Abstract
Human cytomegalovirus (HCMV) glycoprotein B (gB), encoded by the UL55 open reading frame, is an essential envelope glycoprotein involved in cell attachment and entry. Previously, we identified residue serine 900 (Ser900) as a unique site of reversible casein kinase 2 phosphorylation in the cytoplasmic domain of HCMV gB. We have also recently shown that gB is localized to the trans-Golgi network (TGN) in HCMV-permissive cells, thereby identifying the TGN as a possible site of virus envelopment. The aim of the current study was to determine the role of Ser900 phosphorylation in transport of gB to the TGN and in HCMV biogenesis. Recombinant HCMV strains were constructed that expressed gB molecules containing either an aspartic acid (gBAsp900) or alanine residue (gBAla900) substitution at Ser900 to mimic the phosphorylated or nonphosphorylated form, respectively. Immunofluorescence analysis of the trafficking of gB mutant molecules in fibroblasts infected with the HCMV recombinants revealed that gBAsp900 was localized to the TGN. In contrast, gBAla900 was partially mislocalized from the TGN, indicating that phosphorylation of gB at Ser900 was necessary for TGN localization. The increased TGN localization of gBAsp900 was due to a decreased transport of the molecule to post-TGN compartments. Remarkably, the substitution of an aspartic acid residue for Ser900 also resulted in an increase in levels of progeny virus production during HCMV infection of fibroblasts. Together, these results demonstrate that phosphorylation of gB at Ser900 is necessary for gB localization to the TGN, as well as for efficient viral replication, and further support the TGN as a site of HCMV envelopment.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus that can cause devastating disease, primarily in immunosuppressed individuals such as AIDS patients, patients undergoing iatrogenic immunosuppression, and neonates (26). HCMV is an enveloped DNA virus consisting of a large (230 kb) encapsidated genome wrapped within an inner proteinaceous tegument and an outer lipid envelope studded with viral glycoproteins (24). Currently, a “reenvelopment” model is believed to most accurately describe the assembly process of HCMV, as well as many other herpesviruses. In this model, after initial encapsidation within the nucleus, the nucleocapsid enters the cytoplasm by a process of envelopment and deenvelopment at the inner and outer nuclear membranes, respectively. Final envelopment and acquisition of tegument by the cytoplasmic nucleocapsid are then believed to occur at specific membranes of the cellular secretory system. Studies from a number of laboratories indicate that the final envelopment of many herpesviruses, including varicella-zoster virus (VZV), pseudorabies virus (PRV) and herpes simplex virus type 1 (HSV-1), occurs at the trans-Golgi network (TGN) (8, 14, 15, 30, 35, 36). Consistent with the TGN site of virus assembly, the major envelope glycoproteins of these herpesviruses have been shown to accumulate at the TGN (1, 2, 37). The recent demonstration that the major envelope glycoprotein of HCMV, glycoprotein B (gB), as well as other components of the HCMV virion, is localized to the TGN or a closely apposed intracellular compartment similarly supports the TGN as a site of HCMV assembly (17, 29).
The targeting of glycoproteins to specific organelles of the secretory pathway is modulated, at least in part, by the interaction of cis-acting sequence motifs in the cytoplasmic domain of these proteins with components of the cellular secretory machinery. Studies of a number of TGN-localized cargo proteins (e.g., furin endoprotease, VZV gE, HSV gE, and PRV Us9) have identified phosphorylatable acidic clusters (AC) as motifs critical for localization of proteins to this compartment (1, 2, 7, 37). The AC motif targets proteins to the TGN by binding to a cellular connector protein, phosphofurin acidic cluster sorting protein 1 (PACS-1), which serves to connect the cargo protein to cellular adaptor proteins (AP1 and AP3) (10, 34). Phosphorylation of AC motifs has been shown to play a critical role in mediating the interaction of cargo proteins with PACS-1 and thereby modulating the localization of these proteins to the TGN. A model has been proposed wherein cargo proteins bud from the TGN via an interaction of tyrosine and dileucine motifs. PACS-1 then serves to retrieve these cargo proteins back to the TGN in an AC phosphorylation-dependent manner resulting in steady-state TGN localization of the cargo protein (for a review, see reference 25). Using a vaccinia virus expression system, we have previously shown that HCMV gB contains a conserved AC motif in the cytoplasmic domain that undergoes casein kinase 2 (CK2) phosphorylation on the serine 900 residue (Ser900) (11). Importantly, phosphorylation of the HCMV gB AC at this site has recently been shown to be required for PACS-1 in vitro binding (9), suggesting that this AC motif may be important for the accumulation of gB in the TGN.
The aim of the present study was to determine the role of Ser900 phosphorylation in gB trafficking and HCMV biogenesis by using recombinant HCMV expressing either mimic phosphorylated (gBAsp900) or nonphosphorylated (gBAla900) forms of the protein. Amino acid substitution at Ser900 did not affect protein stability, and Ser900 was shown to be the only site of phosphorylation in the cytoplasmic tail of gB during HCMV infection. In comparison to gBAla900 and gBSer900, the gBAsp900 molecule expressed during HCMV infection showed an increased localization to the TGN, which resulted from a decreased transport of the protein to the plasma membrane (PM) and other post-TGN compartments. HCMV expressing gBAsp900 also exhibited an increased level of production of infectious virus in both single-step and multistep growth assays (compared to HCMV expressing gBAla900 or gBSer900). These results demonstrate that phosphorylation of gB at the CK2 site (Ser900) is necessary for efficient viral replication and suggest that phosphorylation at Ser900 may mediate localization of gB to the TGN, a proposed site of virus assembly, possibly through an interaction with PACS-1.
