Abstract
Numerous studies have demonstrated that consuming high calorie food leads to subsequent overeating by chronic dieters. The present study investigates the neural correlates of such self-regulatory failures using functional magnetic resonance imaging (fMRI). Chronic dieters (N=50) and non-dieters (N=50) consumed either a 15-oz glass of cold water or a 15-oz milkshake and were subsequently imaged while viewing pictures of animals, environmental scenes, people, and appetizing food items. Results revealed a functional dissociation in nucleus accumbens (NAcc) and amygdala activity that paralleled well-established behavioral patterns of eating observed in dieters and non-dieters. Whereas non-dieters showed the greatest NAcc activity in response to food items following water consumption, dieters showed the greatest activity after consuming the milkshake. Activity in the left amygdala demonstrated the reverse interaction. Considered together with previously reported behavioral findings, the present results offer a suggested neural substrate for diet failure.
Obesity is a major health problem with both physical and psychological consequences, yet treatment success remains elusive. Nationally representative samples indicate that obesity rates have increased dramatically in the United States from fewer than 15% of the population meeting criteria for obesity in 1980 (body mass index [BMI] ≥ 30) to more than 33% meeting criteria in 2004 (Ogden, et al., 2006). Moreover, current estimates suggest that nearly 67% of the US population is overweight or obese (Ogden, et al., 2006). As such, there have been urgent calls for research to understand the epidemic of obesity and how it can be prevented or treated (Volkow & O'Brien, 2007). This manuscript examines the neural basis of one possible reason that obesity remains an entrenched problem, namely, maladaptive responses to dietary violations.
An interesting feature of the apparent obesity epidemic is that it is occurring even while most people are familiar with the health risks of excessive body weight and many have a strong desire to lose weight. Indeed, many are actively dieting in attempts to lose weight or at least deter weight gain. For instance, in one study more than two-thirds of women and more than half of men reported wanting to lose weight (Heatherton, Mahamedi, Striepe, Field, & Keel, 1997). Similarly, one study of a large representative sample found that 29% of men and 44% of women were currently dieting to lose weight, with an equal number controlling eating in attempt not to gain weight (Serdula, et al., 1999). Although many people diet in an attempt to lose weight, there are reasons to be pessimistic about the extent to which they are able to maintain weight loss, regardless of the diet’s nutritional emphasis (Mann, et al., 2007; Sacks, et al., 2009). The typical pattern is that people tend to lose weight within the first months on a diet, but within a year or two they return to their original weight due to steady weight regain, or they even end up weighing more than when they began dieting (Aronne, Wadden, Isoldi, & Woodworth, 2009; Dansinger, Tatsioni, Wong, Chung, & Balk, 2007). Thus, it is clear that dieters can lose weight over the short term, presumably by controlling food intake, yet various factors appear to sabotage their weight loss efforts over the long term. Likewise, the continuing increase in the rate of obesity implies that many people are unable to follow diets that prevent weight gain. Thus, it is of crucial importance to understand the factors that interfere with dietary success and thereby contribute to obesity. The goal of this research was to identify neural patterns of activity associated with dietary violations. Dieting is common among people who are not clinically obese and the behavioral patterns following dietary violations are similar between the two groups (Baumeister, Heatherton, & Tice, 1994). Here we assess neural activity in chronic dieters who are not obese because we are primarily interested in the response to dietary violations rather than obesity per se.
Insight into diet failure has been obtained through behavioral research examining eating in the laboratory. In one of the first studies of its kind, Herman and Mack (1975) experimentally violated the diets of dieters by requiring them to eat a high calorie food as part of a supposed perception study. College-aged females (dieters and non-dieters, as assessed by a self-report measure of frequent dieting) participated in a “taste test” experiment that was represented to subjects as a study of the influence of one sensory experience upon another. Subjects were told that they would have from zero to two taste experiences in the form of a milkshake before sampling flavors of ice cream and that the experimenters were interested in the taste perception of the flavors. In reality, the taste ratings were of no consequence and the milkshake preload was intended to disrupt the dieters’ diets. Following the mock taste test, the total amount of ice cream consumed was covertly measured. Although non-dieters ate less after consuming the milkshakes, presumably because they were full, dieters paradoxically consumed the most ice cream after having the milkshake preload. This disinhibition of dietary restraint has been replicated numerous times (Heatherton & Baumeister, 1991; Herman & Polivy, 2004) and demonstrates that dieters often eat a great deal after they perceive their diets to be broken. Indeed, chronic dieters often oscillate between periods of restraint, marked by restricted caloric intake, and bouts of excessive caloric intake, or disinhibited overeating, that thwarts their dietary goals (Heatherton, Herman, & Polivy, 1991, 1992; Heatherton, Polivy, & Herman, 1989; Herman & Mack, 1975; Herman & Polivy, 1975; Polivy, Heatherton, & Herman, 1988). Thus, dietary disinhibition is likely one of the key components of why many people struggle with their weight (Heatherton & Polivy, 1992; Polivy & Herman, 1987).
To study the neural correlates of diet failure, we adopted a cue-reactivity paradigm that has been successfully implemented in studies of appetitive behaviors (Passamonti, et al., 2009; Rothemund, et al., 2007). Both human and animal studies have demonstrated that exposure to drug cues increases the likelihood that the cued substance will be consumed (Drummond, Cooper, & Glautier, 1990; Glautier & Drummond, 1994; Jansen, 1998; Stewart, de Wit, & Eikelboom, 1984), and additionally increases cravings, attention, and physiological responses such as changes in heart rate (Drobes & Tiffany, 1997; Payne, Smith, Adams, & Diefenbach, 2006; Stewart, et al., 1984). Recent neuroimaging research has identified a distributed network of brain regions that are active during exposure to relevant drug cues (for reviews see (Jentsch & Taylor, 1999; Wilson, Sayette, & Fiez, 2004). Across a number of studies, activity in the amygdala, hippocampus, nucleus accumbens (NAcc), and ventral tegmental area (VTA) has been observed, perhaps implicating the experience of drug reinforcement and the memory of the learned association between the drug and its rewarding properties. In addition, activity in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and dorsolateral prefrontal cortex (dlPFC) has also been observed in response to rewarding drug cues, and it has been suggested that these responses reflect the craving and cognitive control aspects of drug cue-reactivity (Franken, 2003; Goldstein & Volkow, 2002; See, 2002; Wilson, et al., 2004).
