Abstract
Goals
Oral mucositis can be a significant and dose-limiting complication of high-dose cancer therapy. Mucositis is a particularly severe problem in patients receiving myeloablative chemotherapy prior to bone marrow or hematopoetic stem cell transplant (HSCT). The cyclooxygenase (COX) pathway mediates tissue injury and pain through upregulation of pro-inflammatory prostaglandins, including prostaglandin E2 (PGE2) and prostacyclin (PGI2). The objective of this small (n=3) pilot study was to examine the role of the COX pathway in causing mucosal injury and pain in chemotherapy-induced oral mucositis.
Materials and methods
We collected blood, saliva, and oral mucosal biopsy specimens from three autologous HSCT patients at the following time-points before and after administration of conditioning chemotherapy: Day −10, +10, +28, and +100, where day 0 is day of transplant. RNA extracted from full-thickness tissue samples was measured by RT-PCR for the following: COX-1, COX-2, microsomal prostaglandin E synthase (mPGES), IL-1β, and TNF-α. Blood and saliva samples were measured by ELISA for PGE2 and PGI2, which are markers of COX activity. Severity of oral mucositis was determined using the Oral Mucositis Index. Severity of pain due to oral mucositis was measured using a Visual Analog Scale. Relationships between the different variables were examined using Spearman rank correlation coefficients.
Main results
Mean mucositis and pain scores increased significantly after administration of chemotherapy and then gradually declined. The correlation between changes in mucositis and pain scores was strong and statistically significant. The following additional correlations were statistically significant: between tissue COX-1 and pain; between tissue mPGES and pain; between salivary PGE1 and pain; between salivary PGI2 and pain. Other relationships were not statistically significant.
Conclusions
Our finding of significant associations of pain scores with tissue COX-1 and mPGES, as well as salivary prostaglandins, is suggestive of a role for the cyclooxygenase pathway in mucositis, possibly via upregulation of pro-inflammatory prostaglandins. However, our small sample size may have contributed to the lack of significant associations between COX-2 and other inflammatory mediators with mucosal injury and pain. Thus, additional studies with larger numbers of subjects are warranted to confirm the involvement of the cyclooxygenase pathway in chemotherapy-induced mucositis.
Keywords: Mucositis, Chemotherapy, Hematopoetic stem cell transplant, Cyclooxygenase, Prostaglandin
Introduction
Oral mucositis refers to erythematous, erosive, and ulcerative lesions of the oral mucosa seen in oncology populations, such as head and neck cancer patients undergoing radiation therapy (RT) which includes fields involving the oral cavity, and patients receiving high-dose chemotherapy for cancer, including those receiving myeloablative chemotherapy as conditioning for hematopoietic cell transplantation [12–14].
Oral mucositis is an especially severe and common problem in patients who receive myeloablative chemotherapy as conditioning for hematopoietic stem cell transplantation (HSCT). Among patients receiving conditioning regimens containing high-dose melphalan or total body irradiation, severe mucositis (WHO grades 3 or 4) has been reported to be almost ubiquitous [28].These patients typically have severe pain due to oral mucositis which significantly impacts eating, drinking, speaking, mouthcare, and overall quality of life [2, 3]. Severe mucositis pain commonly necessitates the use of systemic opioid analgesics and total parenteral nutrition [8]. From the patient’s point of view, oral mucositis is often the single most debilitating complication of a transplant [2]. Since these patients are typically severely immunosuppressed, secondary infections of oral lesions, and more importantly bacteremia and sepsis of oral origin pose a significantly increased risk of morbidity and mortality [19, 21]. A singlepoint increase in peak mucositis scores in HSCT patients is associated with one additional day of fever, a 2.1-fold increase in risk of significant infection, 2.7 additional days of total parenteral nutrition, 2.6 additional days of injectable narcotic therapy, 2.6 additional days in hospital, $25,405 in additional hospital charges and a 3.9-fold increase in 100-day mortality risk [26].
In a recent study of patients undergoing chemotherapy for solid tumors or lymphomas, 303 of 599 patients (over 50%) developed oral and/or gastrointestinal (GI) mucositis. OM developed during 22% of 1,236 cycles of chemotherapy, GI mucositis during 7% of cycles and both oral and GI mucositis during 8% of cycles [7]. The risk of infection in these immunosuppressed patients was significantly higher (over twofold) during cycles with mucositis than during cycles without mucositis even though the level and duration of neutropenia was similar. The risk of infection increased with increasing severity of mucositis. Infection-related deaths were significantly more common during cycles with both oral and GI mucositis [7]. During chemotherapy cycles with mucositis, the average duration of hospitalization was significantly longer. The use of liquid diets, total parenteral nutrition, fluid replacement and antifungal or antiviral prophylaxis/therapy were more common in cycles with mucositis. It was estimated that the cost of hospitalization was $3,893 per chemotherapy cycle without mucositis, $6,277 per cycle with oral mucositis and $9,132 per cycle with both oral and GI mucositis. Perhaps most importantly, a reduction in the next dose of chemotherapy was twice as common after cycles with mucositis as compared to cycles without mucositis [7]. This confirms the role of oral mucositis as a dose-limiting toxicity of cancer chemotherapy with direct effects on patient survival.