MATERIALS AND METHODS
Cell lines and virus.
Wild-type (WT) HCMV and HCMV recombinants were propagated in human foreskin fibroblasts (HFF) by standard methods (19). HFF were cultured at 37°C in an atmosphere of 5% CO2 in complete medium (Dulbecco modified essential medium containing 10% fetal bovine serum and supplemented with 4 mM l-glutamine, 200 μg of penicillin G/ml, and 200 μg of streptomycin sulfate/ml).
HCMV titration.
Cells were infected with HCMV by the addition of virus to cell monolayers at the multiplicity of infection (MOI) indicated, followed by incubation for 1 h at 37°C in an atmosphere of 5% CO2. Monolayers were then washed three times with Dulbecco phosphate-buffered saline (DPBS), fresh complete medium was added, and cells were cultured at 37°C in an atmosphere of 5% CO2. Supernatant and cell fractions were harvested at various times postinfection (p.i.), and the level of progeny virus was determined by titration on HFF by standard methodology.
Generation of recombinant HCMV.
Homologous recombination was used to introduce point mutations into gB (UL55), substituting aspartic acid or alanine codons for Ser900 in the gB cytoplasmic domain (Fig. 1A). Plasmid pgHBg10pA-US11(3optx) was used as the parental vector background for construction of the recombination plasmids (Fig. 1B). A 1,769-bp AseI-SspI fragment derived from pHindIII-F was cloned into the SmaI site of pgHBg10pA-US11(3optx). The gBSer900 (gBWT) recombination plasmid was constructed by cloning a 2,113-bp SspI-AseI fragment from pHindIII-F into the HindIII site of pgHBg10pA-US11(3optx). The gBAsp900 and gBAla900 recombination plasmids were created by cloning a 2,083-bp NotI-AseI fragment from either pRep4Δe/gBAsp900 or pRep4Δe/gBAla900 (11) into the HindIII site of pgHBg10pA-US11(3optx). Recombinant virus was isolated after cotransfection of HFF with full-length HCMV AD169 DNA, the appropriate recombination plasmid, and pCMV71 (to increase viral DNA infectivity) as described previously (4, 20). In addition to substitution of the gB Ser900 codon, each recombination plasmid was designed to introduce the β-glucuronidase gene into the noncoding region between DNA polymerase (UL54) and UL55 to enable colorimetric identification of recombinant viruses. Within the HCMV genome, the promoter and transcriptional start site for DNA polymerase (UL54) are embedded within the 3′ coding region of UL55 (22), and substitution of Ser900 within this region may alter the normal function of the UL54 promoter. Consequently, we placed UL54 expression under the control of a heterologous US11 promoter (Fig. 1C). Similar to DNA polymerase, US11 is expressed with early gene kinetics. For control purposes, a similar recombinant (HCMVgBWT) containing the reporter cassette, but without any gB mutation, was also constructed. Recombinant viral plaques were identified, isolated and propagated by multiple rounds of plaque purification (20). Insertion of the recombination cassette within the correct region of the viral genome was verified by Southern blot analysis (data not shown). Presence of Asp900 and Ala900 codon substitutions were confirmed by sequence analysis of recombinant viral DNA, and steady-state protein labeling experiments confirmed that mutation of gB did not affect gB protein stability or maturation (data not shown).
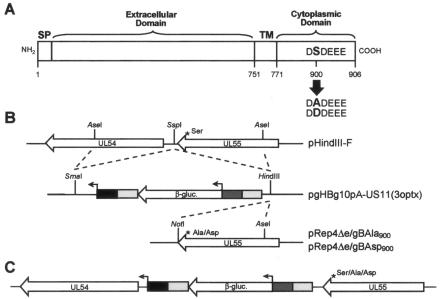
FIG. 1.
Construction of HCMV gBSer900 mutants. (A) Schematic diagram of the HCMV gB protein. HCMV mutants expressing gB with either an aspartic acid or alanine residue substitution in place of Ser900 were constructed to mimic the phosphorylated or nonphosphorylated form of the protein, respectively. The amino acid sequence of the AC is shown. SP, signal peptide; TM, transmembrane domain. Numbers refer to amino acids. (B) Construction of recombination plasmids used to make the HCMV gB mutants. HCMV sequences used for homologous recombination were derived from plasmids pHindIII-F, pRep4Δe/gBAla900, and pRep4Δe/gBAsp900 and cloned into pgHBg10pA-US11(3optx) as described in Materials and Methods. β-gluc., β-glucuronidase. (C) Diagram of recombinant HCMV genomic DNA. The HSV-1 glycoprotein H promoter (dark gray box) controls the expression of the β-glucuronidase gene (β-gluc.). The HCMV US11 promoter (black box) upstream of UL54 (DNA polymerase) is used to maintain correct expression of the UL54 gene. The light gray box represents the polyadenylation signal. Recombinant viruses were plaque purified, and the presence of the mutational cassette was confirmed by Southern analysis.
Generation of adenovirus vectors.
