Abstract
Cannabinoids have long been shown to have a range of potential therapeutic effects, including antiemetic actions, analgesia, and anxiolysis. However, psychomimetic and memory disruptive side effects, as well as the potential for abuse and dependence, have restricted their clinical development. Endogenous cannabinoids (i.e., endocannabinoids; eCBs), such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), are produced throughout the limbic system and other brain regions associated with emotionality and are believed to modulate behavioral responses to stress-related conditions. AEA and 2-AG are rapidly metabolized by the respective enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). Accordingly, inhibition of each enzyme increases brain levels of the appropriate eCB. Although FAAH inhibition has been established to decrease anxiety-like behavior, the role of 2-AG has been difficult to ascertain until the recent synthesis of JZL184, a potent and selective MAGL inhibitor. In the present study, we investigated the effects of inhibiting FAAH or MAGL on anxiety-like behavior in marble burying, a model of repetitive, compulsive behaviors germane to anxiety disorders such as obsessive-compulsive disorder. The FAAH inhibitor PF-3845, the MAGL inhibitor JZL184, and the benzodiazepine diazepam decreased marble burying at doses that did not affect locomotor activity. In contrast, Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent of marijuana, did not consistently reduce marble burying without also eliciting profound decreases in locomotor behavior. The CB1 cannabinoid receptor antagonist rimonabant blocked the reduction in marble burying caused by FAAH and MAGL inhibitors, but not by diazepam, indicating a CB1 receptor mechanism of action. These data indicate that elevation of AEA or 2-AG reduces marble burying behavior and suggest that their catabolic enzymes represent potential targets for the development of new classes of pharmacotherapeutics to treat anxiety-related disorders.
Keywords: endogenous cannabinoid, anxiety, marble burying, fatty acid amide hydrolase, monoacylglycerol lipase, anandamide, 2-arachidonoylglycerol
1. Introduction
Marijuana is commonly smoked to reduce feelings of stress and anxiety (Kogan and Mechoulam, 2007), though paradoxically its primary psychoactive constituent, Δ9-tetrahydrocannabinol (THC), can also increase anxiogenic-like behaviors (Onaivi et al., 1990). Other adverse side effects of cannabis use, including cognitive deficits, abuse potential, and dependence liability, have dampened enthusiasm for the therapeutic development of THC and other cannabinoid receptor agonists. Instead, much interest has been generated by the discovery of the endogenous cannabinoid (i.e. endocannabinoid; eCB) system as a source of targets for the development of new therapeutic treatments of a range of ailments including anxiety and depression (Pacher et al., 2006).
The eCB system is comprised of lipid signaling molecules, anandamide (N-arachidonoylethanolamide; AEA (Devane et al., 1992)) and 2-arachidonoylglycerol (2-AG (Mechoulam et al., 1995)), the receptors for these lipids, CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993), and biosynthetic and catabolic enzymes that regulate eCB levels. AEA is hydrolyzed by the enzyme fatty acid amide hydrolase (FAAH (Cravatt et al., 1996; Deutsch and Chin, 1993)), and 2-AG is predominantly metabolized by monoacylglycerol lipase (MAGL (Blankman et al., 2007; Dinh et al., 2002)). While eCBs are rapidly metabolized in vivo, limiting the efficacy of exogenous administration, inhibition of their catabolic enzymes results in elevated eCB brain levels (Kathuria et al., 2003; Long et al., 2009). Thus, the availability of selective FAAH and MAGL inhibitors has made it possible to study the impact of elevating eCB levels in the whole animal.