Recent neuroimaging studies assessing reactivity to food cues have also found activity in human reward circuitry, including ventral striatum and mesolimbic and mesocortical dopamine circuits (DelParigi, et al., 2007; Killgore, et al., 2003; Passamonti, et al., 2009; Schur, et al., 2009; Simmons, Martin, & Barsalou, 2005; Small, 2002; Stoeckel, et al., 2008). Some evidence suggests that this response to food cues may be more potent for images of high calorie food (Schur, et al., 2009; Stoeckel, et al., 2008) and that personality traits, such as how sensitive an individual is to the sights and smells of food, relate to differences in the neural response to food cues (Passamonti, et al., 2009). Importantly, obese women exhibit greater activity in reward areas including the NAcc in response to high calorie food items than healthy weight control subjects (Stoeckel, et al., 2008).
An open question, however, is whether responsivity to food cues changes as a function of whether a diet is intact or broken. In the present study, chronic dieters and non-dieters were asked to consume either a 15-oz glass of water or a 15-oz milkshake under the guise that the experimenters were interested in the effects of mouth temperature on signal quality in functional brain imaging. During scanning, subjects viewed images of animals, environmental scenes, people, and food and made simple person perception judgments (i.e., whether there were people present in the image or not). We sought to identify brain regions whose activity mirrored the behavioral patterns of eating observed in the literature, and thus may underlie dietary restraint violations. Given that chronic dieters overeat when their diets are broken, we hypothesize that any event that disrupts the diet will produce heightened reward activity in response to food cues. Specifically, then, we predict that a milkshake preload, known to lead to greater eating among dieters, will produce heightened cue reactivity to food cues in brain reward regions. Conversely, satiating an appetitive state is associated with diminished reward responding. Thus, a similar milkshake given to non-dieters should suppress cue reactivity to food cues in reward regions. We used a between group factorial design with a large sample size to test these hypotheses.
Methods
Subjects
A total of 109 native English-speaking females from the Dartmouth community between the ages of 18 and 35 (mean age = 19 years) participated in this experiment. Participants were classified as dieters or non-dieters as a function of their scores on the Restraint Scale (Herman & Mack, 1975; Polivy, Herman, & Howard, 1988), a well-validated measure that is widely used in the eating literature (for a discussion of its psychometric features see Heatherton, Herman, Polivy, King, & McGree, 1988). We recruited only female participants because men and women differ in how and why they gain and lose weight (Holm-Denoma, Joiner, Vohs, & Heatherton, 2008) and college-aged females are more likely to strive for an ‘ideal’ body weight and thus more apt to exhibit restraint in their eating behaviors (Herman & Mack, 1975). No subjects reported abnormal neurological history and all had normal or corrected-to-normal visual acuity. Each subject provided informed consent in accordance with the guidelines set by the Committee for the Protection of Human Subjects at Dartmouth College, and each subject received either course credit or monetary compensation for participating.
Data from five subjects were excluded due to excessive artifact and noise in imaging data and four subjects were excluded due to obesity (BMI ≥ 30). Obese participants were excluded because of potential neuroanatomical differences that vary as a function of BMI (Gunstad, et al., 2008). Although height and weight was initially reported in a pre-screen interview, these four subjects weighed more than they self-reported when actually weighed on a medical scale following the scan session (which was done because weight feedback may lead to negative affect and subsequent disinhibition, see (McFarlane, Polivy, & Herman, 1998; Stice, Maxfield, & Wells, 2003). Analyses reported herein are therefore derived from a total of 100 participants, which included 50 dieters (25 in the water condition, 25 in the preload condition) and 50 non-dieters (25 in the water condition, 25 in the preload condition).
Apparatus
Imaging was performed on a Philips Intera Achieva 3-Tesla scanner (Phillips Medical Systems, Bothell, WA) with a SENSE (SENSEitivity Encoding) head coil. During scanning, visual stimuli were generated with an Apple MacBook Pro Laptop computer running SuperLab 4.0 software (Cedrus Corporation, San Pedro, CA). An Epson (model ELP-7000) LCD projector was used to display stimuli on a screen positioned at the head end of the scanner bore which subjects viewed through a mirror mounted on top of the head coil. A fiber-optic, light-sensitive key press interfacing with the Cedrus Lumina Box recorded subjects’ responses. Cushions were placed around the head to minimize movement during scanning and increase comfort. Following scanning subjects were tested behaviorally using Apple iMacs running SuperLab software.
Imaging
Anatomic images were acquired using a high-resolution 3-D magnetization-prepared rapid gradient echo sequence (MPRAGE; 60 sagittal slices, TE = 4.6 ms, TR = 9.9 ms, flip angle = 8°, voxel size = 1×1×1 mm). Functional images were collected using T2* fast field echo, echo planar functional images (EPIs) sensitive to BOLD contrast (TR = 2500 ms, TE= 35 ms, flip angle = 90°, 3×3 mm in-plane resolution, sense factor of 2). During each of the four functional runs, 160 axial images (36 slices, 3.5 mm slice thickness, 0.5 mm skip between slices) were acquired allowing complete brain coverage.
Procedure
Subjects took part in a mass testing in which their scores on the Restraint Scale and self-reported height and weight were obtained. This mass testing included numerous unrelated questionnaires, and when individuals were contacted to participate in the present study they were simply informed they were eligible based on their responses to the questionnaires in general and were therefore not aware they were recruited based on dieting status. Consistent with past research (e.g., Heatherton et al., 1991), participants were eligible to participate if they scored over 15 on the Restraint Scale (dieters) or below 12 (non-dieters). In order to reduce potential differences in hunger level and time since the participants’ last meal, each participant was asked to refrain from eating, consuming alcohol or caffeine, and from smoking for two hours prior to the fMRI session. To measure participants’ compliance with these instructions and to assess their current hunger level, immediately prior to scanning each subject provided responses to questions regarding their current state wherein they listed food and drink consumption, activity level, and current hunger level on a 1–5 scale.
Following completion of this ‘current state’ questionnaire, each participant was given a cover story in which she was told that the aim of the present study was to investigate social perception and to test technical methods for increasing fMRI signal in the frontal cortex, an area of the brain that is often implicated in such tasks but is near the sinus cavity and thus highly susceptible to signal loss. Subjects were shown an fMRI image in which there was apparent signal loss in the frontal cortex and were led to believe that, among other things (e.g., biting on a graphite bar), significantly cooling the roof of the mouth (and thus lowering the temperature of the air in nearby sinus space) may produce better signal recovery in this brain area and that the purpose of the study was to test this possibility. All participants were informed that they were in this ‘cold mouth condition,’ and this portion of the cover story did not differ across participants. Critically, however, half of the participants (N = 50; 25 dieters, 25 non-dieters) were then given a 15 oz. [425 g] chocolate milkshake (approximate calories = 885) in order to ‘cool their mouths’, whereas the other half of participants (N = 50; 25 dieters, 25 non-dieters) were given a 15 oz. [425 g] glass of water to ‘cool their mouths’.
