Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5% of urothelial malignancies [1]. The disease-free survival for patients with upper tract tumors is variable and ranges from 45–90%. Survival probabilities for patients undergoing nephroureterectomy are closely tied to disease stage and grade [2]. Patients diagnosed with pTa or pT1 disease have a 90% or higher cure rate with surgery compared to only 40% of those with pT3 disease [3,4].
In the absence of clinical lymphadenopathy or metastatic disease, it is difficult to clinically stage UTUC because biopsy specimens can only reveal tumor grade, not tumor invasion. Traditionally, all patients with upper tract tumors were treated with extirpative surgery. Advances in endoscopic technology have permitted successful management for selected patients using ureteroscopy or percutaneous surgery, but nephroureterectomy remains the standard of care for large volume or high grade lesions in patients with bilaterally functioning kidneys [5].
Despite extirpative surgery, disease-specific survival for high-grade disease is only 50–60% at five years, whereas five-year disease-specific survival is greater than 80% and 90%, respectively, for grades 2 and 1 [6,7]. High-grade disease has an 82% association with disease burden ≥pT2, itself a strong negative prognostic factor for survival [3,7]. The positive effects of neoadjuvant chemotherapy on advanced bladder cancer has prompted many to examine the effects of treating locally advanced or metastatic UTUC with perioperative chemotherapy [4,8,9]. Neoadjuvant chemotherapy has the advantage of providing potential prognostic information based on the response of the primary tumor, and the presence of both kidneys maximizes the oncologist’s ability to deliver effective cytotoxic doses of chemotherapy.
Opponents of neoadjuvant chemotherapy express a concern that the use of neoadjuvant chemotherapy delays extirpative surgery and can result in decreased survival. In addition, some urologists remain reluctant to utilize initial endoscopic management over immediate nephroureterectomy. In this study, we sought to evaluate whether such a delay in time to extirpative surgery had any effect on survival of these patients with UTUC.
Patients and Methods
This study was performed with the approval and oversight of the local Institutional Review Board for the Protection of Human Subjects. Retrospective chart review identified a total of 247 patients with UTUC treated with nephroureterectomy or ureterectomy at the University of Texas MD Anderson Cancer Center between 1990 and 2007. Patients with evidence of distant metastases at presentation were excluded from this analysis (n=7). The indications for surgery were large volume, high grade, tumor invasion into the muscularis propria, or Ta, T1, or carcinoma in-situ refractory to ureteroscopic resection with or without BCG therapy.
Clinical tumor stage was determined by the highest tumor stage on endoscopic specimen or cytology obtained prior to nephroureterectomy or ureterectomy. Specialized genitourinary pathologists examined all specimens according to local institutional procedure. All slides were re-examined for this study to ensure homogeneity and standard pathologic readout. Clinical and pathologic stage was assigned according to the 1997 TNM staging system. Pathologic grade was classified according to the 2004 WHO Classification system.
Patients were divided into two groups based on the timing from presentation to nephrouretectomy or ureterectomy. Patients in the “early” surgery group underwent nephroureterectomy or ureterectomy less than three months after diagnosis. Patients in the “delayed” surgery group underwent surgery three months or more after diagnosis. The cut-point of 3 months was selected based on previous reports which declared 3 months or more as a delay in time to definitive therapy for upper tract and bladder urothelial carcinoma [10,11]. Selected patients in each group also underwent neoadjuvant chemotherapy. The chemotherapeutic regimen of choice included 4–6 cycles of dose-dense methotrexate, vinblastine, adriamycin, and cisplatin (M-VAC) [12]. Determination of timing of surgery and receipt of neoadjuvant treatment was based on individual decision-making between patients and their respective physicians. Surgical approach (open or laparoscopic) and the extent of lymphadenectomy was based on surgeon preference.
