, 7, 14 and 21 days in culture using an enzyme-linked immunosorbent assay (ELISA; Wang et al. 2004).
GDF5 ELISA
The wells of ELISA plates (Corning Costar, NY, USA) were coated with 100 μl of a mouse monoclonal anti-GDF5 antibody (2μg/ml in eBioscience (San Diego, CA, USA) Coating Buffer) at 4°C for 12 to 18 h. The wells were then washed three times with washing buffer (1 × PBS with 0.05% tween-20) and blocked with 200μl/well of Assay Diluent (eBioscience), for 1 h at room temperature. Following three rinses with washing buffer, wells were treated with 100 μl of cell culture supernatant or GDF5 standards (0, 10, 100, 200, 400, or 1000 ng/ml) and incubated at room temperature for 2 h. After three rinses with the washing buffer, a biotin-conjugated anti-GDF5 monoclonal antibody was added to each well at a concentration of 1μg/ml in 100 μl and incubated at room temperature for 1 h. Following rinsing, avidin horse radish peroxidase (AV-HRP; eBioscience) at a 1:500 dilution (100 μl/well) was added and the plates were incubated at room temperature for 30 minutes. Wells were again rinsed three times with washing buffer and 100 μl of ABTS Substrate Solution (eBioscience) was added to each well and incubated at room temperature for 30 minutes, and optical densities were measured at 405 nm in a spectrophotometer (Molecular Devices, Sunnydale, CA, USA).
Chondrogenic differentiation of ADSCs
Chondrogenesisof ADSCs was induced in passage 4 cell cultures as described previously. Briefly, 2.0 × 105 cells were gently centrifuged for 5 minutes at 500g in a 15-ml polypropylene tube. Without disturbing the resulting pellet, the cells were cultured under five different culture conditions: (i) basal media control (BM) consisting of high glucose DMEM (Gibco), 1% FBS, 37.5 μg/ml ascorbic-2-phosphate, and 1% penicillin/streptomycin, (ii) chondrogenic media (CM) in which 10 nM dexamethasone and 1% ITS-Premix (Collaborative Biomedical, Becton Dickinson, Bedford, MA, USA) was added to the BM control conditions, and three growth factor containing medias in which (iii) 10 ng/ml TGF-β1 or (iv) 100 ng/ml GDF5 were supplemented to CM, or (v) cells previously transduced with Ad-GDF5 (MOI of 150) were pelleted and cultured in CM. The selection of GDF-5 concentration at 100 ng/ml is based on our former study (data not shown) on dose effects of GDF-5 on ADSCs, which showed that the most optimal concentration is 100 ng/ml. All pellet cultures were maintained at 37°C in a humidified incubator with 5% CO2, and culture media was changed twice weekly.
Analysis of mRNA expression levels
Total RNA from ADSCs pellet was obtained using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). cDNA was then synthesized from the total RNA using the Reverse Transcription System kit (Promega, Madison, WI, USA). Real-time polymerase chain reaction (PCR) analysis was then performed with the Quantitect SYBR Green PCR master mix (Qiagen). Standard curves were generated, and quantities of each transcript were normalized to 18S as an internal control. Amplification primers are listed in Table I.
Table I.
Primers used for polymerase chain reaction amplification.
| Molecule | Primers | Product size (bp) |
|---|---|---|
| Collagen I | 5′-GCTGAA TCC TTC CGT GTT-3′ (sense) | 105 |
| 5′-AGG GAG GGG ACT TAT CTG-3′ (antisense) | ||
| Collagen II | 5′-AGA GCG GAG ACT ACT GGA TTG-3′ (sense) | 243 |
| 5′-TCT GGA CGT TAG CGG TGT T-3′ (antisense) | ||
| Collagen X | 5′-TCT GGG ATG CCT CTT GTC-3′ (sense) | 153 |
| 5′-TCT TGG GTC ATA GTG CTG-3′ (antisense) | ||
| Aggrecan | 5′-ACC CGA CAA TTT CTT TGC-3′ (sense) | 345 |
| 5′-GGT CTC ATC GTC CGC TTC-3′ (antisense) | ||
| 18S | 5′-CGG CGA CGA CCC ATT CGA AC-3′ (sense) | 99 |
| 5′-GAA TCG AAC CCT GAT TCC CCG TC-3′ (antisense) |
Biochemical analyses
Cell pellets were digested in 200 μl of papain digestion buffer (125 μg/ml in sterile PBS, pH6.0 with 5 mM cysteine hydrochloride) for 18 h at 60°C. Sulfated GAG (sGAG) levels were measured spectrophotometrically after incubation with 1,9-dimethylmethylene blue-chloride (DMMB) dye and normalized to total DNA, as measured fluorometrically using the bisbenzimide Hoechst 33258 DNA quantitation kit (Sigma). Standard curves for each assay were generated with chondroitin 6-sulfate sodium salt from shark cartilage and calf thymus DNA, respectively.