Subjects were then scanned while viewing images of animals (100), appetizing food (100), people (100), and environmental scenes (100) in an event-related design (Figure 1). These images were compiled from the Internet and scaled in size using Adobe Photoshop® 7.0 (San Jose, CA) to be 480 by 360 pixels. During scanning subjects were asked to simply determine whether or not each image contained a person and to use key presses to make their responses (left-handed response for ‘non-person’; right-handed response for ‘person’). This was done to both disguise the primary goals of the study and to ensure that participants were attending to the images. Images were presented for 2000 ms followed by a fixation crosshair (500 ms) and were randomly intermixed with jittered periods of fixation (jittered fixation 0 – 15000 ms; mean Inter-trial Interval [ITI] = 3825 ms). Of interest was the neural response to viewing food images in each group of individuals.
Figure 1.
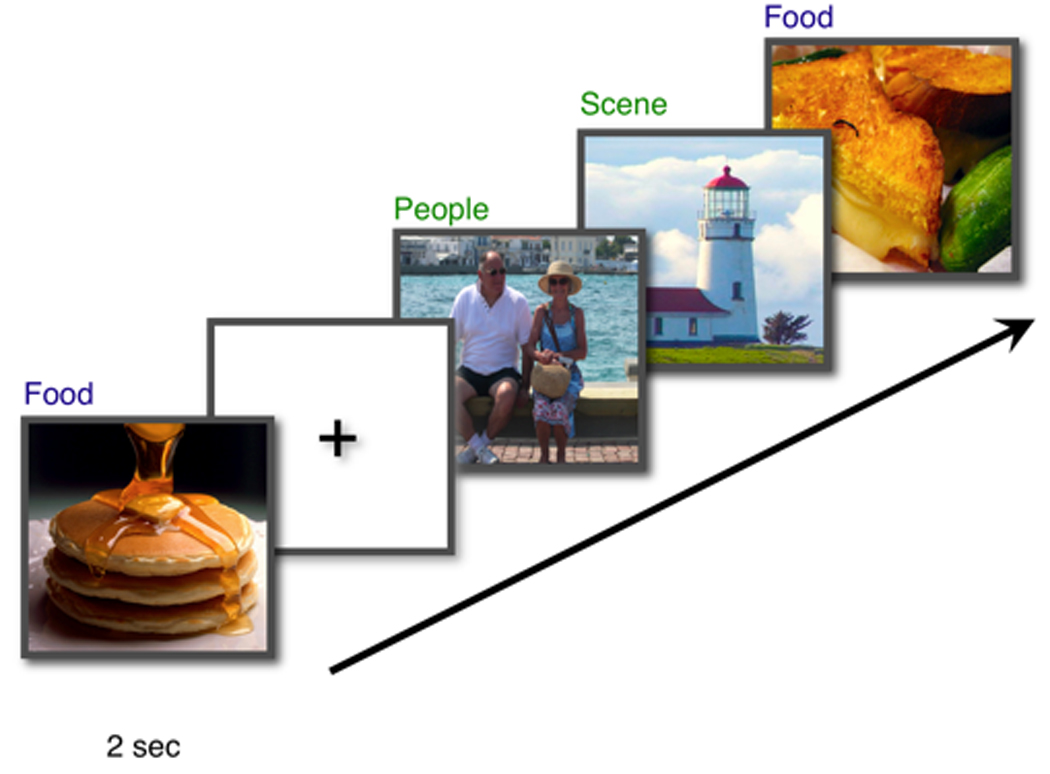
The event-related fMRI paradigm is depicated. Images of food, people, scenes, and animals (not shown) were displayed for 2 seconds each and interspersed with jittered periods of fixation baseline. In total, participants viewed 100 images of animals, 100 images of food, 100 images of people, and 100 images of environmental scenes.
Post-Scanning Behavioral Testing
One day after the scanning session, each subject returned to the lab for behavioral testing, including (1) likeability ratings for all previously viewed stimuli (rated 1–7), (2) detailed journaling of their day following the scanning session, and (3) body measurement (height and weight). The final sample consisted of 100 participants (dieters N = 50, mean RS = 19.6; non-dieters N = 50; mean RS = 8.7).
fMRI Data Analyses
fMRI data were analyzed using Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, London, UK) (Friston, et al., 1995). For each functional run, data were preprocessed to remove sources of noise and artifact. Functional data were realigned within and across runs to correct for head movement, co-registered with each participant’s anatomic data, and transformed into a standard anatomic space (3-mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute), which closely approximates the Talairach & Tournoux (1988) atlas space. Normalized data were then spatially smoothed (6-mm full width at half maximum [FWHM] using a Gaussian kernel, and globally scaled to permit between group comparisons. Analyses of fMRI data took place at two levels for this experiment: formation of statistical images via a whole-brain voxel-wise 2×2 ANOVA, and regional analysis of hemodynamic responses.
For each participant, a general linear model incorporating task effects (modeled as an event-related function convolved with the canonical hemodynamic response function), a mean, and a linear trend were used to compute t-contrast images (weighted parameter estimates) for each trial type at each voxel. Individual contrast images comparing each condition to the baseline control (fixation) were then used to compute a whole-brain voxelwise ANOVAs (with between factors of dietary status [dieters, non-dieters] and preload [milkshake, water]) that yielded F-statistical maps for both main effects and the interaction (thresholded at p < 0.05, corrected for False Discovery Rate, minimum extent threshold: k = 5 contiguous voxels). Functionally defined regions of interest (ROIs: 6mm spheres centered on the peak of activation) were acquired by using an automated peak-search algorithm within these whole-brain F-statistical maps. Given that the F-statistical maps are unidirectional (i.e., there is no information regarding the direction of main effects or interactions) and thus unbiased, parameter estimates were extracted for each subject and each condition and submitted to offline ANOVAs in SPSS to determine the direction, but not magnitude, of such effects.