Surveillance after (nephro)ureterectomy included routine history, physical, and cystoscopy every 3 months in the first post-operative year, every 6 months in years 2–3, and yearly thereafter. For patients with high-grade or invasive disease, urine cytology, serum chemistries, and liver enzymes were obtained at the same schedule. Evaluation of the contralateral urinary tract (and remnant ureter when applicable) was by retrograde pyelography or ureteroscopy on a biannual or yearly basis. Abdominal and pelvic CT or MRI was performed every 6 months in the first 2 post-operative years and yearly in the next 3 years for patients with high-grade or invasive disease. Bone scan was performed in the setting of symptoms or elevated alkaline phosphatase.
From the chart review database, descriptive statistics were obtained on the following variables: age at diagnosis; gender; race; tumor location, grade, and stage; performance status; surgical factors; time from diagnosis to surgery; receipt of neoadjuvant therapy; and recurrence and survival outcomes. The interval of time from diagnosis to nephroureterectomy was analyzed as both a continuous and categorical variable. The association between categorical data was tested using the Chi-square test or Kruskal-Wallis test. Differences in continuous variables across nonparametric variables were tested using the Mann-Whitney U test. Survival outcomes and time to recurrence were evaluated from the date of surgery. Kaplan-Meier survival analyses were used to determine recurrence-free, disease-specific, and overall survival. The disease-free survival between the two groups was compared using log-rank analysis. Univariate and multivariate analyses were performed with Cox-proportional hazards model. The level of statistical significance in this study was set at p<0.05 and all reported p values were two-sided. All analyses were performed with SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL).
Results
All Patients
Tumor and treatment characteristics for all patients are summarized in Table I. Of 240 patients studied, 186 (78%) patients received definitive surgical treatment less than three months from diagnosis, and 54 (22%) patients underwent surgery at least 3 months after diagnosis. Nephroureterectomy was performed in 226 (94%) patients and ureterectomy in the remainder. Among all patients’ tumors, 21% were histologically low grade, and 79% were high grade. Stage was pT2 or higher in 50% of patients. Pathologic features such as CIS, lymphovascular invasion, and necrosis within the tumor were present in 42%, 31%, and 34% of patients, respectively. Multifocality characterized 41% of tumors. Tumors were located in the renal pelvis 58% of the time and in the proximal ureter in 36% of cases.
Table I.
Patient, Tumor, and Treatment Characteristics
| All Patients (n = 240) |
Early Surgery (n = 186) |
Delayed Surgery (n = 54) |
P | |
|---|---|---|---|---|
| Median Age (IQR) | 69 (16) | 67 (17) | 69 (14) | |
| Median months follow-up (IQR) | 29 (46) | 35 (51) | 19 (28) | <0.0001 |
| Mean Delay to Surgery (mo) | -- | 0.8 | 14.4 | <0.0001 |
| Gender (%) | ||||
| Male | 157 (65) | 119 (64) | 38 (70) | 0.3868 |
| Female | 83 (35) | 67 (36) | 16 (30) | |
| Race (%) | ||||
| White | 210 (88) | 161 (87) | 49 (91) | 0.5845 |
| Black | 10 (4.2) | 9 (4.8) | 1 (1.9) | 0.3297 |
| Other | 20 (7.8) | 16 (8.2 | 4 (7.4) | 0.7809 |
| Performance Status (%) | ||||
| 0 | 137 (57) | 109 (59) | 28 (52) | 0.3797 |
| 1 | 102 (43) | 77 (41) | 25 (46) | 0.5325 |
| 2 | 1 (0.4) | 0 (0) | 1 (1.9) | |
| Clinical Nodal stage (%) | ||||
| N1 | 13 (5.4) | 8 (4.3) | 5 (9.3) | 0.1578 |
| N2 | 17 (7.1) | 12 (6.5) | 5 (9.3) | 0.4810 |
| N3 | 0 | 0 (0) | 0 (0) | |
| Pathologic Node Positive (%) | 30 (8.8) | 21 (11.3) | 9 (16.7) | 0.2949 |
| Pathologic T stage (%) | ||||
| pT0 | 4 (1.7) | 1 (0.5) | 3 (5.6) | 0.0111 |