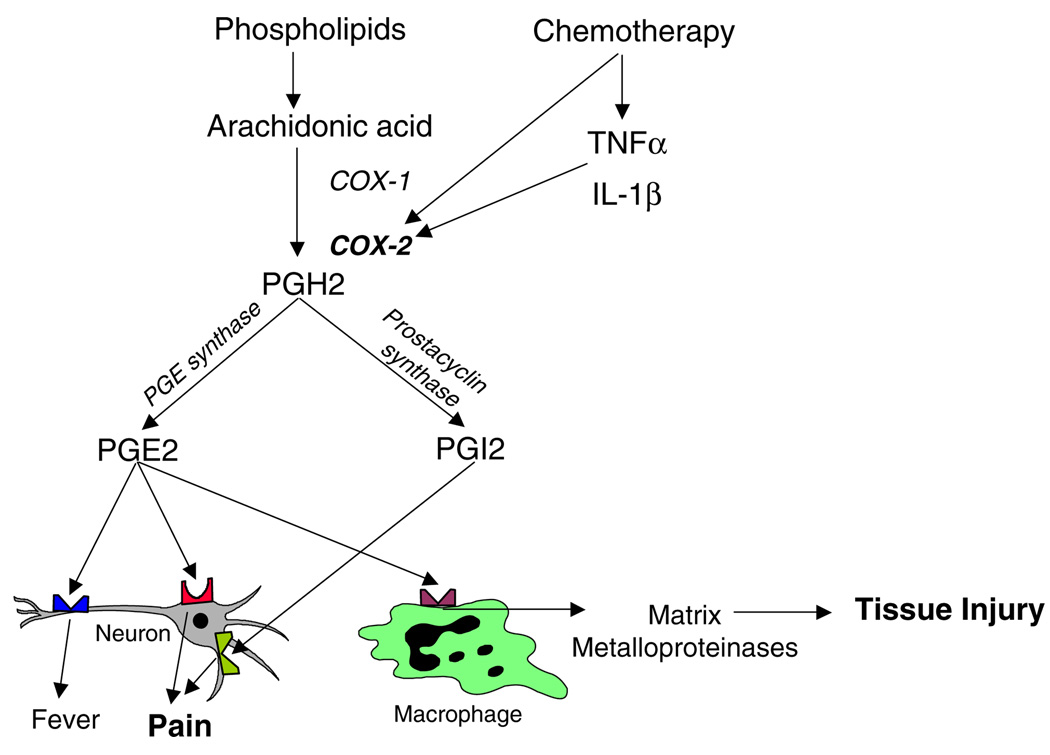
The COX pathway is an important pathway involved in mediating the inflammatory response (Fig. 1) and can be directly activated by a number of chemotherapeutic drugs, including in the oral mucosa [16]. In addition, antineoplastic agents also cause the release of pro-inflammatory cytokines, such as IL-1β and TNF-α, which can amplify further COX activation. COX-1 and COX-2 mediate the conversion of arachidonic acid into Prostaglandin H2 (PGH2), which is converted into PGE2 by PGE synthase and into PGI2 by prostacyclin synthase. Since both PGE2 and PGI2 cause pain by acting at prostaglandin receptors on neurons [6, 15], and because PGE2 also mediates tissue injury via release of matrix metalloproteinases [24], it seems likely that the COX enzymes play a role in both the pathogenesis and symptom complex associated with mucositis.
Fig. 1.
Mechanisms whereby the cyclooxygenase pathway mediates tissue injury and pain
A number of studies support a role for the cyclooxygenase pathway in the pathogenesis of oral mucositis. Administration of radiation/chemotherapy has been demonstrated to cause significant elevations in the release of pro-inflammatory cytokines including TNF-α, interleukin-1 alpha (IL-1α) and interleukin-6 (IL-6) from several different tissues [10, 30]. In a hamster cheek pouch model of radiation mucositis, mRNA levels of TNF- α and interleukin-1 beta (IL-1β) in oral mucosal tissue correlated with severity of mucosal injury. Further, animals treated with the anti-inflammatory cytokine interleukin -11 (IL-11) demonstrated a significant reduction in mucosal injury accompanied by reduced levels of TNF-α and IL-1β [25]. TNF-α and IL-1β both induce the expression of cyclooxygenase-2 (COX-2) which is a key enzyme involved in the inflammatory process [5]. COX-2 expression is upregulated in irradiated hamster oral mucosa and is highest during peak mucositis severity [27]. COX-2 expression and protein levels in irradiated rat mucosa were also found to correlate with severity of oral mucositis [9].
A human study correlated the levels of prostaglandins in plasma with severity of oral mucositis. Patients treated with synchronous radiotherapy and chemotherapy for head and neck cancer had elevated levels of PGE2 in plasma. Further, the plasma levels of PGE2 correlated with the severity of oral mucosal injury [29].
Thus, evidence from animal models indicates that activation of the COX pathway correlates with the pathogenesis of oral mucositis. However, there is a need to confirm these findings in human studies. The goal of this pilot study was thus to examine the role of the COX pathway in chemotherapy-induced oral mucositis in humans receiving stomatotoxic chemotherapy.
Materials and methods
This pilot study was originally designed as a multicenter study with three sites and a total enrollment of 20 subjects. It was estimated that this sample size would allow detection of moderate to large correlations between four to five observation points. However, two of the sites were unable to obtain IRB approval due to concerns about oral mucosal biopsies in neutropenic subjects. The study was therefore terminated at the third site (University of Connecticut Health Center), after three subjects had completed the study over an 8-month period, with IRB approval. No additional patients were screened. The following inclusion criteria were used: willingness and ability to provide written informed consent; Karnofsky score greater than or equal to 60 at enrollment; scheduled to receive high-dose conditioning chemotherapy in preparation for a HSCT for treatment of a solid tumor. The following exclusion criteria were used: less than 18 years of age; positive pregnancy test; inclusion of total body irradiation in the conditioning regimen; WBC count less than 1,000/mm3, granulocyte count less than 500/mm3 or platelet count less than 20,000/ mm3 at enrollment; other serious coexisting disease in addition to malignancy; enrolled on another investigational study.
Assessment of oral mucositis
Oral examinations were performed by calibrated clinicians experienced in evaluating oral mucositis. A slide set demonstrating the possible range of oral mucosal changes post-chemotherapy was reviewed by all examiners for calibration. Oral mucositis was scored three times a week during hospital admission for hematopoetic cell transplant as well as on days oral mucosal biopsy was performed as an out-patient procedure (see Table 1). Oral mucositis was scored using the 20-item Oral Mucositis Index (OMI), derived from the original OMI published by Schubert et al. in 1992 [17, 23]. This validated scale assigns a score for ulceration/pseudomembrane at each of nine specific intra-oral anatomic sites based on estimated surface area involved (0, none; 1, >0 but ≤1 cm2; 2, >1 cm2 but ≤2 cm2; and 3, >2 cm2). Erythema was also scored at the same nine sites according to the following scale (0, normal/no change; 1, mild; 2, moderate; and 3, severe change). In addition, atrophy of the dorsal tongue and edema of the lateral tongue were scored, using the same scale as for erythema. The 20 individual scores were added to determine the total oral mucosal injury score for each assessment for each subject.
Table 1.