RESULTS
Participant Information
As per the design of the study, there was a significant difference in restraint score between chronic dieters (M = 19.6, SD = 2.8) and non-dieters (M = 8.7, SD = 2.8; t(98) = 19.5, p < 0.0001), although scores within subject group did not differ by condition (p’s > .10). At the beginning of the study participants indicated their current hunger level and the number of hours since they last consumed food. There were no significant differences between dieters and non-dieters on either measure (see Table 1, Fs < 1). As is typical in the eating literature, the chronic dieters (BMI = 23.5, SD =2.7) were slightly heavier than the non-dieters (BMI = 22.6, SD =2.4), F(3,96) = 3.0 p = 0.086), although there were no differences as a function of preload condition, F < 1.
Table 1.
Summary of participant information for each condition.
| Condition | Mean Weight |
Mean Height |
Mean BMI |
Mean Restraint Score |
Reported lbs – Actual lbs |
Mean Hunger |
Mean Hours Since Meal |
|---|---|---|---|---|---|---|---|
| Diet Water |
141.8 lbs |
64.67 in |
23.84 | 18.6 | 4.78 | 2.7 | 2.74 |
| Non-Diet Water |
130.1 lbs |
63.74 in |
22.50 | 8.88 | 2.5 | 2.90 | 3.20 |
| Diet Shake |
143.8 lbs |
65.48 in |
23.07 | 20.56 | 3.39 | 2.82 | 3.04 |
| Non-Diet Shake |
134.8 lbs |
64.72 in |
22.64 | 8.44 | 2.24 | 2.5 | 3.42 |
Reaction Times and Ratings
Although the classification task participants performed during scanning (“are there people present in the image or not?”) was intended to simply hold participants’ attention and to bolster the idea that the study was concerned with social perception, it is possible that reaction times to the food images could differ as a function of dietary status or preload condition. Importantly, reaction times to the food items did not differ across groups (Figure 2; main effect of restraint: F < 1; main effect of preload: F[3,96] = 1.77, p = 0.19; restraint by preload interaction: F < 1). Thus, any differences between these groups observed in the brain imaging data cannot be attributed to time-on-task effect. Similarly, post-scan likeability ratings for food items did not differ across the four groups (Main effect of restraint: F < 1; main effect of preload: F < 1; restraint by preload interaction: F [3,96] = 1.62, p = 0.21).
Figure 2.
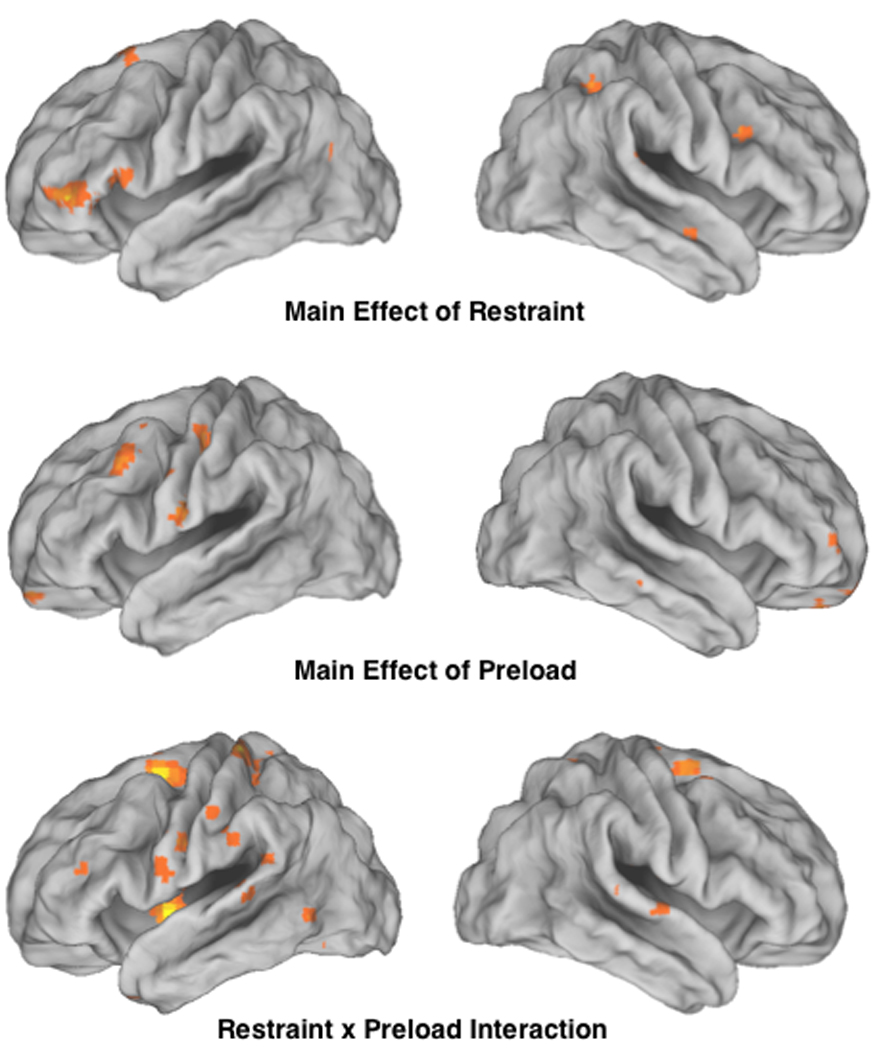
A voxel-by-voxel whole brain ANOVA was used to compute F-statistical maps for the main effect of restraint (top panel), the main effect of preload (middle panel), and the restraint by preload interaction (bottom panel). Statistical images thresholded at p < 0.05, corrected for False Discovery Rate with a minimum extent threshold (k) = 5 contiguous voxels, were superimposed on a fiducial cortical rendering of the left and right cortical surfaces (Van Essen et al., 2001).
fMRI Results
Two analyses were performed in order to investigate the neural response to food items. The first analysis examined neural signatures associated with dietary restraint, preloading, and the interaction between them. Contrast images comparing the response to food items to baseline fixation for each subject were examined using a voxel-wise whole brain ANOVA (2 × 2 between-subjects design with the factors of dietary status (dieters versus non-dieters) and preload (water versus shake). Figure 2 and Table 2 summarize brain regions that revealed a main effect of dietary status, a main effect of preload, and an interaction between dietary status and preload in response to food items. To explore the directionality of each effect, regions identified in the statistical F-maps were examined further using ROI analyses.
Table 2.