| pTis | 15 (6.3) | 9 (4.8) | 6 (11) | 0.0640 |
| pTa | 47 (20) | 40 (21.5) | 7 (13) | 0.1651 |
| pT1 | 54 (23) | 42 (22.5) | 12 (22) | 0.9559 |
| pT2 | 40 (17) | 29 (15.6) | 11 (20) | 0.4089 |
| pT3 | 67 (28) | 56 (30.1) | 11 (20) | 0.1616 |
| pT4 | 13 (5.4) | 9 (4.8) | 4 (7.4) | 0.4649 |
| High Grade (%) | 189 (79) | 147 (79) | 42 (78) | 0.8435 |
| Tumor Location (%) | ||||
| Renal Pelvis | 140 (58) | 114 (61) | 26 (48) | 0.0853 |
| Proximal Ureter | 87 (36) | 63 (34) | 24 (44) | 0.1561 |
| Distal Ureter | 13 (5) | 9 (5) | 4 (8) | 0.4649 |
| Mean Tumor Size (cm) | 3.4 | 3.8 | 2.8 | 0.2284 |
| Necrosis (%) | 82 (34) | 64 (34) | 18 (33) | 0.8643 |
| Lymphovascular Invasión (%) | 74 (31) | 60 (32) | 14 (26) | 0.3650 |
| CIS (%) | 101 (42) | 72 (39) | 29 (54) | 0.0497 |
| Multifocal Tumor (%) | 98 (41) | 74 (40) | 24 (44) | 0.5417 |
| Mean No. Lymph Nodes Removed | 4.4 | 4.1 | 5.3 | 0.2008 |
| Neoadjuvant Chemotherapy (%) | 33 (14) | 6 (3.2) | 27 (50) | <0.0001 |
| Coincident bladder cancer | ||||
| All Types (%) | 132 (55) | 101 (54) | 31 (57) | 0.6878 |
| High Grade (%) | 83 (35) | 58 (57) | 25 (81) | 0.3548 |
| Stage ≥ T2 (%) | 18 (8) | 16 (16) | 2 (7) | 0.1746 |
Thirty-three patients received neoadjuvant chemotherapy: Of the 54 patients undergoing delayed surgery, 50% (27) were delayed because they received neoadjuvant chemotherapy. Six of the 33 patients who received neoadjuvant chemotherapy were not included in the delayed surgery group since they did not complete all of their treatment cycles and instead underwent early surgery. An additional 9/54 (17%) in the delayed group were surveilled and managed endoscopically. Other reasons that contributed to delayed surgery included referral patterns, medical co-morbidities, decisions to proceed with surgery based on radiographic response and stability of disease following chemotherapy. Of patients in the early group, 4/186 (2%) underwent initial endoscopic management. Of patients undergoing early surgery, 61% of tumors were in the renal pelvis, whereas 48% of tumors were in the renal pelvis among patients undergoing delayed surgery. Lymphadenectomy was performed in 133 (55%) of 240 patients, including 33 (61%) patients who underwent delayed surgery. The mean number of lymph nodes resected per patient overall and in the delayed surgery groups were 4.0 and 4.9, respectively.
Incidentally, 38 of the 240 studied patients also underwent adjuvant chemotherapy (15.8%). In the early surgery group, 30 of 186 patients received adjuvant chemotherapy (16.1%), as did 8 of the 54 patients who underwent delayed surgery (14.8%). A single patient in the early surgery group received both adjuvant and neoadjuvant chemotherapy, as did four patients in the delayed surgery group. All five patients who got both neoadjuvant and adjuvant treatment had node-positive disease and underwent open nephroureterectomy. Of this small group of five, two patients (who underwent delayed surgery group) were still alive at follow up, including a single patient who did not relapse.
The early surgery group had slightly better ECOG performance status, with 59% having a PS of 0, compared to 52% in the delayed surgery group. Median follow-up (range) was 35 (0–186) months in the patients who underwent early surgery and 19 months (3–134) in the delayed surgery group. Patient and tumor characteristics were otherwise similar between the two groups of patients.
On univariable analysis, pathologic tumor stage, grade, presence of tumor necrosis, lymphovascular invastion, and worse ECOG performance status were significantly associated with death from disease. In multivariable model, pathologic stage, ECOG performance status, and lymphovascular invasion retained statistical significance. Delay in treatment was not associated with a worse outcome alone or following adjustment for potential confounding factors, including pathologic stage.