Construction and characterization of adenovirus expressing the gBWT with a Flag epitope inserted immediately downstream from the cleavage site (gBFlagWT) have been previously described (18). Recombinant adenoviruses expressing either gBFlagWT containing substitution of an aspartic acid (AdgBFlagAsp900) or alanine residue (AdgBFlagAla900) for gB Ser900 were produced as previously described (31). Recombinant viruses were screened by PCR, and protein expression was confirmed by Western analysis of infected cell lysates by using the appropriate antibody. All recombinant adenoviruses were plaque purified, and viral stocks were grown and titers were determined on 293 cells. Recombinant protein expression was under the control of a Tet-responsive promoter/enhancer element with protein expression driven by coinfection with an adenovirus (AdtTa) expressing the “Tet-off” transactivator (tTa), and protein levels were regulated by altering the MOI of AdtTa used to infect cells. For the expression of recombinant proteins, adenoviruses expressing the protein of interest and AdtTa were added to cells at the MOIs indicated and then incubated for 2 h at 37°C. Monolayers were then washed twice with DPBS, and cells were cultured for the indicated times prior to harvest.
Metabolic labeling and immunoprecipitation analysis.
HFF were infected with HCMV recombinants at an MOI of 3. Steady-state protein labeling was performed by adding either [35S]methionine-cysteine (35S-Met-Cys) or inorganic phosphate (32Pi) to the media at 48 h p.i. The cells were further incubated for 18 h. After three washes with cold DPBS, cells were scraped from the surface of the dish, pelleted by centrifugation, and resuspended in radioimmunoprecipitation assay lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate). After a 10-min incubation on ice, the lysate was clarified by centrifugation at 16,000 × g for 5 min. Pulse-chase analysis was performed by adding 35S-Met-Cys to infected cells at 3 days p.i. After incubation for 1 h, medium containing label was removed, and cells were chased for the time indicated in complete medium containing an excess of unlabeled methionine. Cell lysates were prepared at the indicated times, incubated with a gB monoclonal antibody (MAb; ABI, Columbia, Md.), immunoprecipitated with protein A-Sepharose, and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography.
Western analysis of purified HCMV particles.
Viral particles were purified by glycerol-tartrate centrifugation as described previously (3). Viral proteins were separated by SDS-PAGE and analyzed by Western blotting with either an MAb to gB or a polyclonal antibody raised against whole HCMV particles.
Immunofluorescence microscopy.
Localization of viral and cellular proteins in HFF was determined by indirect immunofluorescence as previously described (17). Where indicated, cells were incubated in the presence of cycloheximide (100 μg/ml) prior to fixation to visualize steady-state gB distribution. The primary antibodies used were a mouse anti-gB MAb 27-156 (used at a dilution of 1/150), mouse anti-FLAG MAbs M1 and M2 (Sigma-Aldrich, St. Louis, Mo.; used at a dilution of 1/200), and a rabbit polyclonal anti-TGN46 antibody (used at a dilution of 1/300) (28). Epifluoresence was visualized by using a Nikon Optiphot fluorescence microscope.
Analysis of post-TGN gB transport.
Cells were infected with AdgBFlag adenovirus recombinants and AdtTa at MOIs of 100 and 10, respectively. At 2 days p.i., transport of gB to the PM and post-TGN compartments was determined as previously described (17). Briefly, cells were incubated with MAb M1 in the medium for 3 h at 37°C prior to fixation and staining. The gB that was transported to the PM and other post-TGN compartments was then visualized by using an MAb M1 isotype-specific secondary antibody. After fixation and permeabilization of cells, total gB was visualized by using MAb M2 and the respective isotype-specific secondary antibody. Epifluorescence was visualized by using a Nikon Optiphot fluorescence microscope.
RESULTS
The gB Ser900 residue is the only site of gB phosphorylation during HCMV infection.
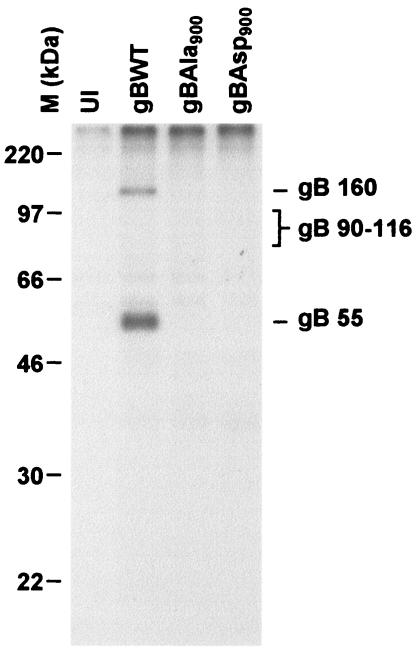
HCMV gB contains a phosphorylated AC at the carboxyl terminus of the cytoplasmic domain. To address the role of phosphorylation within this AC at Ser900, recombinant HCMV expressing gB with either an aspartic acid (HCMVgBAsp900) or alanine (HCMVgBAla900) substitution at gB Ser900 were constructed to mimic the phosphorylated and nonphosphorylated forms of the protein, respectively (Fig. 1A). In a previous study with recombinant vaccinia viruses expressing either gBWT, gBAla900, or gBAsp900, the Ser900 residue was identified as the only site of phosphorylation within the gB molecule (11). Consequently, 32P labeling experiments were performed to determine whether Ser900 is the only site of gB phosphorylation during actual HCMV infection. HFF were infected with HCMVgBWT, HCMVgBAsp900, or HCMVgBAla900. At 48 h p.i., cells were pulsed with inorganic 32P for 18 h. The gB was then immunoprecipitated from cell lysates, and 32P-labeled gB was visualized by SDS-PAGE and autoradiography. As shown in Fig. 2, the presence of 32P-labeled gB in lysates from HCMVgBWT-infected cells, but not HCMVgBAsp900- or HCMVgBAla900-infected cells, demonstrates that Ser900 is the only site of gB phosphorylation during HCMV infection of HFF. Interestingly, both the 160-kDa and the 55-kDa gB subunits were phosphorylated, indicating that some level of gB undergoes phosphorylation during the maturation process prior to proteolytic processing. Since gB proteolysis is known to occur in the TGN (23), this indicates that a proportion of gB is phosphorylated within the endoplasmic reticulum or Golgi before it reaches the TGN.