Identification of BOLD signal changes in response to food items associated with the main effects of restraint, preload, and the restraint by preload interaction.
| Brain Region | F | x | y | z | |
|---|---|---|---|---|---|
| Main Effect of Dietary Status –Dieters > Non-dieters | |||||
| BA 39 | L Middle Temporal Gyrus | 11.9 | −54 | −72 | 23 |
| BA 47 | L Inferior Frontal Gyrus | 11.3 | −48 | 41 | −2 |
| BA 7 | R Lateral Parietal Sulcus | 10.1 | 36 | −50 | 49 |
| BA 6 | L Superior Frontal Gyrus | 9.4 | −15 | 18 | 60 |
| BA 44/45 | L Inferior Frontal Gyrus | 8.3 | −54 | 19 | 7 |
| Insula | R Insula | 7.1 | 36 | 4 | 14 |
| Main Effect of Dietary Status –Non-dieters > Dieters | |||||
| BA 18 | R Inferior Occipital Gyrus | 7.8 | 29 | −86 | −7 |
| BA 9 | R Middle Frontal Gyrus | 7.7 | 36 | 19 | 27 |
| Main Effect of Preload –Milkshake > Water | |||||
| BA 10 | R Orbital Frontal Cortex | 8.2 | 15 | 60 | 21 |
| BA 17 | R Cuneus | 7.1 | 9 | −96 | 2 |
| BA 32 | R Ventral Anterior Cingulate | 6.9 | 15 | 26 | −9 |
| Main Effect of Preload –Water > Milkshake | |||||
| BA 4 | L Precentral Gyrus | 11.5 | −42 | −18 | 45 |
| BA 21 | R Middle Temporal Gyrus | 9.9 | 74 | −41 | −6 |
| BA 10 | L Lateral Orbital Frontal Cortex | 9.8 | 42 | 58 | −5 |
| BA 4 | L Precentral Gyrus | 9.2 | −24 | −15 | 48 |
| BA 32/8 | Dorsal Anterior Cingulate | 5.9 | 6 | 34 | 34 |
| Restraint × Preload Interaction | |||||
| Striatum | R Nucleus Accumbens | 8.2 | 12 | 9 | −3 |
| Striatum | L Putamen | 7.2 | −18 | 6 | 8 |
| Striatum | L Nucleus Accumbens/Putamen | 6.5 | −15 | 3 | −8 |
| Striatum | L Caudate | 6.2 | −12 | 15 | 16 |
| Amygdala | L Amygdala | 5.6 | −27 | −4 | −20 |
Activations determined to be significant (thresholded at p < 0.05, corrected for False Discovery Rate, minimum extent threshold: k = 5 contiguous voxels) are listed along with the best estimate of their location. BA = Brodmann’s area location. Coordinates are from the Talairach & Tournoux (1988) atlas. Locations of the activations are determined based upon the functional responses superimposed on averaged anatomical MRI images and are referenced to the Talairach atlas.
Brain Regions preferentially sensitive to Dietary Status
Several regions exhibited differential activity in response to food items for dieters compared to non-dieters, including two regions of the ventral lateral prefrontal cortex along the left inferior frontal gyrus (vlPFC; BA 47; -48 41 -2, and BA 44 extending into BA 45 (-54 19 7). Similar effects were observed in a region of the left superior frontal gyrus (BA 6; -15 18 60), left middle temporal gyrus (BA 39; -54 -72 23), right lingual gyrus (BA 7; 36 -50 49), and a region within the right insular cortex (36 4 14). Each region showed greater activity for dieters compared to non-dieters (see Table 2). There were no differences as a function of preload, or the interaction between preload and dietary status in any of these regions.
Non-dieters showed increased activity (compared to their dieting counterparts) in the right middle frontal gyrus (BA 9; 36 19 27) and the right inferior occipital gyrus (BA 18; 29 -86 -7).
Brain Regions preferentially sensitive to Preload
A number of brain regions exhibited differential responses to food items as a function of preloading, with subjects given the milkshakes showing selective greater activity in the right ventral anterior cingulate (vACC; 15 26 -9), the right orbital frontal cortex (OFC; 15 60 -21), and the right cuneus (BA 17; 9 -96 2) (Table 2).
Regions demonstrating selective increased responses to food items in subjects given the water preload include two regions of the left precentral gyrus (BA4: -42 -18 45 and -24 -15 48), left lateral OFC (BA10: 42 58 -5), right middle temporal gyrus (BA 21: 74 -41 -6), and a region of the dorsal ACC (BA 32/8: 6 34 34).
Regions Exhibiting an Interaction of Dietary Status and Preload
Two regions within the ventral striatum, including the right nucleus accumbens (12 9 -3) and the left nucleus accumbens extending into the putamen (-15 3 -8) demonstrated a cross-over interaction such that responses to food images in these regions were greatest for dieters that received a milkshake preload and non-dieters that received a water preload (see Figure 3). As can be observed in Figure 3, non-dieters in the water condition showed a robust NAcc to food cues, whereas they showed minimal activity following the milkshake preload. Conversely, dieters in the water condition showed minimal NAcc activity, but dieters in the milkshake preload condition showed robust NAcc activity. Other regions of the striatum, including the left putamen (-18 6 8) and left caudate (-12 15 16) demonstrated a similar restraint by preload interaction.
Figure 3.
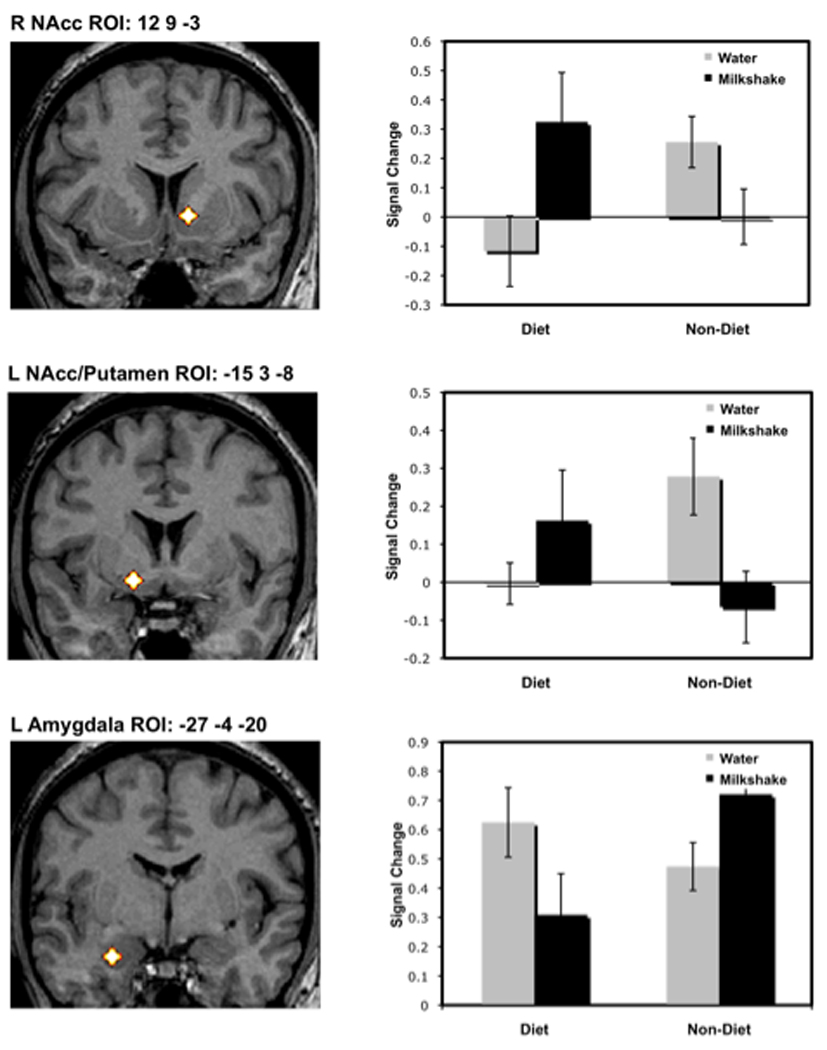
Coronal sections display right nucleus accumbens (top), left nucleus accumbens/putamen (middle), and left amygdala (bottom) spherical regions of interest superimposed on a normalized anatomic image. Graphs to the right of each image display signal chance (parameter estimates) across the four conditions relative to baseline fixation. Error bars indicate standard error of the mean (SEM). Activity in each region exhibits an interaction of restraint by preload.