Overall, 61 (25%) of 240 patients experienced disease relapse. This included 25.3% of the early group and 25.9% of the delayed surgery group. Overall, there were no differences between the early and delayed surgery groups with respect to recurrence-free, disease-specific, and overall survival (Figure I). The 5-yr cumulative disease-specific survival in the early and delayed groups was 71.6% (95%CI 63–78.4%) and 70.6% (95%CI 49–84%), respectively (p=NS). The 5-yr cumulative overall survival was also similar: 59.5% (95%CI 51–67%) in the early group and 69% (95%CI 48–83%) in the delayed group, respectively (p=NS).
Figure I.
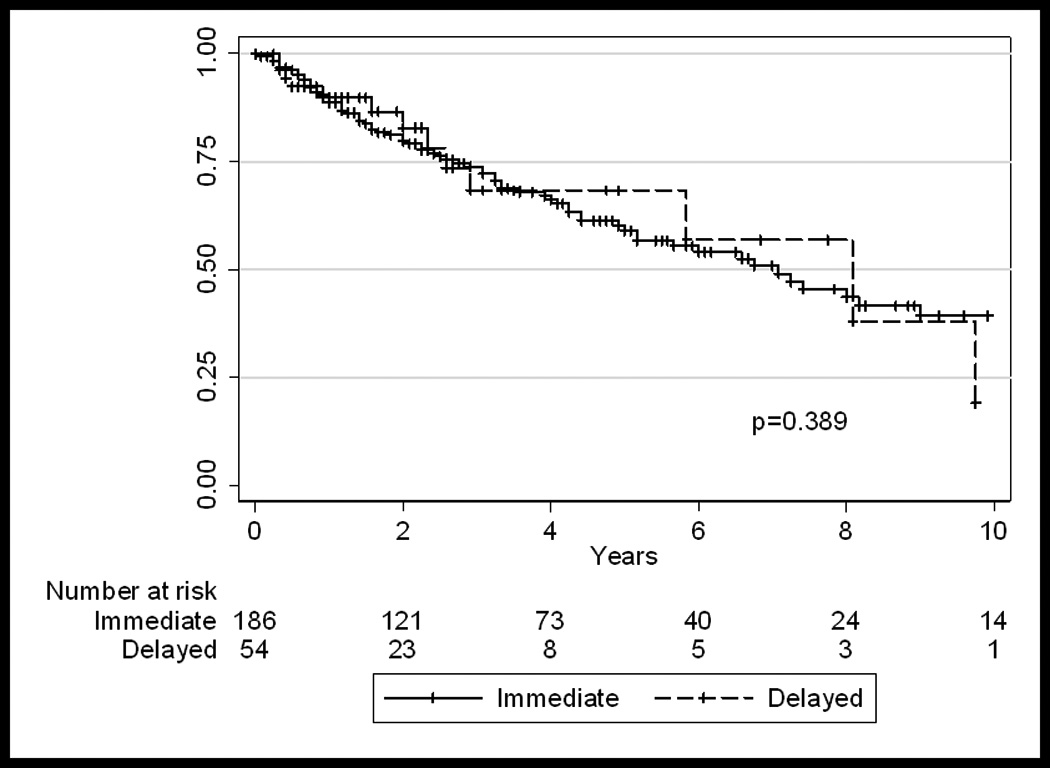
Overall-survival in all patients (n=240), who underwent early (<3 months) or delayed (≥3 months) extirpative surgery.
Patients not Receiving Neoadjuvant Treatment
While the primary goal of our report is to study the effects of time to surgery, since a significant proportion of patients received chemotherapy – and administration of chemotherapy could obviously have been an independent determinant of patient outcomes – we repeated the analyses after excluding patients who underwent neoadjuvant chemotherapy. Overall, 33 (14%) of 210 of patients received neoadjuvant chemotherapy. 27 of the 33 patients who underwent neoadjuvant chemotherapy fell into the delayed surgery group, thus comprising 50% of the 54 patients who underwent delayed surgery.
