Abstract
This study demonstrates the ability of magnolol, a hydroxylated biphenyl compound isolated from Magnolia officinalis, to inhibit LPS-induced expression of iNOS gene and activation of NF-κB/Rel in RAW 264.7 cells. Immunohisto-chemical staining of iNOS and Western blot analysis showed magnolol to inhibit iNOS gene expression. Reporter gene assay and electrophoretic mobility shift assay showed that magnolol inhibited NF-κB/Rel transcriptional activation and DNA binding, respectively. Since p38 is important in the regulation of iNOS gene expression, we investigated the possibility that magnolol to target p38 for its anti-inflammatory effects. A molecular modeling study proposed a binding position for magnolol that targets the ATP binding site of p38 kinase (3GC7). Direct interaction of magnolol and p38 was further confirmed by pull down assay using magnolol conjugated to Sepharose 4B beads. The specific p38 inhibitor SB203580 abrogated the LPS-induced NF-κB/Rel activation, whereas the selective MEK-1 inhibitor PD98059 did not affect the NF-κB/Rel. Collectively, the results of the series of experiments indicate that magnolol inhibits iNOS gene expression by blocking NF-κB/Rel and p38 kinase signaling.
Keywords: Magnolol, Macrophages, p38 kinase, iNOS, NF-κB/Rel
INTRODUCTION
Magnolia officinalis (Magnoliaceae) has long been used for the treatment of fever, headache, anxiety, diarrhea, asthma, and stroke, and possesses potent anti-inflammatory effects [1]. It has been reported that magnolol, a compound purified from Magnolia officinalis, relaxes rat vascular smooth muscle [2], scavenges hydroxyl radicals [3], inhibits neutrophil aggregation and superoxide anion generation [4,5], suppresses the expression of vascular cell adhesion molecule-1 in endothelial cells [6], inhibits nitric oxide (NO) production in lipopolysaccharide (LPS)-activated macrophages [7]. Recently, it has been reported that the anti-inflammatory effects of magnolol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-κB activation signaling [8]. Bacterial LPS is a potent the immune system activator which induces local inflammation, antibody production, and, in severe infections, septic shock [9]. Macrophages play a central role in a host's defense against bacterial infection and are major cellular targets for LPS action. Stimulation of murine macrophages by LPS results in the expression of an iNOS, which catalyzes the production of large amounts of NO from L-arginine and molecular oxygen [10]. NO, in turn, participates in the inflammatory response of macrophages [11]. The promoter of the murine gene encoding iNOS contains two κB binding sites, located at 55 and 971 bp upstream of the TATA box, respectively [12]. It has been reported that protein binding to the κB site is necessary to confer inducibility by LPS [13].
The factor p38 kinase is an important mediator of stress-induced gene expression [14]. In particular, the p38 kinase is known to play a key role in LPS-induced signal transduction pathways leading to cytokine synthesis [15]. It was deomonstrated that p38 kinase activation is involved in iNOS expression in tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1)-stimulated mouse astrocytes, as well as in LPS-stimulated mouse macrophages [16,17].
In the present study, we attempted to determine the effects of magnolol on the expression of iNOS, an important indicator of inflammation. To further investigate the mechanism by which magnolol inhibits the expression of iNOS gene, we studied the possibility that magnolol targets p38 for its anti-inflammatory effects. The present study demonstrates the potential of magnolol in inhibiting iNOS gene expression through the suppression of NF-κB and p38 kinase pathways.
METHODS
Materials
Magnolol and LPS from Salmonella thyposa was purchased from Sigma (St. Louis, MO). Reagents used for cell culture were purchased from Gibco BRL (Grand Island, NY). Anti-iNOS was purchased from Ustate Biotechnology (Lake Placid, NY). CNBr-Sepharose 4B was purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Cell culture
RAW 264.7 cells (murine macrophage line) were purchased from American Type Culture Collection (Bethesda, MD). Cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were then cultured in the presence of 5% CO2 at 37℃.