Interestingly, the left amygdala (-27 -4 -20) showed a restraint × preload interaction that was opposite those observed in the ventral striatum. Specifically, amygdala response to food images was greatest for dieters receiving the water preload and non-dieters receiving the milkshake preload (Figure 3). Thus, as with the NAcc findings, dieters and non-dieters responded differentially to the water and milkshake preloads.
Importantly, the interaction observed in each of these regions was specific to food images. None of these regions demonstrated differential responses to the non-food images (All F’s < 1).
DISCUSSION
Consistent with the hypothesis that dietary violations are associated with increased reactivity to food cues in brain reward regions, dieters who consumed a milkshake showed greater ventral striatal activity when viewing pictures of appetizing food than did dieters who consumed only water. Also as expected, non-dieters showed the reverse pattern of greater striatal activity to appetizing food images when they consumed water compared to when they consumed the milkshake preload. Prior imaging studies of food cue reactivity have generally not focused on dieters or challenged dietary standards. Hence, these findings provide new insights into the neural basis of dietary failure.
The results for the non-dieters are consistent with past research demonstrating robust ventral striatal activity associated with viewing images of appetizing food (DelParigi, et al., 2007; Killgore, et al., 2003; Passamonti, et al., 2009; Schur, et al., 2009; Small, 2002; Stoeckel, et al., 2008). This is not surprising; food is a primary reward and an inherent source of pleasure, and thus food and food-related cues are powerful motivation cues, the neural basis of which has been shown to rely on dopaminergic pathways (see reviews by Jentsch & Taylor, 1999; Wilson, Sayette, & Fiez, 2004). These findings support the general proposition that mesolimbic and mesocortical systems are generally involved in reward. For instance, activity in reward regions has been observed when people view attractive faces (Cloutier, Heatherton, Whalen, & Kelley, 2008) erotic images (Hamann, Herman, Nolan, & Wallen, 2004) and anticipate monetary reward (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006; Knutson, Adams, Fong, & Hommer, 2001; Knutson, Fong, Adams, Varner, & Hommer, 2001). It has long been known that food is less rewarding when one is full (Cabanac, 1971). Thus, the absence of NAcc activity following the large milkshake preload likely reflects the normal reduction in palatability associated with satiety, at least for nondieters.
The findings also implicate dopaminergic systems in maladaptive behaviors (such as overeating) following dietary violation, as dieters showed increased NAcc activity to food cues after they were required to drink a large chocolate milkshake. Likewise, prior work has shown that obese women generally exhibit heightened activity NAcc in response to high calorie food items (Stoeckel, et al., 2008), collectively suggesting that NAcc activity is associated with overeating. This notion is also consistent with recent work by Stice and colleagues (2008) who identified a region of the dorsal striatum that was negatively correlated with weight change in individuals who possess the TaqlA A1 gene allele, a genotype that has been linked to obesity. In this study, participants received food rewards during scanning. A growing body of research suggests that the dorsal striatum may be more involved in the direct consummatory response to rewards (e.g., eating) (Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001; Stice, Spoor, Bohon, & Small, 2008), whereas the ventral striatum is more often recruited during the anticipation of reward (e.g., cue reactivity) (Gottfried, O'Doherty, & Dolan, 2003; Small, 2002; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008). These results converge nicely with the data herein, further suggesting a role for motivation and reward circuitry in dieting and dietary restraint failure.
Interestingly, the amygdala followed an opposite pattern of activation from the striatum such that activity in the amygdala was greatest when NAcc was weak (i.e., for dieters who received water and non-dieters who received the milkshake). Such activity may reflect an avoidance response to the food images, as both non-dieters who are sated and dieters with unbroken diets avoid rich, indulgent foods. Research by LaBar and colleagues (2001) suggests a hunger/motivational state-dependent response to food items in the amygdala. In their study, participants were food deprived for eight hours prior to scanning and viewed food and non-food images (tools) during scanning. Subjects then consumed a satiating meal and were scanned again. Participants showed an increased amygdala response to food images during the hungry state compared to the sated state, and the authors interpret these results to suggest a role for the amygdala in mediating the real-time significance of food stimuli. In the current study, the amygdala response of dieters following a water preload is consistent with this finding and may reflect a dieter’s overall state of hunger. However, non-dieters who were sated by a milkshake preload show a similarly high level of amygdala activation in response to food images. It is difficult to reconcile the somewhat disparate findings, in part because the prior work enrolled both male and female participants and without regard to their dietary status. One possible alternative explanation for the differential amygdala response to food images is that it reflects a general arousal response to the food. For the dieters, it is a potentially aversive response because dieters are actively trying to avoid such foods. For the non-dieters, amygdala activity may also index a similar arousal response to food items, as the rich milkshake may satiate non-dieters to a point of making additional food aversive (as evidenced by the finding that non-dieters eat very little when they are full). On-line ratings of food items were not taken during scanning, but it is possible that amygdala activity is indicative of the motivational appraisal afforded to food items at the present moment, whereas activity in the NAcc is more indicative of future behavior (i.e., desire to eat the pictured food). A second possibility is that the amygdala plays a more direct role in the self-regulation of eating behavior, as high amygdala activity is accompanied by reduced NAcc activity. However, given the well-established linkage between amygdala responsivity and arousal, a more parsimonious account is that the amygdala is not directly involved in self-regulation per se, but is instead indirectly engaged via an interplay between subcortical regions like amygdala and NAcc with cortical regions of frontal cortex.