On univariable analysis, pathologic tumor stage, grade, presence of tumor necrosis, lymphovascular invasion, and worse ECOG performance status were significantly associated with death from disease. On multivariable analysis, only lymphovascular invasion and ECOG performance status were significantly associated with death from disease. Delay in treatment was not associated with a worse outcome alone or following adjustment for potential confounding factors.
Excluding patients who received neoadjuvant chemotherapy, five-year recurrence-free, disease-specific, and overall survivals were similar between the groups of patients receiving early versus delayed surgical treatment (Figure II). The 5-yr cumulative disease-specific survival in the early and delayed groups was 71.6% (95%CI 63–79%) and 81.5% (95%CI 52–94%), respectively (p=NS). The 5-yr cumulative overall survival was also similar: 61.3% (95%CI 53–69%) in the early group and 77% (95%CI 50–91%) in the delayed group, respectively (p=NS).
Figure II.
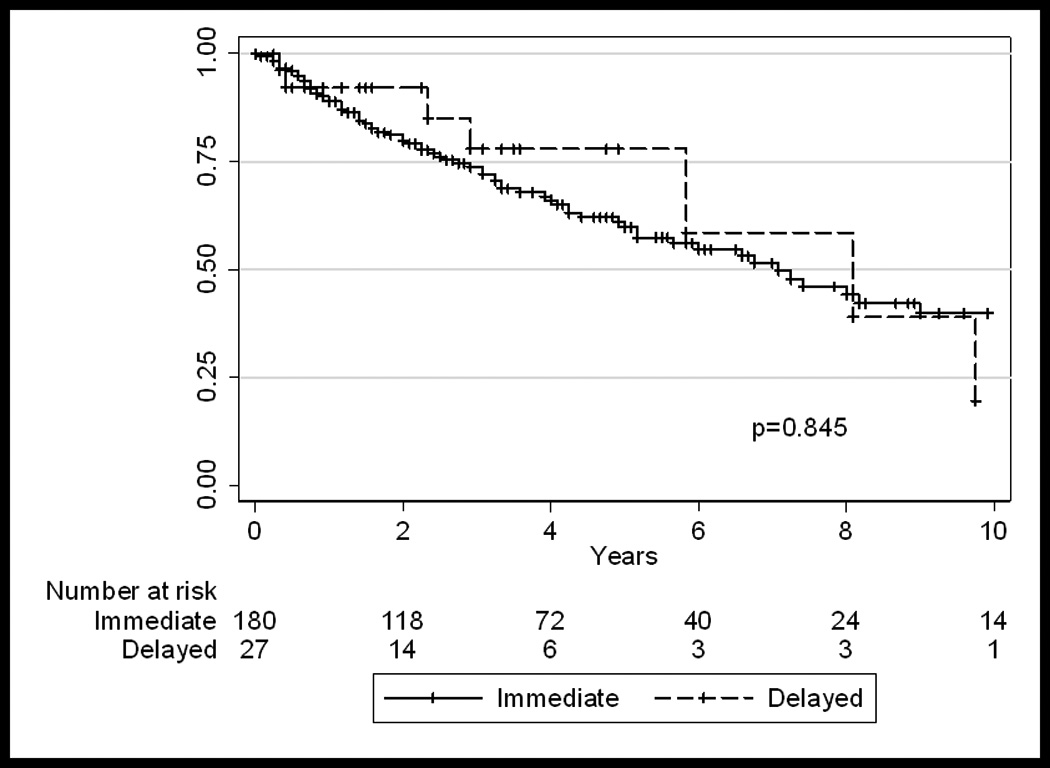
Overall-survival of patients who did not receive neoadjuvant chemotherapy (n=207), who underwent early or delayed surgery.
Discussion
We identified no difference in survival between patients treated with immediate nephroureterectomy/ureterectomy compared to those whose extirpative surgery was delayed by 3 or more months from the time of presentation.