FIG. 2.
The only site of phosphorylation in the cytoplasmic tail of gB during HCMV infection is the Ser900 residue. HFF were infected at an MOI of 3 with HCMV recombinants expressing either gBWT, gBAla900, or gBAsp900. At 48 h p.i., 32Pi was added, and the cells were incubated a further 18 h. The gB was immunoprecipitated from cell lysates, and 32P-labeled gB was visualized by SDS-PAGE and autoradiography. UI, uninfected.
HCMV expression of the mimic-phosphorylated form of gB (gBAsp900) increases localization of gB to the TGN.
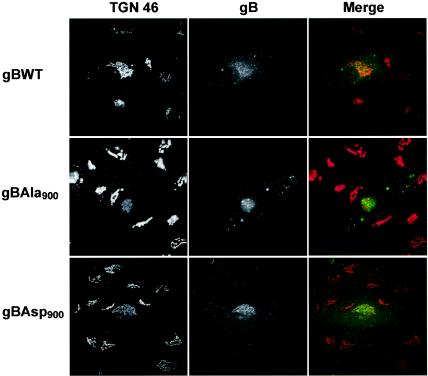
HCMV gB has recently been shown to interact with the PACS-1 cellular connector protein in a phosphorylation-dependent manner (9). The interaction of a number of viral and cellular proteins with PACS-1 has been shown to be required for their localization to the TGN (10, 34). Therefore, we hypothesized that, by having an increased interaction with PACS-1, the mimic-phosphorylated form of gB (gBAsp900) may increase localization of this essential glycoprotein to the TGN. To determine the level of gB localization to the TGN, HFF were infected with recombinant HCMV and gB colocalization with a marker of the TGN (TGN46) was determined by immunofluorescence microscopy at day 3 p.i. To ensure steady-state gB distribution, cells were incubated in the presence of cycloheximide for 2 h prior to fixation. As shown in Fig. 3, the increased TGN localization of gBAsp900 compared to gBAla900 showed that the mimic-phosphorylated form of gB had an increased localization to the TGN. However, gBAla900 still showed a considerable level of TGN localization, indicating that phosphorylation is not the sole determinant of gB localization to the TGN. Together, these results are consistent with an increased association of gBAsp900 with PACS-1 causing an increased localization of gB to the TGN.
FIG. 3.
HCMV expression of the mimic-phosphorylated form of gB (gBAsp900) increases the localization of gB to the TGN. HFF were infected with HCMV recombinants expressing either gBWT, gBAla900, or gBAsp900. At day 3 p.i., cells were fixed and incubated with an MAb against gB (green) and with a polyclonal antibody against TGN46 (red) (a marker of the TGN). Immunoreactivity was detected with an FITC-conjugated anti-mouse and Alexa594-conjugated anti-rabbit secondary antibody. The high level of colocalization of TGN46 and gB in cells infected with HCMVgBAsp900, compared to cells infected with either HCMVgBWT or HCMV gBAla900, shows an increased localization of gBAsp900 to the TGN.
Transport of gB to the TGN is equivalent in HCMV Ser900 mutants.
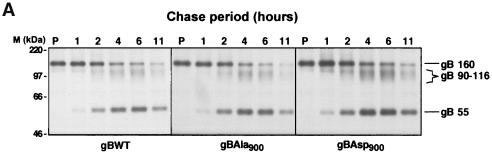
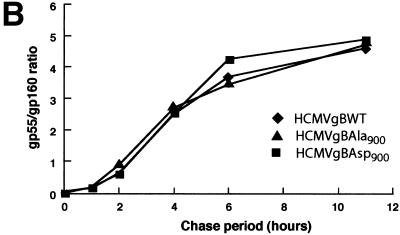
The increased TGN localization of gBAsp900 may result from either an increased rate of transport to the TGN or a decreased rate of transport from the TGN to the PM and other post-TGN compartments. To determine the rate of transport of de novo-synthesized gBWT, gBAsp900, and gBAla900 protein to the TGN, we exploited the knowledge that gB is proteolytically cleaved in the TGN. Consequently, the rate of transport to the TGN can be quantitated by measurement of the rate of formation of the gB cleavage products. HFF were infected with HCMVgBWT, HCMVgBAsp900, or HCMVgBAla900. At day 3 p.i., cells were pulsed with 35S-Met-Cys for 1 h and chased with nonradiolabeled methionine-cysteine. The gB was immunoprecipitated from cell lysates, and 35S-labeled gB was visualized by SDS-PAGE and autoradiography (Fig. 4A) and quantified by phosphorimaging (Fig. 4B). The equivalent rates of gB cleavage visualized by the appearance of the 55-kDa gB subunit during infection with all HCMV recombinants shows that gBWT, gBAsp900, and gBAla900 are all transported to the TGN at comparable rates, indicating that the increased TGN localization of the gBAsp900 is not due to an increased transport of de novo-synthesized gB into this compartment.
FIG. 4.