A growing body of evidence indicates that CB1 receptor agonists reduce anxiety-like behavior (Moreira et al., 2009). The high levels of CB1 receptor expression in the amygdala, hippocampus, hypothalamus, and prefrontal cortex (Mackie, 2006), coupled with evidence that CB1 receptor activation results in decreased release of glutamate and GABA (Steiner and Wotjak, 2008), support the idea that cannabinoids affect anxiety and anxiety-like behavior. FAAH inhibition, as well as genetic deletion of FAAH, have been reported to reduce anxiety-like behavior in the elevated plus maze (Moreira et al., 2008; Naidu et al., 2007; Patel and Hillard, 2006), zero maze (Kathuria et al., 2003), and light/dark box (Moreira et al., 2008), particularly under stressful conditions (Haller et al., 2009). However, investigation of the consequences of elevating endogenous 2-AG levels has not been possible until the recent development of the highly selective MAGL inhibitor, JZL184 (Long et al., 2009). Thus, little is known about the possible anxiolytic and/or anxiogenic effects of 2-AG modulation in vivo.
Marble burying is used as an assay to infer compulsive, anxiety-like behavior and is widely used as a model of obsessive-compulsive disorder (Broekkamp et al., 1986; Njung'e and Handley, 1991a, b). In this test, a mouse is placed into a clean cage filled with a level layer of bedding, covered with glass marbles. The marbles are disturbed and become covered as the mouse digs into the bedding. Thus, the number of marbles buried correlates with the frequency of digging bouts (Deacon, 2006; Thomas et al., 2009). As with other models of anxiety, marble burying is decreased by traditional anxiolytics, such as benzodiazepines (Broekkamp et al., 1986; Njung'e and Handley, 1991b). Marble burying offers advantages over exploratory models in that it measures a repetitive, possibly goal-directed form of anxiety, such as obsessive-compulsive disorder. Importantly, mice do not readily habituate to the assay (Thomas et al., 2009), making within-subjects designs possible and thereby reducing overall animal numbers.
The studies presented herein were designed to test the hypothesis that exogenous and endogenous cannabinoids reduce anxiety-like behavior in the marble burying test. First, we tested whether the plant-derived cannabinoid THC affected marble burying. Next, we tested the consequences of elevating eCB brain levels on marble burying by administering selective inhibitors of MAGL and FAAH. Finally, we used the CB1 receptor antagonist rimonabant to test whether the observed anxiolytic-like effects of MAGL and FAAH inhibitors occurred via a cannabinoid receptor mechanism of action.
2. Materials and Methods
2.1 Animals
Subjects consisted of male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) that were approximately 10 weeks of age and weighed approximately 25 g at the beginning of the study. Mice were group housed 4-6 per cage in a temperature (20-22° C) and humidity controlled, AAALAC-approved facility maintained on a 12:12 light:dark cycle and were provided with ad libitum access to food and water. Mice were randomly assigned to treatment groups. All experiments were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
2.2 Drugs
Diazepam (DZ) was purchased from Sigma-Aldrich (St. Louis, MO). Rimonabant (Rim) and Δ9-tetrahydrocannabinol (THC) were obtained from the National Institute on Drug Abuse (Bethesda, MD). The MAGL inhibitor JZL184 (Long et al., 2009) and the FAAH inhibitor PF-3845 (Ahn et al., 2009) were synthesized as described previously. All drugs were dissolved in a vehicle consisting of equal parts ethanol and Alkamuls-620 (Rhone-Poulenc, Princeton, NJ), then diluted with normal saline to a final ratio of 1:1:18. All compounds were administered intraperitoneally (i.p.), at a volume of 10 μl/g body mass. All solutions were warmed to room temperature prior to injection.
2.3 Behavioral Testing
Mice were allowed to acclimate to the test room for at least 1 h before experimental manipulation. Prior to testing, each mouse was weighed and injected intraperitoneally (i.p.) with drug or vehicle. For the diazepam and THC experiments, pretreatment time was 1 h. For the JZL184 and PF-3845 experiments, pretreatment time was 2 h, based on previous reports that endocannabinoid levels peak 2 h after treatment with either compound (Ahn et al., 2009; Long et al., 2009). The selective CB1 receptor antagonist rimonabant (0.3 mg/kg, i.p.) was administered 10 min prior to JZL184 or PF-3845 treatment. This dose of rimonabant was chosen based on pilot data, which indicated deficits in locomotor activity at higher doses.