Nephroureterectomy is considered the gold standard for high grade, invasive, or bulky upper tract neoplasms [13]. Select patients with disease that is low volume and low grade may experience durable disease control from endoscopic management [14–17]. For example, Boorjian et al. retrospectively examined a series of 155 patients and showed no survival detriment in 12 patients whose nephrouretercotomies were delayed by a mean of 196 days after ureteroscopic biopsy due to management by laser ablation [17]. The comparison groups in this study were 75 patients whose nephroureterectomies were delayed by a mean of 28 days after ureteroscopic biopsy, and 34 patients who underwent surgery on the basis of imaging and cytology findings alone. These and other findings suggest that the delay from ureterocopic management may not be detrimental in UTUC, whereas delayed radical cystectomy for bladder cancer, however, has been shown to be detrimental [10,18,19]. Our study sought to assess the safety of delayed surgery in UTUC in the context of ureteroscopic management or neoadjuvant chemotherapy.
In this study the most common reason for a delay in surgery was the use of neoadjuvant chemotherapy. A limited number of studies have directly evaluated the role of neoadjuvant chemotherapy in patients with UTUC. Igawa et al. reported a series of 15 patients treated with neoadjuvant cisplatin-based chemotherapy: 8 patients responded and 2 patients’ tumors were downstaged to pT0 [20]. Despite the limited data regarding efficacy of neoadjuvant chemotherapy for UTUC, there are compelling reasons for its use in this population. Neoadjuvant chemotherapy may downstage tumors and may treat micro-metastatic disease that is missed by conventional staging [21]. A strategy to downstage disease prior to surgery may improve outcomes in UTUC. We have previously shown that surgery alone for ≥pT2 disease resulted in a 10-year disease-specific survival of only 65%, though the corresponding survival rate was 87% for patients <pT2 [6]. Because radical nephroureterectomy is often the surgical treatment of choice in UTUC, and patients are often advanced in age or have underlying chronic renal insufficiency; a neoadjuvant approach allows the administration of full, effective doses of cytotoxic therapy [21,22].
The failure of current surgical and medical treatments to improve UTUC outcomes, especially for high grade and ≥pT2 disease, obligates that the standard treatment paradigm change to an approach that identifies neoadjuvant candidates by tumor grade on pre-surgical ureteroscopic biopsy [2,6,22]. A significant survival advantage has been demonstrated for patients receiving neoadjuvant cisplatin-based chemotherapy for bladder cancer [12,21]. Randomized controlled trials to properly address the use of neoadjuvant chemotherapy in UTUC may be impractical, but similarities between the histopathology of UTUC and bladder cancer may indicate that responses to cytotoxic treatment may be comparable.
While our study is not designed to measure the effect related to neoadjuvant chemotherapy compared to surgery alone (which would be best done in a prospective fashion), our findings might at least partially allay fears that administration of neoadjuvant chemotherapy in UTUC may cause worse outcome because of the delay to surgery. Our study does have a selection bias in that patients who are selected for neoadjuvant chemotherapy are thought to usually have worse disease at presentation. Seeing that outcomes are equivalent between groups, in this light, makes the prospect of delaying surgery more reassuring.
It is worth emphasizing the point that a delay in surgery in the patients we studied did not necessarily mean a delay in treatment. Half of the delayed patients received neoadjuvant chemotherapy, and still others were managed endoscopically. Based strictly on our results, a delay in surgery (for any reason) may actually be safe. Based on our intuition we only recommend delaying surgery in the setting of an interim treatment such as neoadjuvant chemotherapy or endoscopic management.
We acknowledge certain critical limitations of this study. A selection bias for deciding timing of surgery is inherent in that decisions were made by each patient and his or her respective surgeon. A related limitation is that, due to the difficulty in clinically staging upper tract tumors, the analyses grouped pathologic low- and high-stage disease when comparing the early and delayed surgery cohorts. There was a discrepancy in follow-up time: median was 29 months overall and just 19 months in the delayed surgery group. This difference is likely consequent to temporal practice patterns, as neoadjuvant chemotherapy and delayed surgery have become more common in recent years. It should also be noted that the power of our analyses to detect outcome differences between the early (n=186) and delayed (n=54) groups was attenuated by the disparity in size between those groups. Furthermore the referral-based nature of our practice caused specific limitations in treatment data prior to extirpative surgery, such as number of endoscopic procedures per patient. Finally, while in our practice most patients received pre-operative cisplatinum based therapy, this report was not intended to address issues specific to chemotherapy – whether neoadjuvant or adjuvant – nor to compare chemotherapeutic regimens.