Histological analysis of the pellet cultures
For histological analysis, the pellet cultures from each culture condition were harvested, washed with PBS to remove the medium, and fixed in 10% buffered formalin for 4 h. The samples were then dehydrated by treatment with a series of graded alcohols and embedded in paraffin. Samples were then cut into 5μm sections, rehydrated and stained with Safranin-O for detection of proteoglycan.
Immunochemical staining of collagen II, and immunoflourescent staining of collagen X and aggrecan
Immunochemical staining for collagen II was performed using a collagen staining kit (Chondrex, Redmond, WA, USA), with minor modifications to the manufacturer’s instructions. The paraffin embedded sections were deparaffinized and re-hydrated. Peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol for 10 min, followed by boiling in citrate buffer (10 mM, pH 6.0) for 20 min for antigen retrieval. Sections were treated with 2% bovine testicular hyaluronidase for 30 min at 25°C, and then incubated with blocking buffer for 30 min, followed by Arthrogen-CIA collagen II monoclonal antibodies at a dilution of 1:250 for 1 h at room temperature. Streptavidin peroxidase (50 μl in 10 ml streptavidin peroxidase-dilution buffer) was applied for 1 h at room temperature. Sections were then developed with diaminobenzidine (DAB) for 30 min.
For visualization of collagen X and aggrecan, sections were treated as above, blocked with goat IgG (1:250) for 2 h at room temperature, then incubated with either rabbit anti-rat collagen X polyclonal antibody (5 μg/ml, AXXORA, San Diego, CA, USA) or rabbit anti-rat aggrecan polyclonal antibody (1μg/ml, Gene Tex, Inc., San Antonio, TX, USA) for 12–18 h at 4°C, followed by incubation with Texas red (for collagen X) or Fluoresceinisothiocynate (FITC) (for aggrecan) conjugated secondary antibody for 1 h at room temperature in a moist dark box. The slides were washed and mounted with VectaShield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA).
Statistical analysis
Statistical evaluation was performed using the analysis of variance test then followed by a post hoc Student’s t-test. A p-value of less than 0.05 was considered significant. Data are expressed as mean ± SD.
Results
Infection with Ad-GDF5 induces expression of GDF5
To verify the ability of Ad-GDF5 to induce expression of GDF5, AD293A cells were infected with Ad-GDF5 adenovirus, cell culture supernatant was harvested two days after infection, and Western blot was performed with mouse anti-GDF5 monoclonal antibody. Two bands migrating at approximately 55 and 15 kDa were observed (Figure 1). The GDF5 gene encodes a 501 amino acid precursor protein containing a potential N-glycosylation site in the N-terminal propeptide region and a polybasic processing site (RRKKRR) in the C terminus. Upon processing, the precursor protein is cleaved by subtilisin-like proteases at amino acid 382 to form the mature GDF5 monomeric protein, which is 120 amino acids long (382–501) and has a calculated molecular mass of 13.6 kDa. Under physiological conditions, GDF5 exists as a homodimer, with the two monomers being linked by a disulfide bridge (Hotten et al. 1994; Thomas et al. 1996; Schreuder et al. 2005). Under our reducing conditions, the mature GDF5 showed an apparent molecular mass of approximately 15 kDa, while the band at 55 kDa likely represents the full-length precursor protein. These results suggest that infection with Ad-GDF5 provides expression of mature GDF5 protein.
Figure 1.

Western blotting of GDF5 recovered from the supernatant of cultured AD293A cells, 2 days after transduction with Ad-GDF5. Proteins were separated on 10% Criterion SDS-PAGE gel under reducing conditions, and Western blotting was performed with the mouse anti-GDF5 monoclonal antibody. The primary band migrating at approximately 15 kDa is the mature GDF5 monomer, while the 55 kDa band is full length GDF5 precursor protein.