Dietary Violations and Self-Regulation Failure
One interesting finding in the current research is that chronic dieters did not show increased activity in brain reward regions in response to food cues in the control condition where they consumed only water. This stands in contrast to non-dieters who show robust activity in NAcc to attractive food cues, as has also been found repeatedly in the cue reactivity literature. How is it that chronic dieters were able to ignore the rewarding properties of food cues? Metcalfe and Mischel (1999), in their hot/cool systems analysis of delay of gratification, proposed that ‘hot’ processing is emotional and impulsive and occurs when focus is placed on the stimulus, whereas ‘cool’ processing is more cognitive and emotionally neutral (Metcalfe & Mischel, 1999). Similarly, Bechara (2005) proposes interacting impulsive and reflective systems that, when imbalanced, lead to addiction. One potential reason why diets fail may be that circumstances promote a focus on the rewarding properties of food, such as taste and emotional comfort. In this way, diet success may be rooted in either a shift to more functional ‘cool’ processing of food, or overcoming attention to the strong rewarding aspects of food. Thus, dieters with intact diets may somehow process appetitive cues in a way that removes their reward value.
Similar mechanisms of suppressing reward responses have been recently demonstrated. For example, Delgado and colleagues (2008) observed an attenuation of neural activity in the striatum when participants actively practiced emotion regulation. This may suggest a potential strategy for weight control – by regulating ‘hot’ processing of food stimuli, dieters may be able to diminish reward activity from the nucleus accumbens, which may lead to more effective self-regulation. Recent evidence suggests that frontal regions of the brain often implicated in inhibitory control and self-regulation play a role in promoting food-related control. A recent study by Hare, Camerer, and Rangel (2008) required self-reported dieters to choose between ‘healthy’ and ‘tasty’ foods compared to neutrally rated foods, revealing that activity in the vmPFC tracked the value of a food (i.e., how tasty it was) regardless of the decision outcome, whereas the dlPFC was more active only when participants exerted self control, making decisions based on health rather than taste. Relatedly, successful weight loss maintainers, individuals who have lost a significant amount of weight and have managed to keep it off for at least 10 years demonstrate consistent restraint without disinhibition, and when viewing high-calorie food items show increased activity in frontal regions of the brain relative to both obese and normal weight counterparts (McCaffery et al., 2009).
The notion of a reciprocal relation between cortical and subcortical brain regions has been observed across a number of domains (Drevets & Raichle, 1998). That is, under normal circumstances increased activity in frontal regions is associated with decreased activity in subcortical regions, such as the amygdala (Kim, Somerville, Johnstone, Alexander, & Whalen, 2003). However, as situational cues lead to activity in subcortical regions, such as NAcc and amygdala, there appears to be a concomitant reduction in frontal activity. As such, a sufficiently strong appetitive signal may, at times, overwhelm executive control functions of the prefrontal cortex. In the present work, regions of the left inferior frontal gyrus/ventral lateral prefrontal cortex (BA 47 and BA 44/45) were more active in dieters compared to non-dieters in response to food images, irrespective of the preload manipulation. One interpretation of these results is that dieters attempt to exert self-control whether their diet is intact or not with dietary failure driven by more basic, reward-related mechanisms. That is, once a diet is broken by the milkshake, self-regulation breaks down as subcortical reward regions take precedence over cortical regions associated with executive functions that support inhibition. The left inferior frontal cortex/vlPFC has been implicated in emotional control (Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; Ochsner, Bunge, Gross, & Gabrieli, 2002), and more generally in cognitive control and decision-making (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004) through inhibition of emotional, physical, and social influences. In the present study, activity observed in this area may reflect automatic efforts to exert restraint in response to food items. An open question, however, is why activation in these or other frontal control regions (e.g., dlPFC) did not differ between preloaded and non-preloaded dieters (i.e., LIFG/vlPFC activity was not diminished for ‘disinhibited’ dieters that received the milkshake as might have been expected). Given that participants were not making explicit decisions about the food items, and no demand characteristics were involved, one possibility is that in the case of the cue reactivity paradigm, these control regions are involved in attempts at self-regulatory control at a more sustained, tonic level.
Unfortunately, such effects cannot be readily explored in event-related fMRI paradigms. Whereas responses in the NAcc and amygdala are transient, activating to brief presentations of food cues, regions whose function are to maintain a state of inhibition, will likely remain either tonically active or inactive for the duration of that condition (Visscher, et al., 2003). Recent neuroimaging work has highlighted an elegant method for capturing such sustained state effects while simultaneously measuring the transient responses to individual items in mixed state-item fMRI designs (Burgund, Lugar, Miezin, & Petersen, 2003; Burgund, Lugar, Miezin, Schlaggar, & Petersen, 2006; Donaldson, Petersen, & Buckner, 2001; Donaldson, Petersen, Ollinger, & Buckner, 2001; Velanova, et al., 2003; Visscher, et al., 2003; Wenger, Visscher, Miezin, Petersen, & Schlaggar, 2004). Future research will likely need to capitalize on such designs to better disentangle sustained and transient signals, as it will be important to understand how and when self-regulation breaks down and how subcortical reward regions interact with cortical executive regions to result in differences in inhibition.
Consistent with this notion, in addition to the notable difficulty of accurately imaging the hypothalamus given its size and location using standard imaging parameters, it is possible that the inability of event-related designs to detect sustained responses also accounts for the absence of hypothalamic activity in the present report. Given the role of the hypothalamus in hunger and satiety, differences across dietary and preload conditions may have been expected. However, such differences may not manifest as transient responses to food cues but rather as more general states of either heightened or reduced satiety levels. Indeed, changes in hypothalamic activity occur relatively slowly (approximately 5–10 minutes after intake) and have been shown to persist over relatively long periods of time (i.e., 30 minutes) (Smeets, de Graaf, Stafleu, van Osch, & van der Grond, 2005a, 2005b). Given that participants in the present study were scanned immediately after ingesting either water or high-calorie milkshakes it is probable that differences in the hypothalamic activity had not yet been fully realized.