Conclusions
Patients diagnosed with UTUC can experience a time lag from presentation to extirpative surgery. This analysis found no difference in survival between patients undergoing immediate versus delayed nephroureterectomy surgery. While our observations may allay concerns regarding delaying extirpative surgery for appropriate reasons such as initial endoscopic management or to allow time for the use of neoadjuvant chemotherapy, these findings await independent prospective evaluation to determine their ultimate clinical significance.
Table II.
Regression Analyses – Univariate
| All Patients | Patients not Receiving Neoadjuvant Treatment |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.02 | 0.99, 1.04 | 0.18 | 1.02 | 0.99, 1.05 | 0.17 |
| Male Gender | 0.81 | 0.46, 1.44 | 0.48 | 0.67 | 0.37, 1.23 | 0.20 |
| ECOG Performance Status | 2.09 | 1.19, 3.66 | 0.01 | 2.02 | 1.10, 3.69 | 0.02 |
| High Stage | 6.67 | 3.13, 14.22 | <0.01 | 6.73 | 2.99, 15.12 | <0.01 |
| High Grade | 4.34 | 1.56, 12.08 | 0.01 | 5.39 | 1.67, 17.45 | 0.01 |
| Surgery | 0.33 | 0.05, 2.38 | 0.27 | 0.35 | 0.05, 2.53 | 0.30 |
| Delayed Surgery | 1.07 | 0.52, 2.22 | 0.14 | 0.60 | 0.19, 1.94 | 0.40 |
| Multifocal Disease | 1.53 | 0.88, 2.66 | 0.14 | 1.53 | 0.84, 2.79 | 0.16 |
| CIS | 1.50 | 0.86, 2.62 | 0.15 | 1.33 | 0.73, 2.43 | 0.35 |
| Necrosis | 3.07 | 1.75, 5.40 | <0.01 | 2.87 | 1.56, 5.26 | <0.01 |
| Lymphovascular Invasion | 7.69 | 4.19, 14.13 | <0.01 | 7.60 | 3.95, 14.62 | <0.01 |
Table III.
Regression Analyses – Multivariate
| All Patients | Patients not Receiving Neoadjuvant Treatment |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| ECOG Performance Status | 2.04 | 1.16, 3.58 | 0.01 | 1.87 | 1.01, 3.47 | 0.05 |
| High Stage | 2.93 | 1.10, 7.79 | 0.03 | 2.70 | 0.95, 7.62 | 0.06 |
| High Grade | 0.83 | 0.23, 2.93 | 0.77 | 1.17 | 0.29, 4.78 | 0.82 |
| Delayed Surgery | 1.54 | 0.73, 3.25 | 0.25 | 0.94 | 0.28, 3.08 | 0.92 |
| Necrosis | 1.18 | 0.63, 2.19 | 0.60 | 1.15 | 0.60, 2.21 | 0.68 |
| Lymphovascular Invasion | 4.34 | 2.01, 9.38 | <0.01 | 3.84 | 1.70, 8.67 | <0.01 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmauz R, Cole P. Epidemiology of cancer of the renal pelvis and ureter. J Natl Cancer Inst. 1974;52:1431–1434. doi: 10.1093/jnci/52.5.1431. [DOI] [PubMed] [Google Scholar]
- 2.Brown GA, Matin SF, Busby JE, Dinney CP, Grossman HB, Pettaway CA, Munsell MF, Kamat AM. Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: implications for therapy. Urology. 2007;70:252–256. doi: 10.1016/j.urology.2007.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 4.Bamias A, Deliveliotis C, Fountzilas G, Gika D, Anagnostopoulos A, Zorzou MP, Kastritis E, Constantinides C, Kosmidis P, Dimopoulos MA. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: a study by the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22:2150–2154. doi: 10.1200/JCO.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Lake AM, Roberts WW. Surgical management of upper tract transitional cell carcinoma. J Natl Compr Canc Netw. 2006;4:1015–1018. doi: 10.6004/jnccn.2006.0084. [DOI] [PubMed] [Google Scholar]
- 6.Brown GA, Busby JE, Wood CG, Pisters LL, Dinney CP, Swanson DA, Grossman HB, Pettaway CA, Munsell MF, Kamat AM, et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: Time to change the treatment paradigm? BJU Int. 2006;98:1176–1180. doi: 10.1111/j.1464-410X.2006.06524.x. [DOI] [PubMed] [Google Scholar]