Marble burying behavior was assessed based on published methods (Deacon, 2006; Thomas et al., 2009). The testing apparatus consisted of a polycarbonate mouse cage (internal dimensions: 33 cm long × 21 cm wide × 19 cm high) filled to a depth of 5 cm with pine wood bedding (Harlan Sani-Chip, Indianapolis, IN), and placed in a sound-attenuating chamber lighted by a bank of white LEDs (75 lux). White noise and ventilation were supplied by a PC fan. Prior to each test, twenty clear, glass marbles (10 mm diameter) were evenly spaced and arranged in a grid-like fashion across the surface of the bedding. Then, individual mice were placed into the observation cage, which was then covered with a transparent, Plexiglas lid with air holes. At the conclusion of the 20 min test, the mice were carefully removed from the chamber and the number of buried marbles (50% or more of the marble was covered by bedding) was determined. Inter-observer reliability for assessing marble burying was > 98%.
Locomotor activity was simultaneously captured during the test, using Unibrain Fire-I digital cameras and analyzed in real time using ANY-maze software (Stoelting, Kiel, WI). Immobility was defined as a lack of movement for 1250 ms or longer, and was analyzed in 1 min bins.
2.4 Data Analyses
All data are reported as mean ± SEM and were analyzed using one-way between subjects analysis of variance (ANOVA), with the exception of the antagonist studies, which were analyzed using two-way factorial ANOVA, with antagonist and enzyme inhibitor as the factors. Post hoc comparisons of dose-response data used Dunnett's test to compare each dose to vehicle. Planned comparisons between rimonabant and vehicle were made using T tests. Immobility data were analyzed using repeated measures ANOVA, with time as the within-subjects variable. Differences were considered statistically significant at p < 0.05.
3. Results
3.1 Evaluation of diazepam and THC on marble burying
Mice were injected (i.p.) with various doses of diazepam, THC, or vehicle and then tested in the marble burying assay. Locomotor activity was simultaneously recorded and was quantified as seconds per minute spent immobile. As reported previously, diazepam dose-dependently reduced marble burying [F(3,28) = 37.3; p < 0.0001; Figure 1A]. Post hoc analyses revealed that marble burying was decreased at 1 and 3 mg/kg doses. However, the 3 mg/kg dose also caused a significant increase in immobility, indicating sedation [F(3,532) = 18.6; p < 0.0001; Figure 1B]. Overall immobility increased over time during testing [F(19,532) = 3.8; p < 0.0001].
Figure 1.
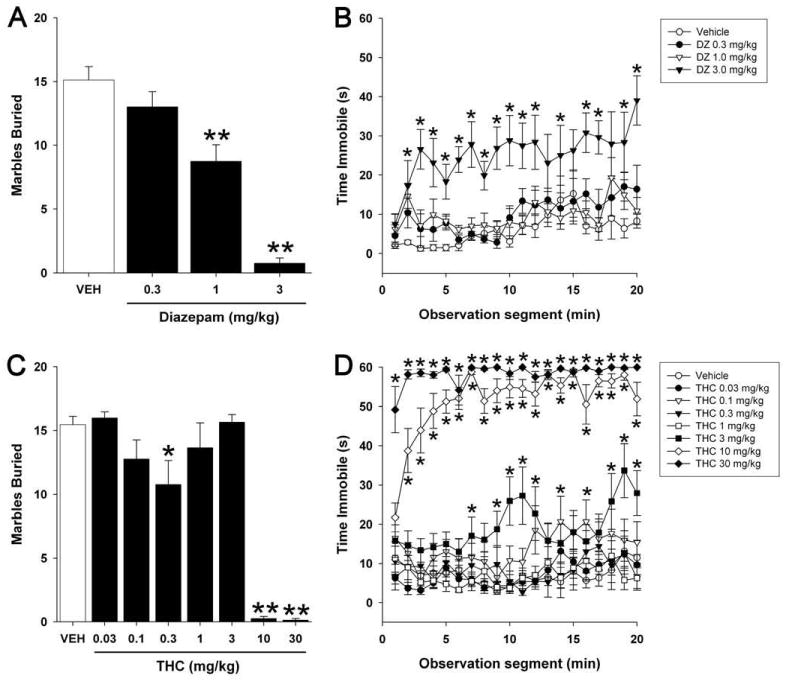
Diazepam and Δ9-THC reduce marble burying behavior. Mice were injected (i.p.) with diazepam (DZ), THC, or vehicle and tested for anxiety-like behavior in the marble burying assay. Locomotor activity was simultaneously recorded and was quantified as seconds per minute spent immobile. (A,C) Diazepam and THC dose-dependently reduced marble burying. (B,D) Both diazepam and THC also caused sedation at higher doses than required to reduce marble burying. Data are presented as the mean ± SEM (n = 8-11). * p < 0.05, ** p < 0.01 vs. vehicle.