Transport of gB to the TGN is equivalent in HCMV Ser900 mutants. HCMV gB is cleaved in the TGN. Therefore, proteolytic processing of gB was used to determine the rate of gB transport to the TGN. HFF were infected at an MOI of 3 with HCMV mutants expressing either gBWT, gBAla900, or gBAsp900. At day 3 p.i., cells were pulsed with 35S-Met-Cys for 1 h and chased with nonradiolabeled methionine for the indicated times. The gB was immunoprecipitated from cell lysates, and 35S-labeled gB was visualized by SDS-PAGE and phosphorimaging (A) and quantified by densitometry (B). gB processing was measured by calculating the gp55/gp160 ratio, and values for gBWT (♦), gBAla900 (▴), and gBAsp900 (▪) are as indicated.
Increased TGN localization of gBAsp900 corresponds to a decreased transport to the PM and other post-TGN compartments.
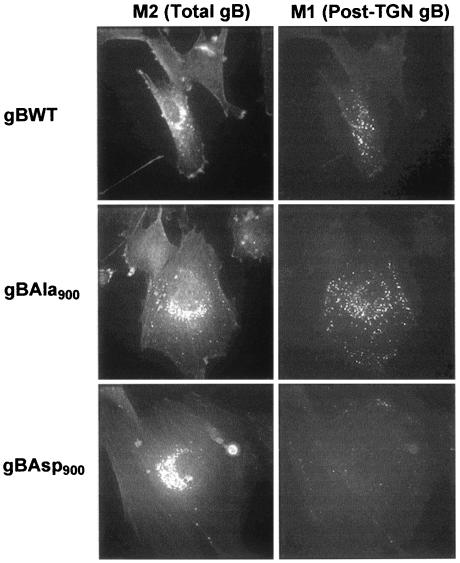
Recombinant adenovirus vectors expressing Flag epitope-tagged versions of gBWT, gBAsp900, and gBAla900 proteins combined with surface labeling were utilized to determine whether the increased TGN localization of gBAsp900 resulted from a decreased transport from the TGN to the PM and other post-TGN compartments. The Flag epitope was inserted immediately adjacent to the furin cleavage site within the gBAsp900 and gBAla900 molecule as previously described (18). HFF were infected with AdgBFlagWT, AdgBFlagAsp900, or AdgBFlagAla900. Cells were then cultured in the presence of anti-Flag epitope antibody (M1) for 3 h prior to fixation. The level of gB transported to the PM over the 3-h period was visualized by using a fluorescein isothiocyanate (FITC)-conjugated anti-M1 isotype-specific antibody. Total cellular gB was visualized in permeabilized, postfixed cells with a second anti-Flag epitope antibody (M2) and a Texas r-conjugated anti-M2 isotype-specific antibody. The absence of M1 staining in cells expressing gBAsp900 compared to gBWT and gBAla900 shows that the mimic-phosphorylated gBAsp900 exhibited a dramatic reduction in transport to the PM even though the total expression level of gBAsp900 was comparable or even higher than that of gBWT or gBAla900 (Fig. 5). PACS-1 has been shown to function by retrieving cargo glycoproteins back to the TGN after their AP-1-dependent budding from this compartment (34). Consequently, this increased TGN localization of gBAsp900 due to a reduced transport of the gB molecule to the PM and post-TGN compartments is consistent with an increased interaction of the mimic-phosphorylated gBAsp900 with the PACS-1 cellular connector.
FIG. 5.
The gBAsp900 molecule shows a decreased transport to the cell surface and post-TGN compartments. HFF were infected with an adenovirus expressing recombinant Flag-tagged gB containing either gBWT, gBAla900, or gBAsp900. Cells were then cultured in the presence of anti-Flag epitope antibody (M1) for 3 h prior to fixation. The level of gB transported to the cell surface and post-TGN compartments over the 3-h period was visualized by using a FITC-conjugated anti-M1 isotype specific antibody. Total cellular gB was visualized in postfixed cells with a second anti-Flag epitope antibody (M2) and a Texas r-conjugated anti-M2 isotype specific antibody.
HCMV expression of the mimic-phosphorylated form of gB (gBAsp900) increases the level of infectious progeny.
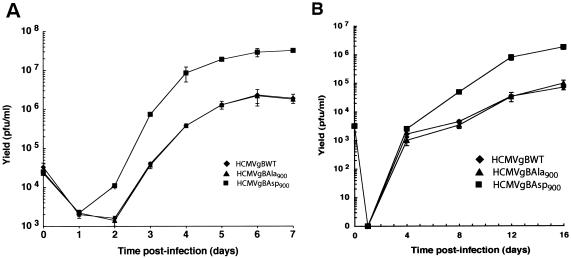
A number of studies have identified the TGN as a potential site of HCMV assembly (17, 29). Consequently, by increasing the localization of the essential gB glycoprotein to the TGN, we hypothesized that the gBAsp900 may increase the production of infectious progeny. The level of progeny virus produced by the HCMV recombinants was determined in single- and multistep growth analysis in HFF. As shown in Fig. 6, HCMVgBAsp900 produced 10-fold more progeny virus than was observed with either the HCMVgBWT or HCMVgBAla900 virus. This capacity of the mimic-phosphorylated gB form to increase virus production is consistent with the increased interaction of the phosphorylated form of gB with PACS-1 directing gB to a TGN site of assembly. The comparable level of virus production of HCMVgBWT or HCMVgBAla900 also suggests that WT gB (gBSer900) has a level of PACS-1 interaction comparable to the mimic-nonphosphorylated form, due either to the level or turnover rate of phosphorylation at Ser900.