- 7.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–1525. [PubMed] [Google Scholar]
- 8.Kwak C, Lee SE, Jeong IG, Ku JH. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68:53–57. doi: 10.1016/j.urology.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Bamias A, Moulopoulos LA, Koutras A, Aravantinos G, Fountzilas G, Pectasides D, Kastritis E, Gika D, Skarlos D, Linardou H, et al. The combination of gemcitabine and carboplatin as first-line treatment in patients with advanced urothelial carcinoma. A Phase II study of the Hellenic Cooperative Oncology Group. Cancer. 2006;106:297–303. doi: 10.1002/cncr.21604. [DOI] [PubMed] [Google Scholar]
- 10.Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, Wood JDP. Cystectomy Delay More Than 3 Months From Initial Bladder Cancer Diagnosis Results in Decreased Disease Specific and Overall Survival. The Journal of Urology. 2006;175:1262–1267. doi: 10.1016/S0022-5347(05)00644-0. [DOI] [PubMed] [Google Scholar]
- 11.Lucas SM, Svatek RS, Olgin G, Arriaga Y, Kabbani W, Sagalowsky AI, Lotan Y. Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int. 2008 doi: 10.1111/j.1464-410X.2008.07535.x. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg CN. Perioperative chemotherapy in muscle-invasive bladder cancer to enhance survival and/or as a strategy for bladder preservation. Seminars in Oncology. 2007;34:122–128. doi: 10.1053/j.seminoncol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Sagalowsky AIJT. Management of urothelial tumors of the renal pelvis and ureter. Philadelphia: WB Saunders; 2002. pp. 2845–2875. [Google Scholar]
- 14.Daneshmand S, Quek ML, Huffman JL. Endoscopic management of upper urinary tract transitional cell carcinoma: long-term experience. Cancer. 2003;98:55–60. doi: 10.1002/cncr.11446. [DOI] [PubMed] [Google Scholar]
- 15.Keeley FX, Jr, Bibbo M, Bagley DH. Ureteroscopic treatment and surveillance of upper urinary tract transitional cell carcinoma. J Urol. 1997;157:1560–1565. [PubMed] [Google Scholar]
- 16.Lam JS, Gupta M. Ureteroscopic management of upper tract transitional cell carcinoma. Urol Clin North Am. 2004;31:115–128. doi: 10.1016/S0094-0143(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 17.Boorjian S, Ng C, Munver R, Palese MA, Vaughan ED, Jr, Sosa RE, Del Pizzo JJ, Scherr DS. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumor ablation of upper tract transitional cell carcinoma. Urology. 2005;66:283–287. doi: 10.1016/j.urology.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 18.May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231–235. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–115. doi: 10.1016/S0022-5347(05)64047-5. discussion 115. [DOI] [PubMed] [Google Scholar]
- 20.Igawa M, Urakami S, Shiina H, Kishi H, Himeno Y, Ishibe T, Kadena H, Usui T. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol Int. 1995;55:74–77. doi: 10.1159/000282755. [DOI] [PubMed] [Google Scholar]
- 21.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr, Raghavan D, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 22.Kirkalia Z. Re: Nephroureterectomy for treating upper urinary tract transitional cell carcinoma. time to change the treatment paradigm? Eur Urol. 2007;51:1141–1142. doi: 10.1016/j.eururo.2007.01.058. [DOI] [PubMed] [Google Scholar]