Study flow sheet
| Intervention | Day −10a | Day 0b | Day ±10a | Day ±28a | Day ±100a |
|---|---|---|---|---|---|
| Mucositis and pain scoring | √ | 3×/week during admission | √ | √ | √ |
| Oral mucosal biopsy | √ | √ | √ | √ | |
| Blood sample collection | √ | √ | √ | √ | |
| Saliva sample collection | √ | √ | √ | √ |
Estimated days, actual days varied slightly
Day 0=day of transplant
Assessment and management of pain
The subjects’ assessment of pain was recorded using a Visual Analog Scale (VAS) [11], three times a week throughout their hospital stay. This VAS asked subjects to describe their current oral pain by drawing a vertical line through a 100-mm horizontal scale, where the left end was labeled “No Pain” and the right end was labeled “Worst Possible Pain”. The distance from the left end of the horizontal scale to the vertical line drawn by the subject was measured as the VAS score for that assessment. To increase consistency and limit mucosal sensitivity, the VAS was completed at approximately the same time of day, prior to oral examination or eating. Mucosal pain control was based on standard hospital protocol and typically included both topical anesthetic solutions and systemic narcotics.
Oral mucosal biopsy
Oral mucosal biopsies were obtained by an experienced oral and maxillofacial surgeon at the following time-points: Day –10, day +10, day +28, and day +100, where day 0 is day of HSCT. The biopsy technique was based on a procedure utilized for lip biopsy in transplant patients [22]. The biopsy technique was reviewed with the attending surgeon to ensure consistency of technique and quality of specimens. Patients were anesthetized using a unilateral infiltration of 2% Xylocaine (with 1:100,000 epinephrine), 1 cm distal to the site of biopsy. Site selection was based on the following schema:
| 1st specimen (day −10) | 1 cm distal to left lip commissure |
| 2nd specimen (day +10) | 1 cm distal to right lip commissure |
| 3rd specimen (day +28) | 2 cm distal to left lip commissure |
| 4th specimen (day +100) | 2 cm distal to right lip commissure |
A 4-mm punch biopsy specimen was harvested from the buccal mucosa, using a gentle, rotating motion to insert the instrument 1 mm in depth. The punch biopsy instrument was then withdrawn, and the specimen separated from underlying tissue with a number 15 scalpel. When necessary, a suture was placed for hemostasis. Mucosal specimens were immediately placed into a cryomold filled with Tissue-Tek OCT (optimal cutting temperature) compound (Sakura Finetek, Torrance, CA), and then put into a mixture of methylbutane and dry ice to flash-freeze the sample. When the OCT media solidified, the cryomold was wrapped, labeled, and transferred to −80°C.
Saliva collection
Saliva samples were collected from each patient by a trained research nurse at the following time-points: Day −10, day +10, day +28, and day +100 (see Table 1). Unstimulated whole saliva was collected by directing patients to not swallow for 1 min, followed by expectoration of the pooled saliva into a 50-cc collection tube. This was repeated three to five times for each time-point. Saliva samples were centrifuged to separate cellular debris and then frozen at −80°C until analyzed.
Blood sample collection
Peripheral blood samples were collected from each subject by a trained research nurse at the following time-points: day −10, day +10, day +28, and day +100 (see Table 1). Blood samples were centrifuged to separate cellular debris and then frozen at −80°C until analyzed.
Quantitative RT-PCR analysis
Biopsies were brought to the laboratory embedded in OCT. Biopsies were first cleared of the water-soluble compound by cutting away most of the OCT followed by several rinses with RNAsefree water. Biopsies were cracked in the frozen state in liquid nitrogen and then homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH) in a Dounce homogenizer. RNA was extracted according to the manufacturer’s procedure. Samples were DNAsed using the Ambion Turbo DNA-free kit (Applied Biosystems, Foster City, CA). RNA was quantified by measuring the optical density at 260 and 280 nm. The semi-quantitative PCR method measures relative RNA expression levels in cellular samples by use of a sequence-specific fluorogenic probe (TaqMan probe). RNA was reverse transcribed into the complementary DNA (cDNA) strand using the Highcapacity cDNA Archive kit (Applied Biosystems, Foster City, CA) which uses MMLV reverse transcriptase in random-primed cDNA syntheses. This cDNA was used in subsequent PCR reactions with primers and probes specific to the cytokine of interest. Primers and probes for Cox-1, Cox-2, and mPGES were designed by Dr. Jonathan Covault, MD, PhD, for use in our laboratory using the Primer Express software (Applied Biosystems, Foster City, CA). RT-PCR conditions and primer/probe concentrations were optimized according to manufacturer recommendations. Primers and probes for IL-1 β, TNFα, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and 18S ribosomal RNA (rRNA) were purchased as commercially available sets (Applied Biosystems, Foster City, CA). A four-point standard curve was run alongside the samples for each gene tested, using the Universal Reference RNA from human tissues (Clontech, Mountain View, CA). Samples and standards were run in triplicate. Amplification was measured throughout the PCR using the ABI 7700 Real-time PCR instrument (Applied Biosystems, Foster City, CA). This system monitors the PCR at every cycle and generates quantitative data based on the PCR at early cycles when PCR fidelity is highest. Analysis was done using SDS software (Applied Biosystems, Foster City, CA) and plotted against the standard curve. Cytokine specific expression levels were then normalized to values obtained from housekeeping genes from the same sample. The normalizing gene was GAPDH and 18 S rRNA was run as well to ensure that results were consistent across multiple housekeeping genes.
Enzyme-linked immunosorbent assay
Plasma and saliva samples were measured for the following using Enzyme-linked immunosorbent assay (ELISA):
PGE2
PGE2 is rapidly converted in vivo to its 13,14-dihydro-15-keto metabolite. This metabolite is not chemically stable and undergoes a variable amount of degradation to other metabolites. Therefore, measurement of the metabolites is necessary to provide a reliable estimate of actual PGE2 production. We used the PGE2 metabolite assay (Cayman Chemical, Ann Arbor, MI), per manufacturer’s instructions [18]. This assay converts all of the immediate PGE2 metabolites to a single, stable derivative that can be easily quantified using enzyme immunoassay.
Prostacyclin (PGI2)
Prostacyclin is rapidly and nonenzymatically hydrated to 6-keto PGF1α. Therefore, the 6-keto PGF1αEIA kit (Cayman Chemical, Ann Arbor, MI) was used to estimate prostacyclin synthesis, per manufacturer’s instructions [1].