The optimal multiplicity of infection of Ad-GDF5 for transduction of ADSCs
To determine the relative level of secreted GDF5, ADSCs were infected with increasing MOI of the Ad-GDF5 adenovirus. The media from the infected cells was then collected at 3, 7, 14, and 21days after infection. As shown in Figure 2, production of GDF5 peaked 7 days after the initial infection. After this 7 day time point, the concentration of GDF5 produced by all the groups decreased over time, although, cells that had been infected with Ad-GDF5 at MOI of 150 and 200 demonstrated GDF5 levels of 17 and 23 ng/ml, respectively, after 21 days. At 7 days post-infection GDF5 expression increased with increasing viral MOI (6.5 ng/ml at MOI of 25, 19 ng/ml at MOI of 50, 68 ng/ml at MOI of 100, and 170 ng/ml at an MOIs of 150 and 200), while detectable levels of GDF5 were not observed in control cells (Figure 2). Since the difference in production of GDF5 was not significantly greater at an MOI of 200 than of 150, an MOI of 150 was used for all the subsequent experiments. Importantly, levels of secreted GDF5 that were measured at an MOI of 150 are within the range of concentrations of GDF5 that are typically used to supplement MSC cultures in models of in vitro chondrogenesis (Hatakeyama et al. 2004).
Figure 2.
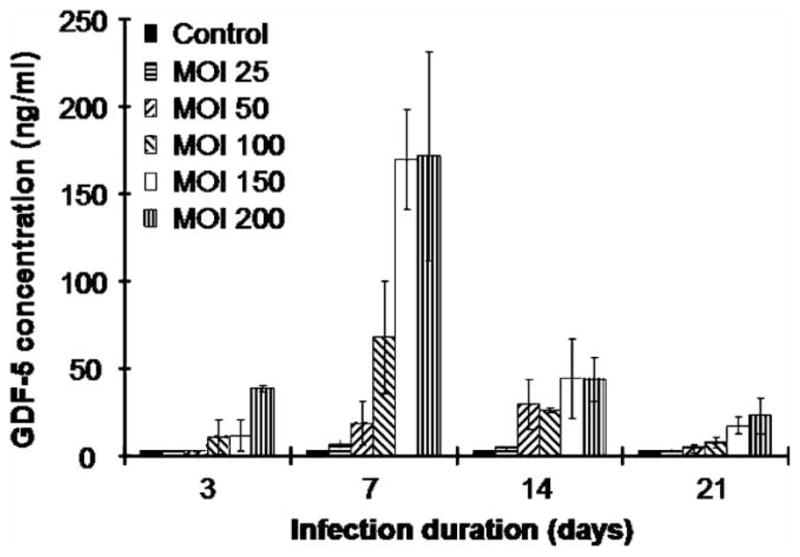
Adenoviral-mediated expression of GDF5 in rat ADSCs as determined by ELISA. Levels of GDF5 were measured from media conditioned by ADSCs that had been infected with increasing amounts of Ad-GDF5 (MOI of 25, 50, 100, 150, and 200) and incubated in DMEM plus 1% FBS for up to 21 days. Media was collected at 3, 7, 14 or 21 days post-infection, and the levels of GDF5 were detected by ELISA. Data are represented as the mean of triplicate experiments. Error bars represent mean ± SD.
Ad-GDF5 promotes the synthesis of sulfated GAG
ADSCs were cultured in a pellet system to determine if gene transfer could be used as an effective means of protein delivery with which to induce chondrogenesis. We compared the ability of GDF5 to induce chondrogenesis when delivered as a recombinant protein or when supplied as the product of a transgene. For the latter, we infected passage 3 monolayer cultures of ADSCs at an MOI of 150 Ad-GDF5 and then transferred the cells into pellet culture 24 h post-infection. Parallel cultures of passage 3 ADSCs (untransduced) were pelleted and treated with either 100 ng/ml GDF5 or 10 ng/ml TGF-β1, as a positive control for chondrogenesis (Goessler et al. 2005; Kawamura et al. 2005; Mehlhorn et al. 2006). Additional control pellets were maintained in either BM or chondrogenic culture media in the absence of prochondrogenic growth factors.
All the ADSC pellets cultures maintained a spherical shape under the different culture conditions (Figure 3(a)). Compared with the BM control, pellets that had been grown in CM, with or without supplementation of TGF-β1, GDF5 or Ad-GDF5, showed an enlarged micromass size. Treatment with either GDF5 or Ad-GDF5 increased the pellet size to a greater extent than CM alone, whereas TGF-β1 did not show a greater effect than the CM alone (Figure 3(b)).