FIG. 6.
HCMV Asp900 produces increased levels of infectious progeny. Replication of HCMV mutants expressing gBWT (♦), gBAla900 (▴), or gBAsp900 (▪) was determined by single-step (A) and multistep (B) growth analysis in HFF. HFF were infected at an MOI of 3 (A) or 0.03 (B). Cells and supernatant were then harvested at designated times p.i., and titers were determined on HFF. The levels of total virus produced are shown.
HCMV expression of the mimic-phosphorylated form of gB (gBAsp900) increases the level of virion particle production.
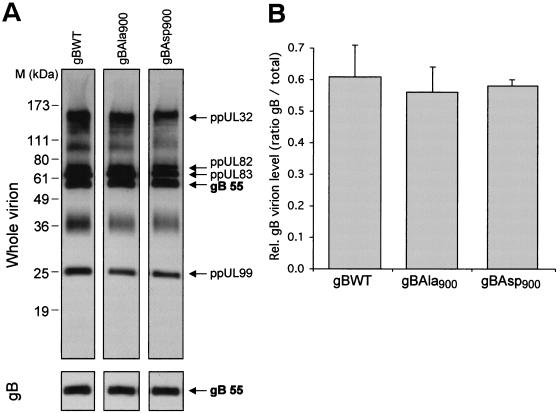
The increased level of infectious progeny virus produced by HCMVgBAsp900 may result from either an increase in the number of infectious particles or from a greater level of gB incorporation within the virion causing an increased ability of each virion to infect HFF. To determine whether an increased level of gBAsp900 was incorporated within the virion, noninfectious enveloped particles (NIEPs), virions, and dense bodies (DBs) were purified on glycerol-tartrate gradients from the supernatant of HFF infected with the HCMV recombinants. Consistent with an increased production of virions, visual inspection of the gradients was suggestive of an increase in the total amount of virus particles in HCMVgBAsp900-infected cell supernatants. However, the relative levels of NIEPs, virions, and DBs produced in the supernatants from cells infected with the different HCMV recombinants were comparable. To enable a determination of the relative levels of gBWT, gBAsp900 gBAla900 incorporation within the virion particle, proteins from the purified virion fraction were separated by SDS-PAGE and analyzed by Western blot and densitometry. The gB was visualized by using an MAb to the 55-kDa subunit of gB, and total HCMV proteins were visualized by using a polyclonal antibody directed against total virion proteins (Fig. 7A). Levels of virion gB normalized to total virion protein level are shown in Fig. 7B. The equivalent levels of gB within the virion particles produced during infection with all HCMV recombinants suggests that the increased level of infectious progeny virus associated with HCMVgBAsp900 infection results from an increased production of virion particles rather than an increase in the level of gB incorporated within each virion.
FIG. 7.
Viral particles produced by HCMV mutants contain equivalent levels of gB. HCMV mutants expressing either gBWT, gBAla900, or gBAsp900 were used to infect HFF at an MOI of 1. Virions, NIEPs, and DBs were purified from the supernatants of infected cells by glycerol-tartrate centrifugation. (A) Virion proteins were visualized by Western blot with a polyclonal antibody raised against whole virus, and gB was visualized with an MAb against gB. For reference, several viral structural proteins are indicated (3). (B) Relative levels of gB compared to total virion proteins quantitated by densitometry of multiple lanes after SDS-PAGE. The values shown are the average of at least three lanes.
DISCUSSION
In the present study, we used a series of recombinant HCMV expressing either a mimic-phosphorylated (gBAsp900) or a mimic-nonphosphorylated (gBAla900) form of the gB molecule to investigate the role of gB AC phosphorylation in modulating gB TGN localization and HCMV biogenesis. During HCMV infection, the mimic-phosphorylated gBAsp900 exhibited an increased localization to the TGN. Remarkably, HCMV expressing the gBAsp900 also exhibited an increased level of infectious virus production. The cellular connector protein, PACS-1, was recently shown to bind the HCMV gB AC motif (DS900DEEE904) in a Ser900 phosphorylation-dependent manner and to be essential for the TGN localization of recombinantly expressed gB (9). Together, these results suggest a role of gBSer900 phosphorylation in directing gB to the TGN site of virion assembly potentially by enhancing an interaction with the cellular connector, PACS-1.
A model for PACS-1-mediated localization of cargo proteins to the TGN has been proposed wherein PACS-1 serves to retrieve cargo proteins back to the TGN after their budding from the TGN in a tyrosine or dileucine motif-dependent manner (for a review, see reference 25). Localization of cargo proteins to the TGN is believed to be modulated by phosphorylation state of the cargo protein AC motif, with AC phosphorylation being necessary for PACS-1 retrieval to this compartment. Consistent with this model, the mimic-phosphorylated form of gB (gBAsp900) displayed an increased level of TGN localization, whereas the mimic-nonphosphorylated gB (gBAla900) was dispersed from this region. However, gBAla900 still showed a substantial level of TGN localization, indicating that AC phosphorylation is not the only requirement for gB localization to the TGN. These observations show that the localization of gB to the TGN during HCMV infection is modulated, in part, by the phosphorylation state of the gB molecule and is consistent with an increased ability of the phosphorylated form of gB to bind the PACS-1 connector protein. Similarly, the comparable transport of de novo synthesized gBWT, gBAsp900, and gBAla900 to the TGN combined with the decreased transport of gBAsp900 from the TGN is consistent with the retrieval-based mechanism of PACS-1-mediated TGN localization. Together, these results suggest that the increased localization of gBAsp900 to the TGN observed during HCMVgBAsp900 infection is due to an enhanced retrieval of this molecule back to the TGN by PACS-1.