Data analysis
Statistical analyses were performed using StatXact software (Cytel Software Corporation, Cambridge, MA). Analyses focused on correlations between temporal changes in mucositis or pain scores and concurrent changes in components of the cyclooxygenase pathway. Spearman rank correlation coefficients (r) were calculated to quantify these relationships. Due to the small sample size, “exact” p values were calculated to evaluate statistical significance of the correlation estimates. A 0.05 significance level was applied in all statistical tests.
Results
Subjects
Three subjects were enrolled to this pilot study. They included a 44-year-old female with breast cancer, a 64-year-old male with non-Hodgkins lymphoma and a 48-year-old female with breast cancer. All subjects received myeloablative chemotherapy consisting of busulfan 16 mg/kg over 4 days, followed by thioTEPA 500 mg/m2 and carboplatin 900 mg/m2 over 4 days. None of the subjects received total body irradiation. Myeloablative chemotherapy was followed by infusion of previously collected autologous CD34 positive stem cells on day 0. Each subject was followed at protocol-specified time-points (see Table 1) until the last time-point at day 100 after transplant. The following samples/data were not obtained and were not included in the data analysis: Day +10 blood sample for subject 01 was not obtained due to an unintentional protocol deviation; day +28 saliva and tissue samples and mucositis and pain scores for subject 03 were not obtained since the subject was febrile and neutropenic. All mucosal biopsies that were performed healed without any complications.
Mucositis and pain scores
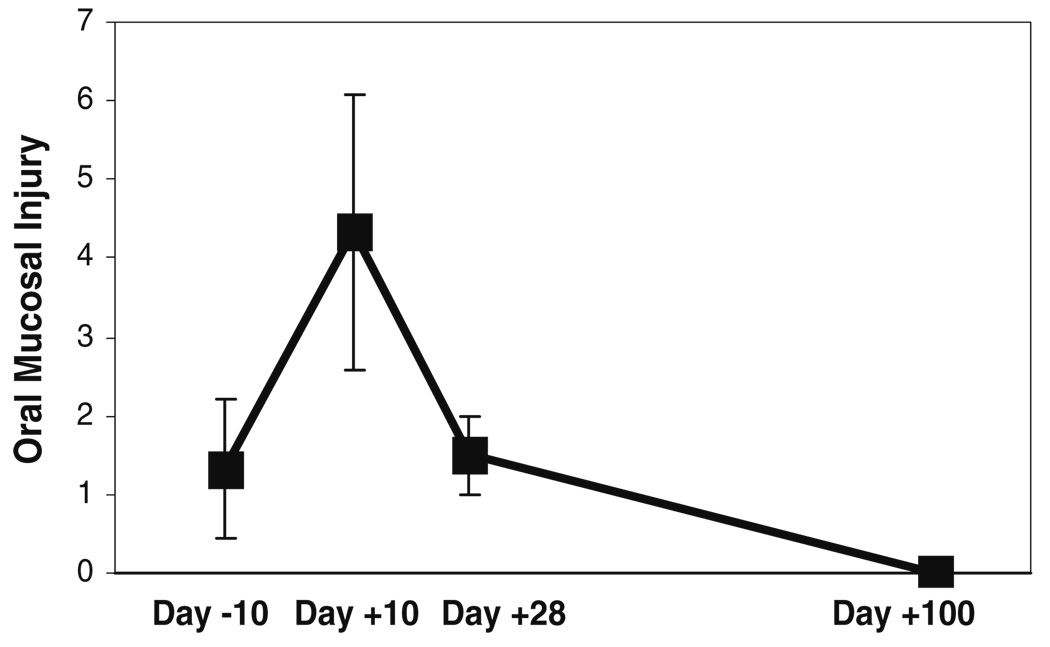
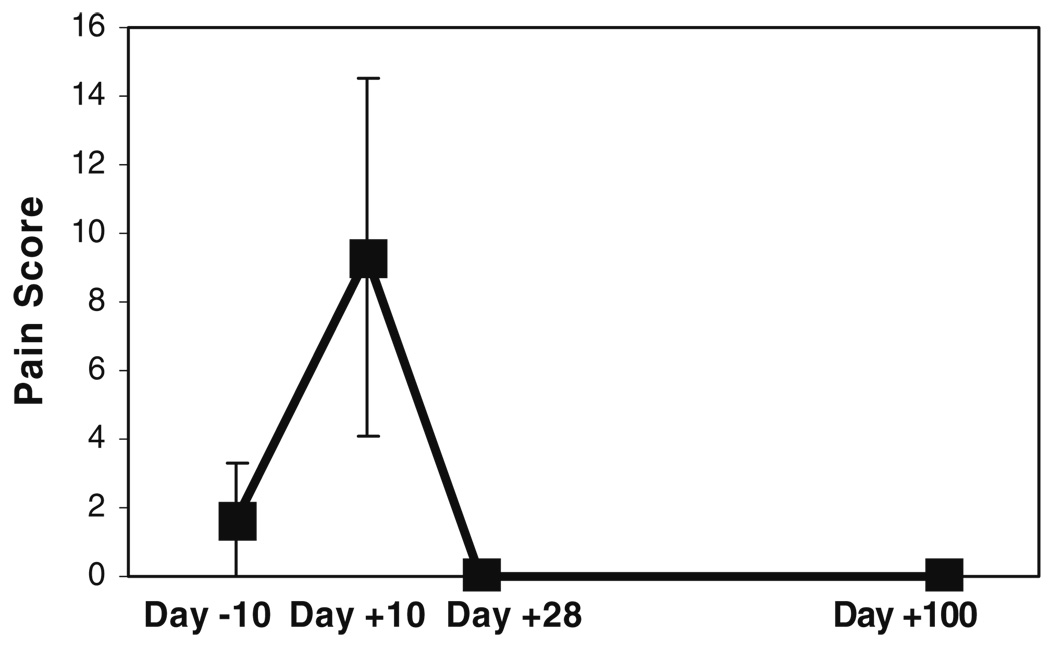
As expected, mean mucositis and pain scores increased significantly after administration of chemotherapy and then gradually declined (Fig. 2 and Fig. 3). Mucositis and pain scores were slightly higher than 0 at baseline, this can be attributed to resolving oral mucosal damage from previous rounds of chemotherapy. Both mucositis and pain scores peaked at day +10. Mucositis scores returned to baseline levels by day +28 and were at 0 by day +100. Pain scores returned to 0 by day +28. The correlation between changes in mucositis and pain scores was strong (r=+0.91) and statistically significant (p=0.01).