Figure 3.
Induction of chondrogenesis of ADSCs in high-density pellet culture. ADSCs were infected at a MOI of 150 Ad-GDF5, while a comparator set of ADSCs was not infected. Twenty-four hours post-transfection, 2.0 × 105 ADSCs were gently centrifuged for 5 min at 500g in a 15 ml polypropylene tube, then cultured in various culture media for 3-weeks. (BM, basal media; CM, chondrogenic media; TGF-β1 = CM + 10 ng/ml; TGF-β1; GDF5 = CM + 100 ng/ml GDF5; Ad-GDF5 = Ad-GDF5 transduced cells in CM) (a) Representative images of ADSCs pellet culture in various treatment groups. (b) Quantification of pellet diameters. Values shown are mean ± SD for triplicate samples. *p < 0.05 vs. BM group; **p < 0.01 vs. BM group; #p < 0.05 vs. CM group.
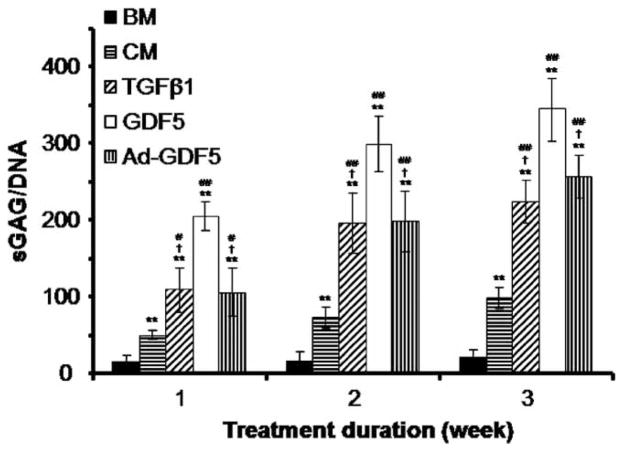
To verify the production of a cartilaginous matrix by ADSCs, the biochemical content of ADSCs pellets was evaluated using the DMMB dye-binding assay for detection of sGAG. Synthesis of sGAG by the pellets cultured in BM was low after 1-week, and did not significantly increase over time. CM alone or in the presence of one of the growth factors (i.e. TGF-β1, GDF5 or Ad-GDF5) induced synthesis of sGAG by 1-week in culture, and the levels of sGAG gradually increased over time in culture (Figure 4). Treatment with GDF5 (100 ng/ml) induced synthesis of sGAG to a significantly greater extent than either TGF-β1 (10 ng/ml) or Ad-GDF5 (MOI of 150) ( p < 0.05).
Figure 4.
Biochemical analysis of expression of sGAG in high density pellet-cultured ADSCs after induction of chondrogenesis. The levels of sGAG were measured spectrophotometrically after reaction with DMMB dye following 1, 2 or 3-weeks in the different culture conditions. The levels of sGAG were normalized to the amount of DNA, which was measured fluorometrically using the bisbenzimide Hoechst 33258 method. Values are given as mean ± SD (n = 3). *p < 0.05 vs. BM group; **p < 0.01 vs. BM group; #p < 0.05 vs. CM group; ##p < 0.01 vs. CM group; †p < 0.05 vs. 100 ng/ml GDF treatment group.
Ad-GDF5 augments chondrogenic extracellular matrix production
The pellet cultures were then assessed histologically via Safranin-O staining, to visualize deposition of a proteoglycan-rich extracellular matrix. TGF-β1, GDF5 and Ad-GDF5 all increased deposition of proteoglycans compared with BM or CM alone (Figure 5).
Figure 5.
Safranin-O and fast green staining of representative ADSCs pellets for detection of proteoglycan-rich extracellular matrix. ADSC pellets cultured in BM alone or CM in the absence or presence of Ad-GDF5, GDF5 (100 ng/ml) or TGF-β1 (10 ng/ml) (Top panel: 100 × magnification, bar = 100 μm; bottom panel: 400 × magnification, bar = 25 μm).