Expression of the gBAsp900 mimic-phosphorylated form of gB during HCMV infection also increased the level of virus progeny production. Previously, we identified the TGN as a major site of gB accumulation in two distinct permissive cell types during HCMV infection (17). The results from another study showed the localization of virion tegument proteins (pp28, pp65, and pp150), as well as glycoproteins (gB and gH), to an intracellular region that contained high levels of infectious virus and was in close proximity to a structure marked by antibodies reactive with TGN46 (29). These findings provide strong evidence that the TGN represents a site of assembly and final envelopment of HCMV. gB is an essential component of the virion envelope involved in attachment and entry processes (5, 16). Consequently, the finding that targeting of the gBAsp900 molecule to the TGN corresponds to an increased production of virus further supports the TGN as an important site of HCMV envelopment.
The increased level of HCMVgBAsp900 infectious progeny may result from either the production of more infectious particles or from a greater level of gB incorporation within each virion, resulting in an enhancement of the virion particle infectivity. Western analysis showed comparable levels of gB in purified virion particles from supernatants of cells infected with HCMVgBWT, HCMVgBAsp900, and HCMVgBAla900. This finding suggests that the increased level of infectious virus is due to an increase in virion particle production and not to enhanced infectivity as a result of a greater level of gB within each virion particle. Viruses can be characterized on the basis of their requirement for nucleocapsid and glycoproteins to drive the envelopment process (for a review, see reference 13). Although preliminary, the observation that targeting gB to a site of virion assembly increases the production of virus particles raises the possibility that the formation of HCMV virion particles may be regulated by the rate of gB delivery to the assembly site. This model is consistent with studies of PRV, wherein deletion of the glycoproteins gE/I and gM resulted in a dramatic envelopment defect and the accumulation of tegumented capsids within the cytoplasm (6). In PRV, deletion of either gE/I or gM individually resulted in only a minimal effect on virus production, indicating a redundancy of glycoprotein function that may not exist for gB of HCMV.
In addition to mediating TGN localization, PACS-1 has been shown to be involved in protein trafficking at a number of levels within the cellular secretory pathway. A model for PACS-1-mediated trafficking of cargo proteins has been presented, wherein proteins are maintained in local cycling loops at either the TGN or PM/early endosomal (EE) compartment by their interaction with PACS-1 (25). Localization to these local cycling loops is phosphorylation dependent, with dephosphorylation being required for movement of cargo proteins between the TGN and PM/EE loops. The results from a series of published studies from our laboratory suggest that gB trafficking may be regulated in a manner that is consistent with this model. We recently showed that gB cycles between the TGN and PM in a similar manner to other PACS-1-dependent TGN-localized proteins (17). In the present study, the decreased staining of gBAsp900 in the antibody uptake assay is consistent with an increased binding of gBAsp900 to PACS-1 causing an increased localization of gB to a localized TGN cycling loop and decreased transport to the PM. However, any gBAsp900 that escapes the TGN would then exhibit increased PM localization due to being “trapped” within a PM/EE cycling loop as a result of binding to PACS-1 at the PM/EE. Concordant with this prediction, vaccinia virus-expressed recombinant gBAsp900 was shown in a study by Fish et al. (11) to have an increased localization at the PM in HFF. In contrast, as a result of decreased binding to PACS-1, the gBAla900 molecule would be expected to be more rapidly transported to the PM and post-TGN compartments. This increased transport from the TGN is shown in the current study by the increased staining of gBAla900 in the uptake assays. The gBAla900 protein would also be more rapidly recycled back to the TGN from the PM/EE compartment, which would result in the decreased gBAla900 PM expression observed in the earlier study (11). Alternatively, absence of PM localization of gBAsp900 observed during HCMV infection in the current study may reflect differences in trafficking of vaccinia virus compared to HCMV-expressed gB. A number of immunofluorescence-based studies (11, 17) and a recent immunoelectron microscopy-based study (12) have shown that, during HCMV infection of HFF, the level of gB localized at the PM is low. In contrast, in the study by Fish et al. (11), significant levels of vaccinia virus-expressed WT gB were observed at the PM. This difference between the surface expression of vaccinia virus compared to HCMV-expressed gB suggests that additional HCMV-expressed or HCMV-induced cellular factors may be further modifying the trafficking of gB during HCMV infection.
Retrieval of gB by endocytosis from the PM has been shown to target HCMV gB to a cytoplasmic EE site of virus assembly in HFF (32), and we have recently shown that this endocytic pathway can similarly target gB to the site of virus assembly in HCMV-infected U373 cells (17). However, prevention of gB endocytosis from the PM during HCMV infection by expression of a dominant-negative dynamin molecule (dynK44A) did not affect the levels of infectious virus produced (17). Consequently, although the trafficking of gBAsp900 in the PM/EE compartment may be altered compared to gBWT and gBAla900, this aspect of the trafficking itinerary of gB plays a minor role, if any, in the assembly and final envelopment of HCMV.