Fig. 2.
Mean (±SD) oral mucosal injury scores of the three subjects, at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the total Oral Mucositis Index score for each subject, at each time-point
Fig. 3.
Mean (±SD) mouth pain scores of the three subjects, at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the Visual Analog Scale pain score for each subject, at each time-point
Levels of inflammatory mediators in oral mucosal biopsies
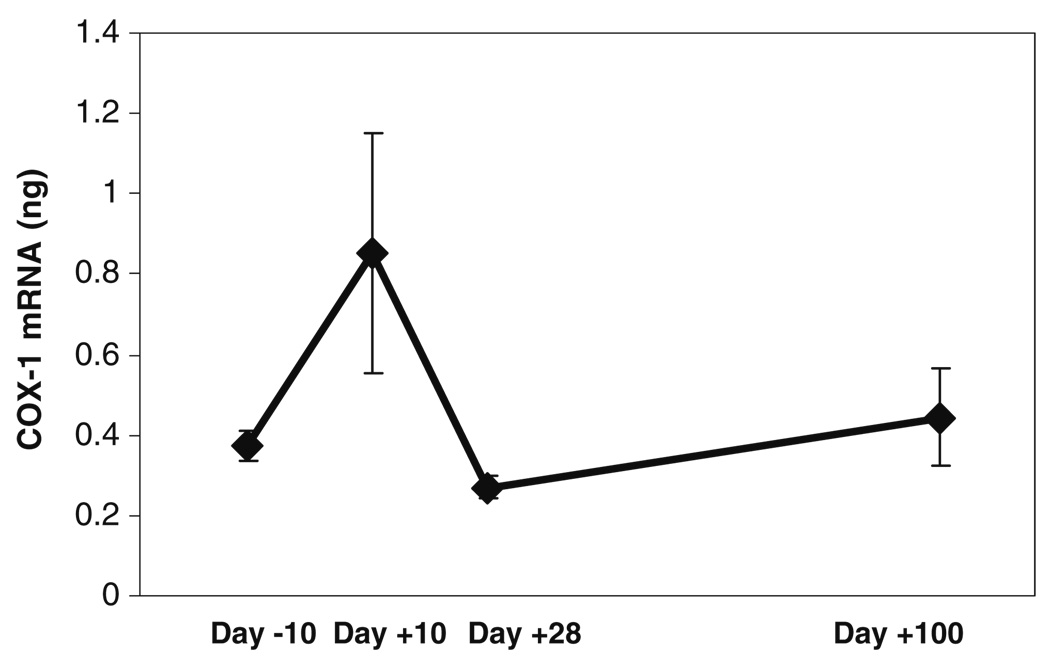
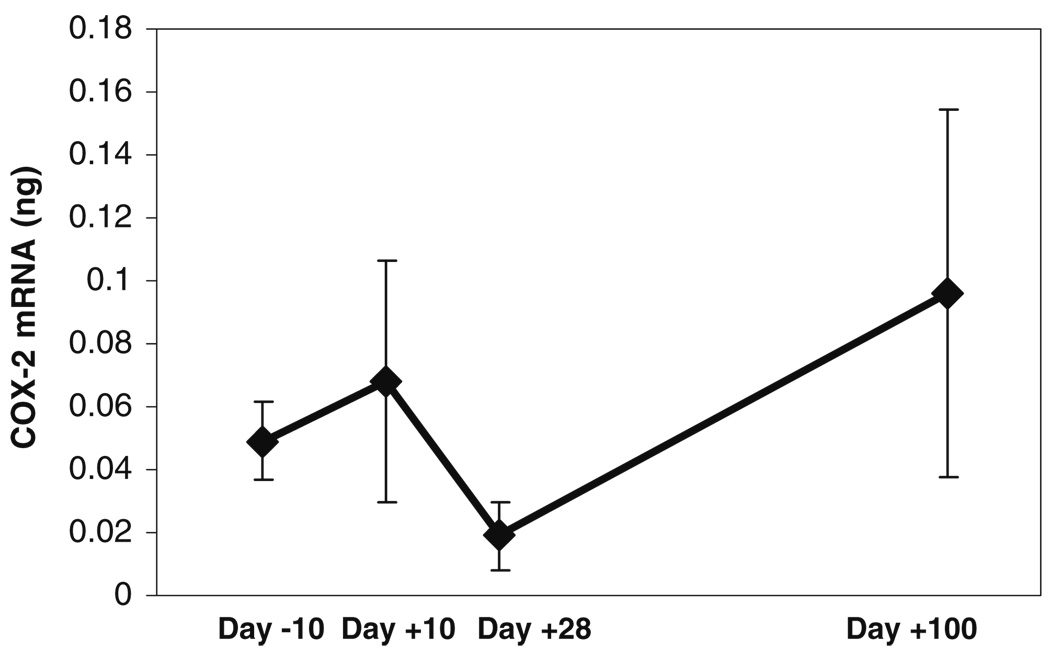
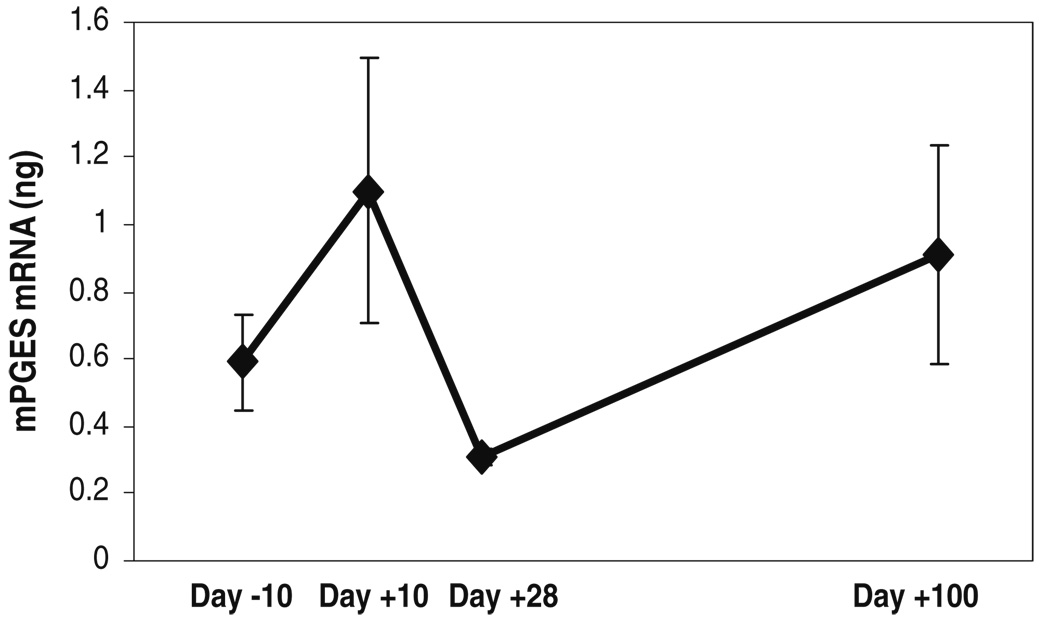
In general, the levels of inflammatory mediators measured in the oral mucosal biopsies showed an increase following chemotherapy, followed by a decline that paralleled mucositis and pain scores (Fig. 4, Fig. 5, and Fig. 6). Correlations between temporal changes in levels of the inflammatory mediators and changes in the mucositis and pain scores are shown in the upper half of Table 2. The correlations of tissue levels of COX-1, COX-2, mPGES, and IL-1β, with the mucositis and pain scores were in the positive direction. Correlations between COX-1 and pain and between mPGES and pain were statistically significant. Further, a positive correlation between COX-1 and mucosal injury showed a trend toward statistical significance. Interestingly, an increase was seen at the day +100 time-point for all of the mediators mentioned above.