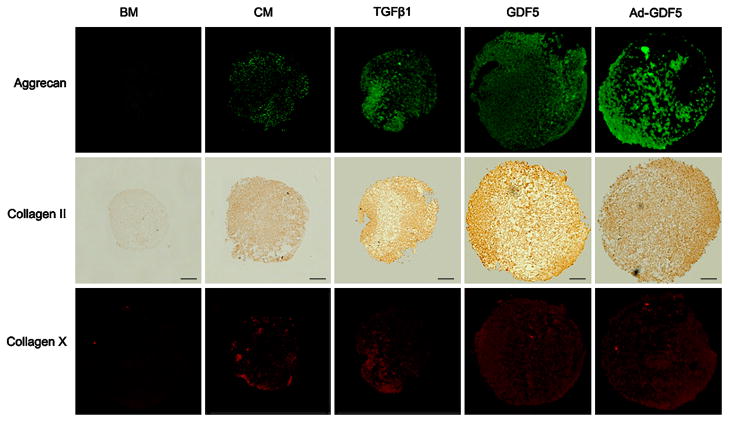
Additional pellets were harvested and immunostained for collagen II (immunochemical staining), aggrecan and collagen X (both by immunofluorescent staining). Pellets cultured in CM in the presence of TGF-β1, GDF5 and Ad-GDF5 all demonstrated robust staining for collagen II and aggrecan. Typically, staining intensity was higher toward the periphery and lower in the central regions of pellets cultured in the presence of TGF-β1 and GDF5. ADSCs that had been pellet cultured in CM showed weaker staining for collagen II and aggrecan compared the GDF5, TGF-β1 and Ad-GDF5 treatment groups. This, along with the biochemical data and Safranin-O staining, clearly demonstrates that chondrogenic transformation of the ADSCs was occurring at an enhanced rate in the presence of growth factor induction or supplementation (Figure 6).
Figure 6.
Immunostaining of ADSC pellet cultures for the presence of collagen II, aggrecan and collagen X in ADSCs following adenoviral-mediated gene transfer of GDF5 or treatment with GDF5 (100 ng/ml) or TGF-β1 (10 ng/ml) for 3-weeks. Regions of positive staining show green fluorescence (aggrecan), dark brown (collagen II), or red fluorescence (collagen X). Bar = 100 μm (Original magnification 100 ×).
ADSCs pellets cultured in CM in the presence or absence of GDF5, TGF-β1 or Ad-GDF5 showed weak staining for collagen X, with low variation in the signal intensities between these groups. On the other hand, pellets maintained in BM were negative for collagen X.
Ad-GDF5 augments expression of chondrogenic genes
To evaluate the gene expression patterns of ADSCs in which chondrogenesis has been induced by TGF-β1, GDF5 or Ad-GDF5, the levels of the mRNA transcripts for collagen I, II, X and aggrecan were determined. BM and CM alone were again used as controls.
Compared with BM, CM alone strongly induced expression of collagen II, aggrecan and collagen X. However, the three different growth factor treatments demonstrated additional prochondrogenic effects beyond that of the CM alone. After 1-week in culture, TGF-β1, GDF5 and Ad-GDF5 treated groups all exhibited elevated collagen II expression levels compared to pellets maintained in BM; but only GDF5 provided a significant enhancement compared to chondrogenic culture media alone ( p < 0.05). However, aggrecan gene expression was significantly up-regulated by all growth factor groups compared to CM alone at this time point (p < 0.05). collagen I mRNA levels were highly increased by TGF-β1 and slightly by Ad-GDF5 at this time point, whereas no significant effect on the expression of collagen X was observed with any of the three growth factor treatments compared with the CM control ( p > 0.05). At 2-weeks, both collagen II and aggrecan mRNA levels were significantly higher for all growth factor-treated groups, with expression peaking at this time point. No differences in collagen I or collagen X expression were observed for any groups at this time point. At 3-weeks, collagen II and aggrecan expression in growth factor-treated groups decreased compared to levels observed at 2-weeks, but all groups were still significantly higher than the CM control group. Finally, no differences were observed for collagen I and collagen X at the 3-week time point (Figure 7).
Figure 7.
Gene expression patterns of high density pellet-cultured ADSCs after induction of chondrogenesis over 1, 2 or 3-week period. Pellets were cultured in BM alone or CM in the absence or presence of Ad-GDF5, GDF5 (100 ng/ml) or TGF-β1 (10 ng/ml). (a) mRNA for collagen II, (b) mRNA for aggrecan, (c) mRNA for collagen I, and (d) mRNA for collagen X. Data are presented as mean ± SD (n = 4). *p < 0.05 vs. BM group; **p < 0.01 vs. basal media group; #p < 0.05 vs. CM group; ##p < 0.01 vs. CM group; †p < 0.05 vs. 100 ng/ml GDF treatment group; ††p < 0.01 vs. 100 ng/ml GDF treatment group.