Similarly, the plant-derived cannabinoid THC reduced marble burying [F(7,64) = 31.1; p < 0.0001; Figure 1C], but caused profound hypomotility at doses greater than 3 mg/kg [F(7,1216) = 70.6; p < 0.0001; Figure 1D]. Post hoc analyses revealed that marble burying was decreased at 0.3, 10, and 30 mg/kg, but not at 1 or 3 mg/kg, THC.
3.2 Inhibition of eCB catabolic enzymes attenuates anxiety-like behavior in the marble burying test
The highly selective FAAH inhibitor PF-3845 (10 mg/kg) significantly reduced marble burying [F(3,44) = 3.199; P < 0.05; Figure 2A], but had no effect on immobility at any dose tested (p = 0.31; Figure 2B).
Figure 2.
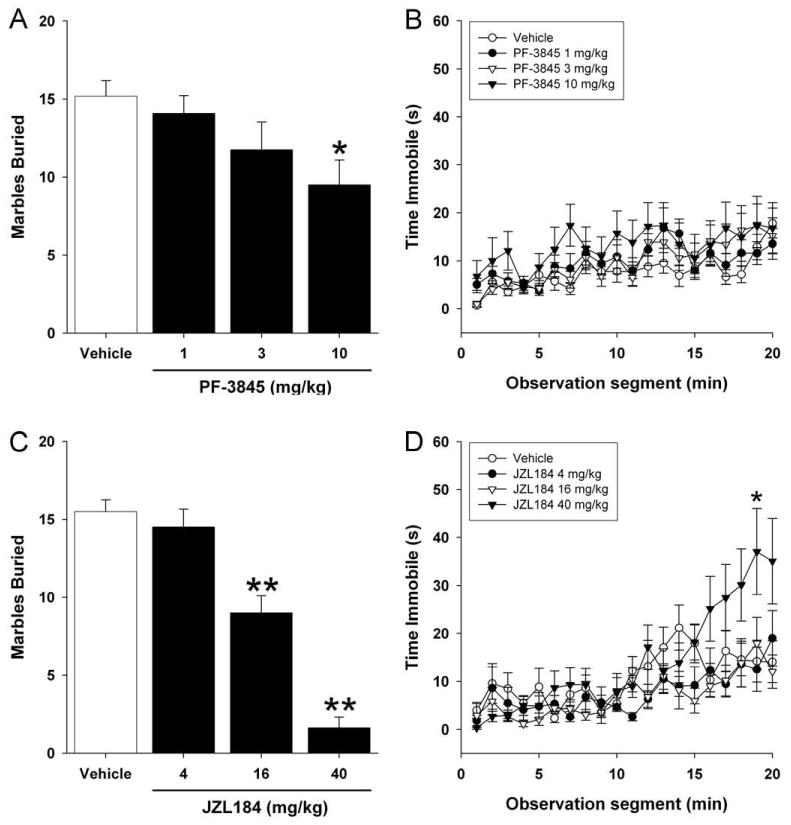
Endocannabinoids reduce marble burying behavior. Mice were injected (i.p.) with the FAAH inhibitor PF-3845, the MAGL inhibitor JZL184, or vehicle and tested in the marble burying assay. Locomotor activity was simultaneously recorded and was quantified as seconds per minute immobile. (A) FAAH dose-dependently reduced marble burying, but (B) had no effect on immobility. (C) JZL184 dose-dependently reduced marble burying, although (D) caused a statistically significant increase in immobility at 40 mg/kg. Data are presented as the mean ± SEM (n = 8). * p < 0.05, ** p < 0.01 vs. vehicle.