Fig. 4.
Mean (±SD) tissue levels of cyclooxygenase-1 (COX-1) mRNA of the three subjects at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the normalized cyclooxygenase-1 mRNA levels (in nanograms) obtained by RT-PCR, for each subject, at each time-point
Fig. 5.
Mean (±SD) tissue levels of cyclooxygenase-2 (COX-2) mRNA of the three subjects at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the normalized cyclooxygenase-2 mRNA levels (in nanograms) obtained by RT-PCR, for each subject, at each time-point
Fig. 6.
Mean (±SD) tissue levels of microsomal prostaglandin E synthase (mPGES) mRNA of the three subjects at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the normalized mPGES mRNA levels (in nanograms) obtained by RT-PCR, for each subject, at each time-point
Table 2.
Correlations of temporal changes in levels of inflammatory mediators and prostaglandins with changes in mucositis and pain scores
| Marker | Mucositis |
Pain |
||
|---|---|---|---|---|
| Correlation | p value | Correlation | p value | |
| Inflammatory mediators | ||||
| Biopsy Cox-1 | 0.75 | 0.07 | 0.91 | 0.01* |
| Biopsy Cox-2 | 0.39 | 0.40 | 0.61 | 0.16 |
| Biopsy mPGES | 0.64 | 0.14 | 0.87 | 0.02* |
| Biopsy TNFα | –0.25 | 0.59 | –0.08 | 0.89 |
| Biopsy IL-1β | 0.46 | 0.30 | 0.61 | 0.16 |
| Salivary IL1β | –0.18 | 0.71 | –0.02 | 1.0 |
| Prostaglandins | ||||
| Salivary PGE1 | 0.77 | 0.10 | 0.88 | 0.03* |
| Blood PGE2 | 0.40 | 0.52 | 0.71 | 0.40 |
| Salivary PGE2 | 0.43 | 0.35 | 0.63 | 0.12 |
| Blood PGI2 | –0.60 | 0.35 | –0.35 | 0.80 |
| Salivary PGI2 | 0.57 | 0.20 | 0.77 | 0.048* |
Positive values in the correlations column indicate positive correlations and negative values indicate negative correlations. Higher correlation values indicate stronger correlations.
P values marked with an asterisk indicate statistical significance for that correlation
Levels of prostaglandins in blood and saliva
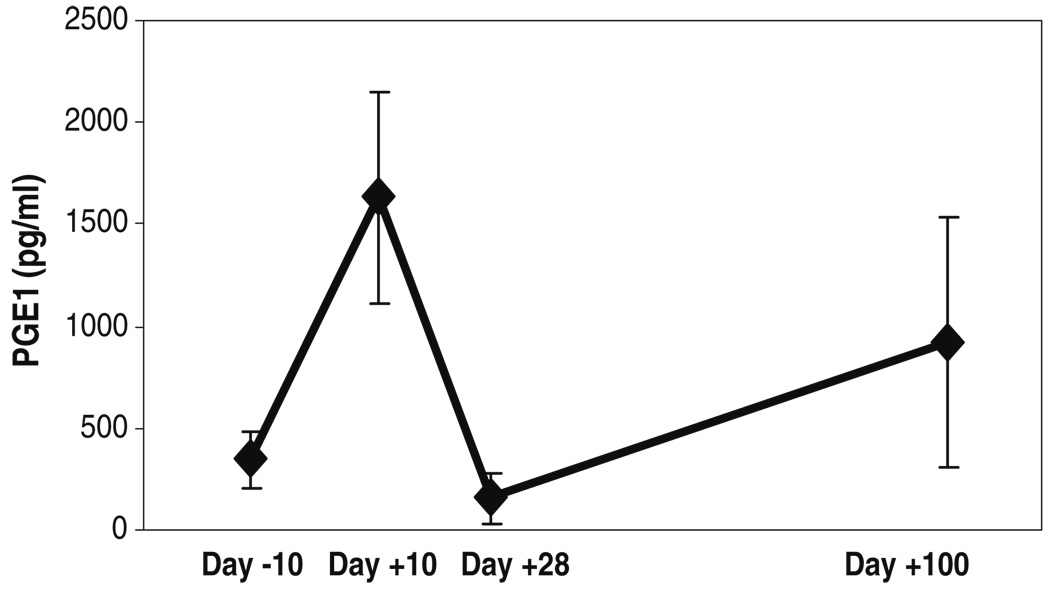
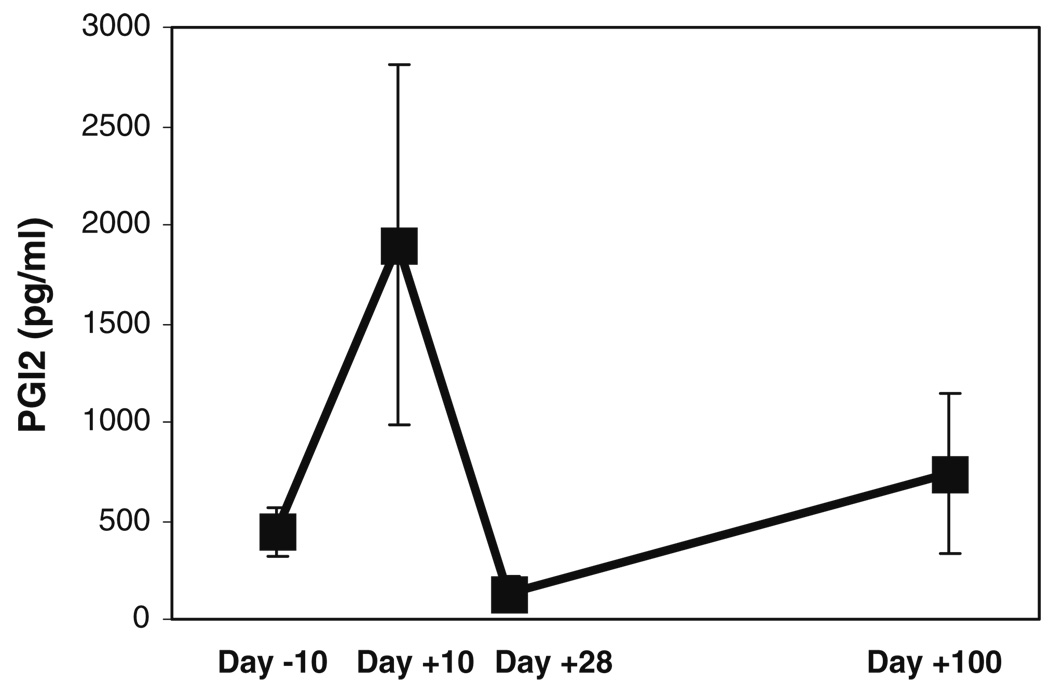
Salivary PGE1 and PGI2 levels were significantly correlated with patient reports of pain (Fig. 7 and Fig. 8, Table 2). Correlations between blood or salivary levels of these prostaglandins and mucositis scores did not achieve statistical significance.
Fig. 7.
Mean (±SD) salivary PGE1 levels of the three subjects at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the PGE1 levels (in picograms per milliliter) obtained by ELISA, for each subject, at each time-point
Fig. 8.
Mean (±SD) salivary prostacyclin (PGI2) levels of the three subjects at the different time-points before and after administration of high-dose chemotherapy. These mean scores were obtained by calculating an average of the PGI2 levels (in picograms per milliliter) obtained by ELISA, for each subject, at each time-point
Discussion
The kinetics of objective mucositis scores and patientreported pain scores noted in this pilot study were consistent with those noted in the literature [17]. Both endpoints peaked at approximately day 10, and the trajectory of tissue levels of COX-1, COX-2, and mPGES was similar. Likewise, salivary levels of PGE1 and PGI2 followed the same pattern. While it seems likely that the small number of subjects precluded statistical confirmation of the seeming correlations between mucositis scores and levels of inflammatory mediators, we were able to demonstrate significant associations of tissue levels of COX-1 and mPGES with scores of pain resulting from oral mucosal injury. COX-1 is usually considered to be the constitutive