In a recent immunoelectron microscopy study, localization of virus particles and HCMV envelope proteins to multivesicular bodies (MVBs) identified MVBs as a potential site of virion envelopment (12). HCMV envelope proteins were also found localized to cytoplasmic membranes, although the exact identity of these membranes was not determined. Similar to the TGN, MVBs are localized to the juxtanuclear region of the cell, being concentrated at the microtubule organizing center (for a review, see reference 27). A direct trafficking route is also believed to exist between the MVBs and the TGN. Consequently, the identification of virion particles within MVBs is not incompatible with the TGN or a closely opposed site of HCMV envelopment. Further immunoelectron microscopy studies by using colocalization of markers of the secretory pathway with HCMV virion proteins will be required to determine the exact relationship of the TGN and MVB compartments regarding HCMV envelopment.
The ability to phosphorylate gB may be critical for modulating aspects of HCMV assembly and egress. For example, the studies of VZV gE trafficking showed that differential phosphorylation of the gE AC targeted gE to either the TGN or cell surface, thereby increasing virus assembly or cell-to-cell spread, respectively (21). Although the role of cell-to-cell spread in the biology of HCMV is unclear, this observation indicates that herpesviruses utilize differential phosphorylation of critical glycoproteins to modulate their assembly and egress processes. HCMV infects a variety of cell types within the host, and the role of gB phosphorylation may be more critical in particular types of cells. For example, we have observed the accumulation of gBAsp900, but not gBWT or gBAla900, in a distinct post-TGN compartment of HCMV-infected endothelial cells, but not HFF. This intracellular site of accumulation corresponded to a decreased release of progeny virus from HCMVgBAsp900-infected endothelial cells, suggesting that gB phosphorylation may be important for the regulation of egress from this cell type (M. A. Jarvis, C. J. Baldick, D. D. Drummond, J. A. Borton, P. P. Smith, W. J. Smith, T. R. Jones, and J. A. Nelson, Abstr. 27th Int. Herpesvirus Workshop, abstr. 7.03, 2002). Endothelial cells have been shown to facilitate the infection of monocytes (33), and an ability of HCMV to regulate its release from endothelial cells to times of close cell-to-cell contact would serve to maximize monocyte infection.
In summary, during HCMV infection expression of the mimic-phosphorylated form of gB (gBAsp900) resulted in an increased targeting of gB to the TGN. This observation was consistent with the previously observed interaction between the phosphorylated form of gB and PACS-1, a cellular connector involved in the retrieval of cargo proteins to the TGN. Expression of the gBAsp900 molecule during HCMV infection also increased production of progeny virus, a finding which further supports the TGN as a site of virion assembly. The precise function of gB phosphorylation in the HCMV replication cycle is currently unclear. However, the ability to modulate intracellular trafficking of an essential glycoprotein by controlling interactions with the cellular secretory machinery may be important for the regulation of various aspects of the HCMV assembly and egress such as virus release and cell-to-cell spread.
Acknowledgments
We are grateful to Aurelie Snyder at the Oregon Health Sciences University Core Laboratory Facility and to Shi-Wu Lee and Jennifer Kindler at Wyeth Research for technical support. We are also thankful to Hanna Välimaa and Sean Molloy for critical review of the manuscript and to Andrew Townsend at Extreme Images for assistance with graphic illustrations.
This study was supported by funding from the Public Health Service (W.J.B., NIAID) and the National Institutes of Health (AI21640 and AI10418 [J.A.N. and M.A.J.]).
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick, C. J., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppul82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein 5. J. Virol. 72:1826-1833. [DOI] [PMC free article] [PubMed]
- 6.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brideau, A. D., T. del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump, C. M., C.-H. Hung, L. Thomas, L. Wan, and G. Thomas. 2003. Role of PACS-1 in the trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77:11105-11113. [DOI] [PMC free article] [PubMed]
- 10.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish, K. N., C. Söderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218-232. [DOI] [PubMed] [Google Scholar]
- 13.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean, F., L. Thomas, S. S. Molloy, G. Liu, M. A. Jarvis, J. A. Nelson, and G. Thomas. 2000. A protein-based therapeutic for human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 97:2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, T. R., and V. P. Muzithras. 1991. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J. Virol. 65:2024-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, T. R., V. P. Muzithras, and Y. Gluzman. 1991. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J. Virol. 65:5860-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerry, J. A., M. A. Priddy, and R. M. Stenberg. 1994. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J. Virol. 68:4167-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall, G. S., D. P. Fenger, G. G. Stout, M. E. Knights, and L. A. Hunt. 1996. Processing of human cytomegalovirus glycoprotein B in recombinant adenovirus-infected cells. J. Gen. Virol. 77:1549-1557. [DOI] [PubMed] [Google Scholar]
- 24.Mocarski, E. S. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In B. N. Fields (ed.), Virology, 4th ed., vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 25.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9:28-35. [DOI] [PubMed] [Google Scholar]
- 26.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 27.Piper, R. C., and J. P. Luzio. 2001. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2:612-621. [DOI] [PubMed] [Google Scholar]
- 28.Ponnambalam, S., C. Rabouille, J. P. Luzio, T. Nilsson, and G. Warren. 1994. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J. Cell Biol. 125:253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streblow, D., C. Soderberg-Naucler, J. Vieira, P. Smith, F. Ruchti, K. Mattison, and J. A. Nelson. 1999. Vascular smooth muscle cell migration is induced by the human cytomegalovirus chemokine receptor US28. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 32.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 33.Waldman, W. J., D. A. Knight, E. H. Huang, and D. D. Sedmak. 1995. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J. Infect. Dis. 171:263-272. [DOI] [PubMed] [Google Scholar]
- 34.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 35.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]