The selective MAGL inhibitor JZL184 significantly reduced marble burying [F(3,28) = 46.3; p < 0.0001; Figure 2C] at doses of 16 and 40 mg/kg. However, 40 mg/kg JZL184 also caused a statistically significant increase in immobility [F(57,532) = 2.26; p < 0.0001; Figure 2D]. Unlike THC or diazepam treatment, however, this decrease in locomotor activity did not manifest until after 10 min in the test apparatus.
3.3 A CB1 receptor mechanism of action mediates the reduction of marble burying caused by FAAH and MAGL inhibitors
The anxiolytic-like effects of eCBs and exogenous cannabinoids have been previously shown to occur via the CB1 receptor, although the possible role of CB1 in marble burying is unknown. As reported above, PF-3845 (10 mg/kg) reduced marble burying [F(1,43) = 14.2; p < 0.001; Figure 3A], and rimonabant pretreatment blocked this reduction in marble burying. None of the treatments affected locomotor activity (p = 0.38; Figure 3B).
Figure 3.
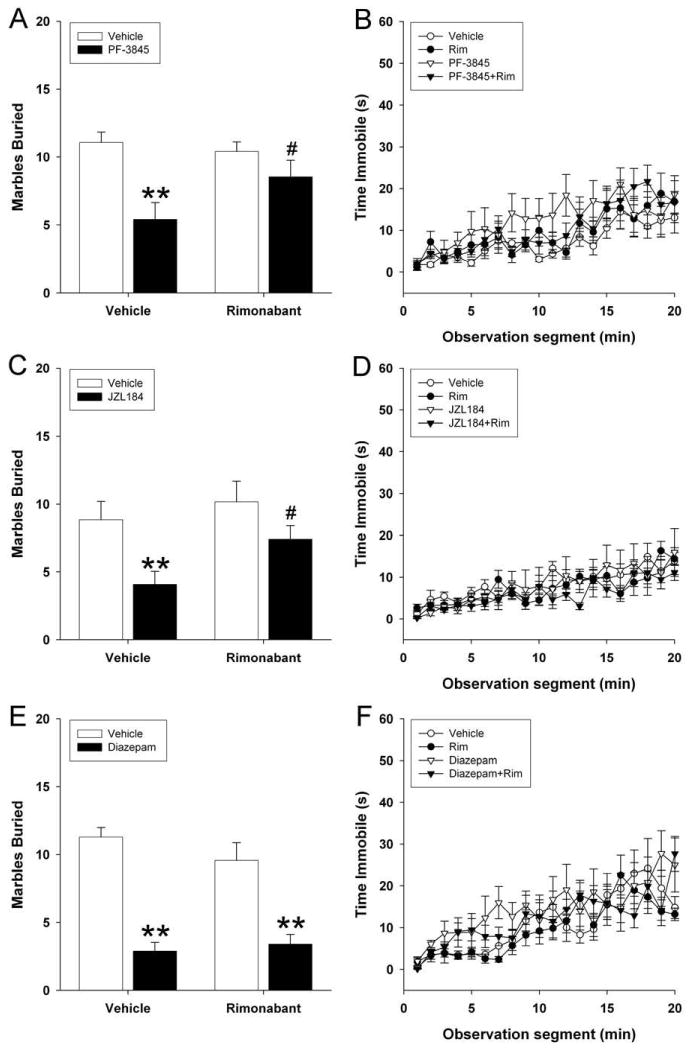
The anxiolytic-like effects of FAAH and MAGL inhibition in the marble burying test are mediated by the CB1 receptor. Mice were injected (i.p.) with PF-3845 (10 mg/kg), JZL184 (16 mg/kg), diazepam (DZ; 1 mg/kg), or vehicle and tested in the marble burying assay. Locomotor activity was simultaneously recorded and was quantified as seconds per minute spent immobile. (A, C, D) Pretreatment with the CB1 receptor selective antagonist rimonabant (Rim) reversed the anxiolytic effects of PF-3845 and JZL184, but had no effect on diazepam. (B, D, E) Rimonabant had no effect on locomotor activity by itself or in conjunction with PF-3845, JZL184, or diazepam. Data are presented as the mean ± SEM (n = 9-16). ** p < 0.01 vs. vehicle; # p < 0.05 vs. PF-3845 or JZL184.