isoform expressed in basal conditions, while COX-2 is considered to be the inducible isoform upregulated in inflammatory and other pathological states. However, recent studies have disputed this traditional paradigm and indicated that both isoforms may be upregulated in pathological states [4, 20, 31]. Our finding of a significant association between COX-1 and pain scores is consistent with this emerging concept. Both COX-1 and COX-2 mediate conversion of arachidonic acid to PGH2, which in turn can be converted to PGE2 by mPGES (Fig. 1). Since PGE2 is known to act at pain receptors on neurons, our finding of a significant association between mPGES and pain scores is consistent with a potential role for the cyclooxygenase pathway in the pathogenesis of mucositis.
Interestingly, the mean tissue levels of these inflammatory mediators showed an unexpected increase at the day +100 time-point. Although the reason for this increase is not known, it may be speculated that there was a sub-clinical inflammatory process in progress at this time-point. In this population of HSCT patients, sub-clinical graft vs. host disease is one possible explanation, although this is less likely with autologous transplants. We also found that salivary levels of the important pro-inflammatory prostaglandin PGI2 closely tracked and were significantly correlated with clinical pain scores. This finding is significant in view of the known abilities of PGI2 to mediate pain (Fig. 1). These results also indicate that measuring salivary levels of symptom mediators, such as PGE2 and PGI2, may be a convenient method to measure pathologic responses leading to pain. The relative ease of sample collection for saliva offers a logistical advantage compared to blood sample collection.
As noted above, the small number of subjects and a loss of data due to missing observations precluded statistical modeling of the temporal relationships between markers of cyclooxygenase pathway activity and the measures of mucositis. In place of modeling methods, we used a rankbased, non-parametric technique that is resistant to the effects of non-normality and outlying observations and combined it with the calculation of exact p values appropriate for small samples. On the one hand, the finding of any statistically significant associations with repeated measurements from only three subjects is note-worthy and may indicate the strength of these associations. On the other hand, there may exist complex associations in the temporal patterns of each pathway marker and between the markers and the mucositis scores that could not be investigated with a limited data set and were not incorporated into our analyses. Additional studies with larger numbers of subjects are needed to more fully explore these relationships.
Our findings are consistent with those of Logan et al., who demonstrated increased levels of COX-2 (by immunohistochemistry) in human oral mucosa following administration of chemotherapy [16]. Sonis et al. demonstrated a significant increase in COX-2 expression (by immunohistochemistry) following radiation in an animal model of radiation mucositis. The kinetics of COX-2 expression paralleled mucositis severity [27]. Our current data extend these findings by quantitative measurement of COX-2 and other inflammatory mediators in human tissues using quantitative RT-PCR, and initially define the relationship between tissue and salivary levels of these inflammatory mediators and the extent of mucosal injury and symptoms in humans.