These data demonstrated that the supplementation of CM with growth factors (recombinant proteins or adenovirus-induced transgenic proteins) potently induces chondrogenic gene expression.
Discussion
In the present study, we demonstrated that adenoviral-mediated expression of GDF5 can serve as an effective means of protein delivery to induce chondrogenesis of rat ADSCs in high density pellet culture. This was verified by the demonstration that, after infection with Ad-GDF5, GDF5 was expressed at levels equivalent to those previously used for application of exogenous protein to pellet cultures. Further, the expression of chondrocyte markers was markedly induced, both at the mRNA and protein level, in the infected ADSCs. This is the first report describing the effects of GDF5 gene therapy on the chondrogenesis of ADSCs.
It has previously been reported that GDF5 augments the chondrogenic differentiation of BMSCs (Bai et al. 2004). Consistent with this finding, we demonstrate robust induction of chondrogenesis in ADSCs following GDF5 exposure, whether via adenoviral overexpression or supplementation of recombinant protein. These results support existing evidence that GDF5 may have significant promise for various clinical applications in cartilage repair and regeneration. The temporal induction of chondrogenic markers in ADSCs at the mRNA level supported the profound role(s) of GDF5 to induce chondrogenic differentiation. Moreover, the observed enhancement of transcriptional message translated into pronounced increases in extracellular matrix production in 3D micromass cultures following treatment with exogenous GDF5 or infection with Ad-GDF5. Parallel cultures were treated with TGF-β1 as a positive control, based on the well documented prochondrogenic effects of TGF-β1 on MSCs throughout the different steps of chondrogenesis, including condensation (Bai et al. 2004; Heng et al. 2004; Kawamura et al. 2005; Toh et al. 2005; Mehlhorn et al. 2006). We discovered that treatment with either GDF5 or Ad-GDF5 increased the ADAS cell pellet size to a greater extent than CM alone, whereas TGF-β1 did not show a greater effect than the CM alone (Figure 3(b)). We also found that application of exogenous GDF5 (100 ng/ml) had a much greater effect on chondrogenesis than TGF-β1 (10 ng/ml), as demonstrated by the significantly higher GAG content in pellet cultures, and the expression profile of chondrogenic marker genes. Thus, it appears that GDF5 is acting to increase both differentiation and proliferation, whereas TGF-β1 is acting to promote condensation and differentiation consistent with its known role at early stages during cartilage development. Insights into the molecular mechanism(s) underlying these phenomena remain to be empirically determined.
In addition to infecting ADSCs and inducing robust expression of GDF5, Ad-GDF5 promotes the expression of collagen II and aggrecan, and accelerates chondrogenic differentiation of ADSCs. Although, Ad-GDF5 did not show the same chondrogenic potential as application of exogenous GDF5 (100 ng/ml), only a single infection of the Ad-GDF5 was applied, whereas the GDF5 was reapplied every other day. The relative effects of Ad-GDF5 may be more potent than the exogenously supplemented GDF5. In addition, the one time infection of Ad-GDF5 show equivalent chondrogenic effects on ADSCs compared with the application of exogenous TGF-β1 (10 ng/ml) every other day.
Gene therapy has several advantages over traditional techniques involving application of recombinant growth factors and ex vivo cell therapies. The induction of stem cell differentiation by application of exogenous growth factors may not be a realistic approach for clinical applications due to the prolonged periods that the cells must be maintained under in vitro conditions (Heng et al. 2004; Strem et al. 2005). Alternatively, stem cell implantation coupled with delivery of recombinant growth factors to induce differentiation in vivo may be quite challenging, as the growth factor pharmacodynamics may be insufficient to provide substantial long-lasting effects given their short half-life in vivo. This is confirmed by the fact that proteomically treated MSCs has not yielded satisfactory regeneration of cartilage after implantation subcutaneously or into the site of the cartilage defect (Trippel et al. 2004), likely due to insufficient local stimulation of the implanted stem cells by growth factors. The genetic manipulation of stem cells to modulate the expression of the required growth factors offers an attractive solution to this problem (Kaul et al. 2006). After a short period of differentiation in vitro, engineered stem cells could continue to differentiate further in vivo under the direction of the transgenic growth factors that they produce, so it is of particular interest to continue evaluation and translation of transgene-induced chondrogenesis into therapeutic practice.