Similarly, JZL184 (16 mg/kg) reduced marble burying [F(1,44) = 11.4; p < 0.01; Figure 3C] and rimonabant prevented this reduction in marble burying. Neither JZL184 nor rimonabant affected immobility (p = 0.60; Figure 3D).
Finally, the possible role of the CB1 receptor in benzodiazepine suppression of marble burying was tested. Diazepam (1 mg/kg) reduced marble burying [F(1,41) = 44.03; p < 0.0001; Figure 3E], however, rimonabant pretreatment had no effect on diazepam suppression of marble burying (p = 0.58). Neither diazepam nor rimonabant affected locomotor activity (p = 0.65; Figure 3F).
4. Discussion
In the present study, we investigated the consequences of inhibiting eCB catabolic enzymes on anxiety-like behavior in the marble burying test. Specifically, we tested the effects of pharmacological inhibition of FAAH or MAGL, as well as Δ9-tetrahydrocannabinol (THC) and the benzodiazepine diazepam, on marble burying and locomotor activity. As reported previously (Broekkamp et al., 1986; Njung'e and Handley, 1991b), diazepam dose-dependently decreased marble burying. The FAAH inhibitor PF-3845 and the MAGL inhibitor JZL184 decreased marble burying, at doses that did not affect locomotor activity, suggesting that eCBs may reduce compulsive or anxiety-like behavior in the marble burying test. The observation that rimonabant completely blocked reductions in marble burying, following FAAH or MAGL inhibition, indicates a CB1 receptor mechanism. The overall pattern of marble burying in response to THC indicated sedative effects in the range of doses tested. It is noteworthy that, as in previous studies, FAAH inhibition had no effect on locomotor activity.
Although irreversible carbamate FAAH inhibitors (e.g., URB597; (Kathuria et al., 2003)) and inhibit FAAH and increase brain levels of anandamide in vivo, they also inhibit other serine hydrolases, calling into question their selectivity for FAAH (Lichtman et al., 2004). Reversible α-ketoheterocycle FAAH inhibitors (e.g. OL-92, OL-135; (Boger et al., 2005; Lichtman et al., 2004)) are more selective for FAAH than URB597, but cause milder elevations of anandamide in vivo. Recently, a new class of highly selective and potent piperidine urea FAAH inhibitors (e.g. PF-3845; (Ahn et al., 2009)) has been synthesized. PF-3845 has a 10-fold higher binding affinity for FAAH than URB597 (Ahn et al., 2009) and inhibits FAAH for at least 24 h in vivo. Similarly, although several compounds inhibit MAGL, these compounds have been criticized because they lack selectivity for MAGL. For example, URB602 lacks potency to systemically elevate 2-AG in vivo (King et al., 2007) and has similar selectivity for both MAGL and FAAH (Vandevoorde et al., 2007). However, JZL184 (Long et al., 2009) potently inhibits MAGL in vivo and is highly selective (200-fold higher for MAGL than for FAAH) and long lasting (> 24 h). Systemic administration of JZL184 significantly increases anandamide in mouse brain and spinal cord and has antihyperalgesic and anti-allodynic effects (Kinsey et al., 2009; Long et al., 2009).