Our finding of significant associations of pain scores with tissue COX-1 and mPGES, as well as salivary prostaglandins, is suggestive of a role for the cyclooxygenase pathway in mucositis, possible via upregulation of pro-inflammatory prostaglandins. However, our small sample size may have contributed to the lack of significant associations between COX-2 and other inflammatory mediators with mucosal injury and pain. Thus, additional studies with larger numbers of subjects are warranted to confirm the involvement of the cyclooxygenase pathway in chemotherapy-inducedmucositis.
Acknowledgments
Dr. Lalla’s effort on this research was supported by grants T32DE007302 and K23DE016946 from the NIH. This research was supported in part by a General Clinical Research Center (GCRC) grant (M01RR06192) from the NIH awarded to the University of Connecticut Health Center. We thank Ms. Pamela Fall, Ms. Christine Abreu and Dr. Jonathan Covault of the GCRC Core Laboratory for implementation of the quantitative RT-PCR and ELISA analyses. We thank Ms. Kim Jennings of the GCRC Clinical Core for study coordination and data entry.
Contributor Information
Rajesh V. Lalla, Email: Lalla@uchc.edu, Section of Oral Medicine, Department of Oral Health and Diagnostic Sciences, Head & Neck/Oral Oncology Program, Neag Comprehensive Cancer Center, University of Connecticut Health Center, MC 1605, Room L6062, 263, Farmington Avenue, Farmington, CT 06030, USA.
Carol C. Pilbeam, Division of Endocrinology and Metabolism, Department of Medicine, University of Connecticut Health Center, 263, Farmington Avenue, Farmington, CT 06030, USA
Stephen J. Walsh, School of Nursing, University of Connecticut, 231 Glenbrook Road, Storrs, CT 06269, USA
Stephen T. Sonis, Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Division of Oral Medicine and Dentistry, Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Boston, MA 02115, USA
Dorothy M. K. Keefe, Discipline of Medicine, Faculty of Health Sciences, University of Adelaide, Adelaide, Australia
Douglas E. Peterson, Section of Oral Medicine, Department of Oral Health and Diagnostic Sciences, Head & Neck/Oral Oncology Program, Neag Comprehensive Cancer Center, University of Connecticut Health Center, MC 1605, Room L6062, 263, Farmington Avenue, Farmington, CT 06030, USA
References
- 1. [Accessed 11 February, 2009];6-keto Prostaglandin F1a EIA Kit. [Internet document] Available at: http://www.caymanchem.com/app/template/Product.vm/catalog/515211/a/z.
- 2.Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–39. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 3.Cheng KK. Oral mucositis and quality of life of Hong Kong Chinese patients with cancer therapy. Eur J Oncol Nurs. 2007;11:36–42. doi: 10.1016/j.ejon.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by interleukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res. 1998;241:222–229. doi: 10.1006/excr.1998.4050. [DOI] [PubMed] [Google Scholar]
- 6.Doi Y, Minami T, Nishizawa M, Mabuchi T, Mori H, Ito S. Central nociceptive role of prostacyclin (IP) receptor induced by peripheral inflammation. Neuroreport. 2002;13:93–96. doi: 10.1097/00001756-200201210-00022. [DOI] [PubMed] [Google Scholar]
- 7.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JB, Schubert MM. Managing pain in mucositis. Semin Oncol Nurs. 2004;20:30–37. doi: 10.1053/j.soncn.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Feng CJ, Guo JB, Jiang HW, et al. Spatio-temporal localization of HIF-1alpha and COX-2 during irradiationinduced oral mucositis in a rat model system. Int J Radiat Biol. 2008;84:35–45. doi: 10.1080/09553000701616080. [DOI] [PubMed] [Google Scholar]
- 10.Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 11.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 12.Lalla RV, Peterson DE. Oral mucositis. Dent Clin North Am. 2005;49:167–184. doi: 10.1016/j.cden.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Lalla RV, Peterson DE. Treatment of mucositis, including new medications. Cancer J. 2006;12:348–354. doi: 10.1097/00130404-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CR, Amaya F, Barrett L, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319:1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 16.Logan RM, Gibson RJ, Sonis ST, Keefe DM. Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007;43:395–401. doi: 10.1016/j.oraloncology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 17.McGuire DB, Peterson DE, Muller S, Owen DC, Slemmons MF, Schubert MM. The 20 item oral mucositis index: reliability and validity in bone marrow and stem cell transplant patients. Cancer Invest. 2002;20:893–903. doi: 10.1081/cnv-120005902. [DOI] [PubMed] [Google Scholar]
- 18.Prostaglandin E. [Accessed 11 February, 2009];Metabolite EIA kit. [Internet document] Available at: http://www.caymanchem.com/app/template/Product.vm/catalog/514531/a/z.
- 19.Rapoport AP, Miller Watelet LF, Linder T, et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol. 1999;17:2446–2453. doi: 10.1200/JCO.1999.17.8.2446. [DOI] [PubMed] [Google Scholar]
- 20.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2008;50 doi: 10.1194/jlr.R800042-JLR200. S29–34S. April 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST. The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer. 1998;82:2275–2281. [PubMed] [Google Scholar]
- 22.Schubert MM, Sullivan KM, Morton TH, et al. Oral manifestations of chronic graft-v-host disease. Arch Intern Med. 1984;144:1591–1595. [PubMed] [Google Scholar]
- 23.Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer. 1992;69:2469–2477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 24.Shankavaram UT, Lai WC, Netzel-Arnett S, et al. Monocyte membrane type 1-matrix metalloproteinase. Prostaglandindependent regulation and role in metalloproteinase-2 activation. J Biol Chem. 2001;276:19027–19032. doi: 10.1074/jbc.M009562200. [DOI] [PubMed] [Google Scholar]
- 25.Sonis ST, Peterson RL, Edwards LJ, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373–381. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 26.Sonis ST, Oster G, Fuchs H, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–2205. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 27.Sonis ST, O'Donnell KE, Popat R, et al. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004;40:170–176. doi: 10.1016/s1368-8375(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 28.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 29.Tanner NS, Stamford IF, Bennett A. Plasma prostaglandins in mucositis due to radiotherapy and chemotherapy for head and neck cancer. Br J Cancer. 1981;43:767–771. doi: 10.1038/bjc.1981.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2- incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 31.Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues - is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 2009 July 24; doi: 10.1111/j.1582-4934.2008.00430.x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]