One potential limitation to the current study is that our dieters were normal to overweight rather than obese, and it is possible that the truly obese might respond differently than our dieters. For instance, it is possible that obese dieters would fail to show the reduced NAcc activity to food images following water preload that we observed here. Studies of adolescents with Prader-Willi syndrome (Holsen, et al., 2006) indicate strong reward responses to food images. However, it is notable that such individuals are obese because of chronic overeating. Similarly, obese women exhibit greater activity in reward areas including the NAcc in response to high calorie food items than healthy weight control subjects (Stoeckel, et al., 2008). Future studies that examine cue reactivity across the range of disordered eating (e.g., anorexia, binge eating disorder) may be particularly informative. Additionally, diets are broken not only by ingesting high calorie food, but also by emotional distress and self-regulatory depletion (Heatherton, et al., 1991; Vohs & Heatherton, 2000). The findings presented here would be particularly compelling if such manipulations produced similar patterns of activity in response to food cues as a diet-breaking food preload.
Obesity is a growing problem in the western world and many dieters struggle to achieve and maintain long-term weight loss. The present study, along with the extant neuroimaging literature on eating, suggests a neural underpinning for diet failure and perhaps self-regulatory failures, such as addiction, more generally. The results of the current study provide initial evidence that self-regulatory failure associated with breaking a diet may be mediated by hyperactive reward and motivational responses to food. At the same time, we uncovered a particularly intriguing finding that dieters somehow manage to observe tempting foods, at least when their diets are intact, without activating reward regions. At the most general level, these data suggest that attempts to regulate behavior, such as trying to overcome addictions, control anger, or avoid other temptations, may rely on a common mechanism centered around brain reward regions. The use of a dieting analogue provides a novel and important way to assess the breakdown of restraints. Given the enormous societal costs of self-regulation failures, drug addiction, obesity, and so forth, understanding the neural mechanisms involved in successful self-regulation and its breakdowns should be a high scientific priority. (Bechara, 2005; Delgado, Gillis, & Phelps, 2008)
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Aronne LJ, Wadden T, Isoldi KK, Woodworth KA. When prevention fails: obesity treatment strategies. Am J Med. 2009;122(4 Suppl 1):S24–S32. doi: 10.1016/j.amjmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(Pt 10):2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Lugar HM, Miezin FM, Petersen SE. Sustained and transient activity during an object-naming task: a mixed blocked and event-related fMRI study. Neuroimage. 2003;19(1):29–41. doi: 10.1016/s1053-8119(03)00061-2. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Lugar HM, Miezin FM, Schlaggar BL, Petersen SE. The development of sustained and transient neural activity. Neuroimage. 2006;29(3):812–821. doi: 10.1016/j.neuroimage.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971 September 17;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci. 2008;20(6):941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11(8):880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31(6):1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13(1):129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict. 1990;85(6):725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55(2):224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, et al. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7(4):411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychol Bull. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol. 1991;60(1):138–143. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J. Effects of distress on eating: the importance of ego-involvement. J Pers Soc Psychol. 1992;62(5):801–803. [PubMed] [Google Scholar]
- Heatherton TF, Mahamedi F, Striepe M, Field AE, Keel P. A 10-year longitudinal study of body weight, dieting, and eating disorder symptoms. J Abnorm Psychol. 1997;106(1):117–125. [PubMed] [Google Scholar]
- Heatherton TF, Polivy J, Herman CP. Restraint and internal responsiveness: effects of placebo manipulations of hunger state on eating. J Abnorm Psychol. 1989;98(1):89–92. doi: 10.1037//0021-843x.98.1.89. [DOI] [PubMed] [Google Scholar]
- Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975;43(4):647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84(6):66–72. [PubMed] [Google Scholar]
- Herman CP, Polivy J. The self-regulation of eating: theoretical and practical problems. In: Baumeister RF, Vohs KD, editors. Handbook of Self-Regulation. New York: Guilford: 2004. pp. 492–508. [Google Scholar]
- Holm-Denoma JM, Joiner TE, Vohs KD, Heatherton TF. The "freshman fifteen" (the "freshman five" actually): predictors and possible explanations. Health Psychol. 2008;27(1 Suppl):S3–S9. doi: 10.1037/0278-6133.27.1.S3. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36(3):257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Mann J, Cummings JH, Englyst HN, Key T, Liu S, Riccardi G, et al. FAO/WHO scientific update on carbohydrates in human nutrition: conclusions. Eur J Clin Nutr. 2007;61 Suppl 1:S132–S137. doi: 10.1038/sj.ejcn.1602943. [DOI] [PubMed] [Google Scholar]
- McFarlane T, Polivy J, Herman CP. Effects of false weight feedback on mood, self-evaluation, and food intake in restrained and unrestrained eaters. J Abnorm Psychol. 1998;107(2):312–318. doi: 10.1037//0021-843x.107.2.312. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;1061:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ. Personality predicts the brain's response to viewing appetizing foods: the neural basis of a risk factor for overeating. J Neurosci. 2009;29(1):43–51. doi: 10.1523/JNEUROSCI.4966-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31(4):702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Polivy J, Heatherton TF, Herman CP. Self-esteem, restraint, and eating behavior. J Abnorm Psychol. 1988;97(3):354–356. doi: 10.1037//0021-843x.97.3.354. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP, Howard K. The restraint scale: Assessment of dieting. In: Herson M, Bellack AS, editors. Dictionary of behavioral assessment techniques. New York: Pergamon: 1988. pp. 377–380. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33(6):653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999;282(14):1353–1358. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Small DM. Toward an understanding of the brain substrates of reward in humans. Neuron. 2002;33(5):668–671. doi: 10.1016/s0896-6273(02)00620-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005a;82(5):1011–1016. doi: 10.1093/ajcn/82.5.1011. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage. 2005b;24(2):363–368. doi: 10.1016/j.neuroimage.2004.07.073. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91(2):251–268. [PubMed] [Google Scholar]
- Stice E, Maxfield J, Wells T. Adverse effects of social pressure to be thin on young women: an experimental investigation of the effects of "fat talk". Int J Eat Disord. 2003;34(1):108–117. doi: 10.1002/eat.10171. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23(24):8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol Sci. 2000;11(3):249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Volkow ND, O'Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry. 2007;164(5):708–710. doi: 10.1176/ajp.2007.164.5.708. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Visscher KM, Miezin FM, Petersen SE, Schlaggar BL. Comparison of sustained and transient activity in children and adults using a mixed blocked/event-related fMRI design. Neuroimage. 2004;22(2):975–985. doi: 10.1016/j.neuroimage.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]