In the present studies, JZL184 caused a significant decrease in marble burying but also increased immobility at the highest dose tested (i.e., 40 mg/kg). Unlike diazepam or THC, however, this increase in immobility was not apparent until 10 min into the test session. These data somewhat contrast with our previous report that JZL184 causes a decrease in spontaneous locomotor activity (Long et al., 2009), however, this discrepancy is likely due to differences in the vehicle used. The original study used a vehicle consisting of 4:1 parts polyethylene glycol (PEG200):TWEEN80) and the present study used a vehicle consisting of 1:1:18 parts ethanol, Alkamuls-620, and saline. Supporting this notion, when dissolved in the same vehicle used in the present study, JZL184 had no effect on muscle coordination in the rotarod test (Schlosburg et al., 2009). Moreover, in the same experiment, high doses of THC disrupted motor coordination. Thus, high doses of JZL184 may produce hypomotility, but not sedation or loss of muscle coordination.
AEA elevation, via FAAH inhibition or genetic deletion, has been reported to have anxiolytic-like effects in exploratory models of anxiety, such as the zero maze (Kathuria et al., 2003), elevated plus maze (Moreira et al., 2008; Naidu et al., 2007; Patel and Hillard, 2006), and light/dark box (Moreira et al., 2008). However, the effects of 2-AG on anxiety have been difficult to ascertain, due to its rapid in vivo metabolism, until the recent synthesis of JZL184. Although MAGL is the primary enzyme responsible for 85% of 2-AG catabolism, other enzymes such as ABHD6 and ABHD12 contribute to 2-AG degradation, and these enzymes have distinct intracellular distributions (Blankman et al., 2007). Thus, it is possible that inhibition of these minor 2-AG catabolic enzymes may also have consequences on anxiety-related behavior that differ from MAGL inhibition.
In humans, the dose response to THC is biphasic with regard to anxiety and anxiety-like behavior. That is, at low to moderate doses, THC elicits anxiolytic effects, whereas at high doses it has anxiogenic effects (D'Souza et al., 2004; Gonzalez, 2007). In the present studies, although THC caused a decrease in marble burying at 0.3 mg/kg, this effect was not dose-related. Higher doses (i.e., 10 and 30 mg/kg), which reduced marble burying behavior, also lead to profound hypomotility, indicating sedation, as reported previously (Martin et al., 1987). Previously, Onaivi et al. (1990) reported that THC produced dose-related, anxiogenic-like effects in both mice and rats, as assessed in the elevated plus maze test. The marble burying test, however, is not designed to assess increased anxiogenic-like behavior.
Endocannabinoids have been shown to have both anxiolytic and anti-depressant properties in clinical and preclinical modes (Hill and Gorzalka, 2009; Moreira et al., 2009). CB1 receptors are expressed throughout the central nervous system, with high densities in the amygdala, prefrontal cortex, hippocampus, and hypothalamus (Herkenham et al., 1991). Activation of CB1 inhibits the presynaptic release of GABA and glutamate (Mackie, 2008). Thus, it is posited that differences in endocannabinoid effects on emotionality occur through regional differences in receptor activation (Hill and Gorzalka, 2009) and/or through differential activation of CB1 and off-target receptors, such as TRPV1 (Rubino et al., 2008).
Although marble burying has long been used as a model to assess anxiolytics, the data derived from this assay do not necessarily correlate directly with exploratory models of anxiety-like behavior, such as the open field test or the light/dark box test (Thomas et al., 2009). Marble burying is also reduced by selective serotonin reuptake inhibitors and tricyclic antidepressants and anti-psychotics (Broekkamp et al., 1986; Njung'e and Handley, 1991a), calling into question its specificity as a test of anxiety. Despite these caveats, given its sensitivity to anxiolytics, marble burying is generally accepted as a model of anxiety disorders marked by compulsive and repetitive behaviors, such as obsessive-compulsive disorder (Thomas et al., 2009).