In the present study, we found transient expression of the mRNA for collagen X in the early stage (1-week) of all pellets grown in CM. This observation is consistent with the results from Mwale et al. (2006) and contrary to what one would expect for the prototypical gene expression profile for chondrogenic differentiation, as collagen X is expressed only during chondrocyte hypertrophy. Unlike collagen II and aggrecan, the addition of growth factor (GDF5, TGF-β1 and Ad-GDF5) did not further enhance the expression of the mRNA for collagen X, indicating that the chondrogenic medium itself is responsible for this up-regulation of collagen X. Such up-regulation of collagen X has previously been reported for MSCs pellet cultured in the presence of CM, where it commenced prior to the up-regulation of the mRNA for collagen II (Barry et al. 2001; Sekiya et al. 2002; Pelttari et al. 2006). Collectively, these studies suggest that current CM formulations to facilitate in vitro induction of chondrogenesis of MSCs is suboptimal and further efforts are required to optimize formulations to promote chondrogenesis of MSCs without premature induction of prototypical markers of chondrocyte hypertrophy. Unfortunately, it is still unclear which reagent(s) is responsible for the transient up-regulation of collagen X. Another possible cause of transient collagen X gene expression may be the fact that the ADSCs extract is a heterogeneous mix of putative multipotential progenitor cells containing different lineage-progenitors (Zuk et al. 2001). Moreover, previous studies from our group had demonstrated that GDF5 protein could promote ADSCs differentiation into osteoblast lineage under osteogenic induction, indicating the effects of GDF5 on ADSCs is context dependent (Zeng et al. 2006, 2007). All these suggest that development of strategies for cloning or selecting for lineage-specific progenitor cells within the heterogeneous population of ADSCs is a crucial next step, if ADSCs are to be considered as a source for chondrocytes in tissue-engineered repair or regeneration of cartilage. Determination of their functional suitability and optimization of their phenotypic stability are imperative for future clinical applications in order to avoid the risk of graft instability.
Future work will focus on the mechanism(s) by which GDF5 (either GDF5 or Ad-GDF5) modulates mesenchymal cell proliferation and differentiation of clonal-derived progenitors. We will also characterize sub-populations of ADSCs according to their specific cell surface markers and functional pathways, as an important step towards clarifying the composition of this heterogeneous population. Further comparison of the responsiveness of these subpopulations will promote identification of the mechanism(s) of mesenchymal cell differentiation.
Conclusions
We demonstrated that GDF5 is a potent inducer of chondrogenesis in ADSCs, and ADSCs genetically engineered to express prochondrogenic growth factors, such as GDF5, may be a promising therapeutic cell source for cartilage tissue engineering.
Acknowledgments
We gratefully thank our grant support from the Musculoskeletal Transplant Foundation, the Arthritis Foundation and FEST of the University of Virginia. We would also like to thank Sarah A De La Rue of the NIH Silvio O Conte Digestive Health Research Center for editorial assistance with this manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bai X, Xiao Z, Pan Y, Hu J, Pohl J, Wen J, Li L. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325:453–460. doi: 10.1016/j.bbrc.2004.10.055. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88:303–308. doi: 10.2106/JBJS.E.00033. [DOI] [PubMed] [Google Scholar]
- Bobacz K, Gruber R, Soleiman A, Graninger WB, Luyten FP, Erlacher L. Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthritis Cartilage. 2002;10:394–401. doi: 10.1053/joca.2002.0522. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Burkart A, Imhoff AB. Treatment of articular cartilage defects with the autologous chondrocyte transplantation (ACT) Surg Technol Int. 2002;10:255–260. [PubMed] [Google Scholar]
- Buxton P, Edwards C, Archer CW, Francis-West P. Growth/differentiation factor-5 (GDF-5) and skeletal development. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S23–S30. [PubMed] [Google Scholar]
- Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: Molecular and cellular aspects. Matrix Biol. 2002;21:449–459. doi: 10.1016/s0945-053x(02)00028-8. [DOI] [PubMed] [Google Scholar]
- Dudics V, Kunstar A, Geher P, Gomor B, Hangody L, Uher F. Mesenchymal stem cells as potential source cartilage repair. Orv Hetil. 2005;146:1201–1208. [PubMed] [Google Scholar]