In the present study, whereas the CB1 receptor antagonist rimonabant blocked the anxiolytic effects of PF-3845 and JZL184, it had no effect on benzodiazepine suppression of marble burying. This is in contrast to a recent report that that the anxiolytic effects of the benzodiazepine alprazolam were blocked by pretreatment with the CB1 antagonist AM251 in the light/dark box (Garcia-Gutierrez and Manzanares, 2010). This apparent discrepancy may be due to intrinsic differences between exploratory tests, such as the light/dark box, and marble burying, as described above.
In conclusion, inhibition of the eCB catabolic enzymes FAAH and MAGL produced anxiolytic-like effects in the marble burying assay. This decrease in marble burying behavior was blocked by rimonabant pretreatment, indicating that the CB1 receptor plays a necessary role in mediating this observed decrease in marble burying. Together with previous reports of the anxiolytic-like effects of FAAH inhibition, these data indicate that elevation of AEA or 2-AG can reduce anxiety-like behavior in mice. Collectively, these results suggest that both FAAH and MAGL are potential therapeutic targets for the development of new classes of anxiolytic drugs.
Acknowledgments
We thank Carlotta Jackson for technical assistance and Dr. Laura Wise for discussions related to this study. This research was supported by the National Institute on Drug Abuse [grants T32DA007027, P01DA009789, P01DA017259, R01 DA015683, and R01DA03672].
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AEA
Anandamide, N-arachidonoyl ethanolamine
- CB1
Cannabinoid receptor type 1
- CB2
Cannabinoid receptor type 2
- DZ
Diazepam, 7-chloro-1,3-dihydro-1-methyl-5-phenyl-1,4-benzodiazepin-2(3H)-one
- eCB
Endocannabinoid
- FAAH
Fatty acid amide hydrolase
- JZL184
4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- MAGL
Monoacylglycerol lipase
- PF-3845
N-(pyridin-3-yl)-4-(3-(5-(trifluoromethyl)pyridin-2-yloxy)benzyl)piperdine-1-carboxamide
- Rim
rimonabant, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl
- THC
Δ9-Tetrahydrocannabinol
Footnotes
The authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven G. Kinsey, Department of Pharmacology and Toxicology, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA 23298-0613
Scott T. O'Neal, Department of Pharmacology and Toxicology, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA 23298-0613
Jonathan Z. Long, The Skaggs Institute for Chemical Biology and Department of Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd. La Jolla, CA 92037
Benjamin F. Cravatt, The Skaggs Institute for Chemical Biology and Department of Chemical Physiology, The Scripps Research Institute, 10550 N. Torrey Pines Rd. La Jolla, CA 92037
Aron H. Lichtman, Department of Pharmacology and Toxicology, Medical College of Virginia Campus, Virginia Commonwealth University, Richmond, VA 23298-0613
References
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, Guimaraes CR, Jorgensen WL, Cravatt BF. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J Med Chem. 2005;48:1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharm. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Sci. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J. The cannabinoid CB1 receptor is involved in the anxiolytic, sedative and amnesic actions of benzodiazepines. J Psychopharmacol. 2010;24:757–765. doi: 10.1177/0269881109106910. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton C, Saghatelian A, Hardouin C, Boger D, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004 doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60–65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Little PJ, Martin TJ, Beardsley PM. Pharmacological evaluation of agonistic and antagonistic activity of cannabinoids. In: Rapaka RS, Makriyannis A, editors. Structure-Activity Relationships of Cannabinoids. Washington, D.C.: U.S. Govt. Printing Office; 1987. pp. 108–122. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski N, Schatz A, Gopher A, Almog S, Martin B, Compton D, Pertwee R, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–64. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991a;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991b;38:63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11:342–352. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–191. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]