- Erlacher L, Ng CK, Ullrich R, Krieger S, Luyten FP. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum. 1998;41:263–273. doi: 10.1002/1529-0131(199802)41:2<263::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Goessler UR, Bugert P, Bieback K, Deml M, Sadick H, Hormann K, Riedel F. In vitro analysis of the expression of TGF beta-superfamily-members during chondrogenic differentiation of mesenchymal stemcells and chondrocytes during dedifferentiation in cell culture. Cell Mol Biol Lett. 2005;10:345–362. [PubMed] [Google Scholar]
- Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208–218. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Zimmermann B, Barrach HJ, Merker HJ. Behaviour of epiphyseal mouse chondrocyte populations in monolayer culture. Morphological and immunohistochemical studies. Virchows Arch A Pathol Anat Histol. 1980;389:167–187. doi: 10.1007/BF00439484. [DOI] [PubMed] [Google Scholar]
- Hardingham T, Tew S, Murdoch A. Tissue engineering: Chondrocytes and cartilage. Arthritis Res. 2002;4(Suppl 3):S63–S68. doi: 10.1186/ar561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91:1204–1217. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152–1167. doi: 10.1634/stemcells.2004-0062. [DOI] [PubMed] [Google Scholar]
- Hotten G, Neidhardt H, Jacobowsky B, Pohl J. Cloning and expression of recombinant human growth/differentiation factor 5. Biochem Biophys Res Commun. 1994;204:646–652. doi: 10.1006/bbrc.1994.2508. [DOI] [PubMed] [Google Scholar]
- Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, Trippel SB, Madry H. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006;8:100–111. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Chu CR, Sobajima S, Robbins PD, Fu FH, Izzo NJ, Niyibizi C. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33:865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron MD, Laurencin CT. Orthopaedic applications of gene therapy. Curr Gene Ther. 2005;5:37–61. doi: 10.2174/1566523052997488. [DOI] [PubMed] [Google Scholar]
- Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000;18:790–799. doi: 10.1002/jor.1100180517. [DOI] [PubMed] [Google Scholar]
- Mallein-Gerin F, Ruggiero F, Garrone R. Proteoglycan core protein and type II collagen gene expressions are not correlated with cell shape changes during low density chondrocyte cultures. Differentiation. 1990;43:204–211. doi: 10.1111/j.1432-0436.1990.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Mehlhorn AT, Schmal H, Kaiser S, Lepski G, Finkenzeller G, Stark GB, Sudkamp NP. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393–1403. doi: 10.1089/ten.2006.12.1393. [DOI] [PubMed] [Google Scholar]
- Mwale F, Stachura D, Roughley P, Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–1798. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Lust G, Vernier-Singer M. Isolation, propagation, and cryopreservation of equine articular chondrocytes. Am J Vet Res. 1992;53:2364–2370. [PubMed] [Google Scholar]
- Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12:219–228. doi: 10.1016/j.ymthe.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Podskubka A, Povysil C, Kubes R, Sprindrich J, Sedlacek R. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation on a hyaluronic acid ester scaffolds (Hyalograft C) Acta Chir Orthop Traumatol Cech. 2006;73:251–263. [PubMed] [Google Scholar]
- Ringe J, Haupl T, Sittinger M. Mesenchymal stem cells for tissue engineering of bone and cartilage. Med Klin (Munich) 2003;98(Suppl 2):35–40. [PubMed] [Google Scholar]
- Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Schreuder H, Liesum A, Pohl J, Kruse M, Koyama M. Crystal structure of recombinant human growth and differentiation factor 5: Evidence for interaction of the type I and type II receptor-binding sites. Biochem Biophys Res Commun. 2005;329:1076–1086. doi: 10.1016/j.bbrc.2005.02.078. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- Toh WS, Liu H, Heng BC, Rufaihah AJ, Ye CP, Cao T. Combined effects of TGFbeta1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors. 2005;23:313–321. doi: 10.1080/08977190500252763. [DOI] [PubMed] [Google Scholar]
- Trippel SB, Ghivizzani SC, Nixon AJ. Gene-based approaches for the repair of articular cartilage. Gene Ther. 2004;11:351–359. doi: 10.1038/sj.gt.3302201. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338–343. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W, Richter W. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82:126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li X, Choi L, Beck G, Balian G, Shen FH. Recombinant growth/differentiation factor-5 stimulates osteogenic differentiation of fat-derived stromal cells in vitro. Connect Tissue Res. 2006;47:264–270. doi: 10.1080/03008200600980769. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li X, Beck G, Balian G, Shen FH. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374–381. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]