Western immunoblot analysis
Whole cell lysates were separated by 10% SDS-PAGE, then electro-transferred to nitrocellulose membranes (Amersham International, Buckinghamshire, UK). The membranes were preincubated for 1 hr at room temperature in Tris-buffered saline (TBS), pH 7.6 containing 0.05% Tween-20 and 3% bovine serum albumin. The nitrocellulose membranes were incubated with iNOS, phosphorylated p38 or p38-specific antibodies. Immunoreactive bands were then detected by incubation with conjugates of anti-rabbit IgG with horseradish peroxidase and enhanced chmiluminescence reagents (Amersham).
Electrophoretic mobility shift assay (EMSA)
Electrophoretic mobility shift assay (EMSA) was performed as described in previous literature [18]. Nuclear extracts were prepared as previously described [19]. Treated and untreated RAW 264.7 cell line was lysed with hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, pH 7.5) and nuclei were pelleted by centrifugation at 3,000 × g for 5 min. Nuclear lysis was performed using a hypertonic buffer (30 mM HEPES, 1.5 mM MgCl2, 450 mM KCl, 0.3 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1 µg/ml of aprotinin, and 1 µg/ml of leupeptin). Following lysis, the samples were centrifuged at 14,500×g for 15 min, and supernatant was retained for use in the DNA binding assay. The double-stranded oligonucleotides were end-labeled with [γ-32P]-ATP. Nuclear extracts (5 µg) were incubated with poly (dI-dC) and the [32P]-labeled DNA probe in binding buffer (100 mM KCl, 30 mM HEPES, 1.5 mM MgCl2, 0.3 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1 µg/ml of aprotinin, and 1 µg/ml of leupeptin) for 10 min. DNA binding activity was separated from free probe using a 4% polyacrylamide gel in 0.5× TBE buffer. Following electrophoresis, the gel was dried and subjected to autoradiography.
Transient transfection of RAW 264.7 cells
Vector constructions were performed as previously described [20]. RAW 264.7 cells were transfected using the DEAE-dextran method, diluted to 5×105 cells per 1 ml of complete media, plated on 24 well plates, and then incubated in the presence of 5% CO2 at 37℃ for 24 hr. The transfectants were treated with LPS and magnolol. Eighteen hours later the cells were lysed with lysis buffer. The lysates were centrifuged (12,000×g for 10 min at 4℃), and the supernatant was assayed for the expression of CAT enzyme using CAT ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions.
Receptor selection
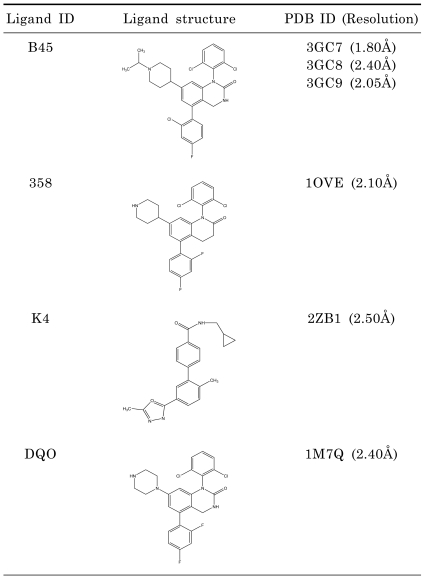
To propose binding positions for p38 kinase inhibitor (Magnolol), p38 co-crystal structures was determined in the PDB that resulted in 127 co-crystal structures. To select best receptor for docking study, we considered the similarity of ligands. Since Magnolol has a biphenyl moiety, we collected the p38 kinase co-crystal structures with ligands which have biphenyl moiety. They are 6 structures (3GC7, 1OVE, 3GC8, 3GC9, 1M7Q, 2ZB0). The six ligand structures and their corresponding PDB codes are listed in Table 1. Out of these six x-ray crystal structures, 3GC7 was selected based on the resolution of the x-ray structure (1.80Å).
Table 1.
Ligands with Biphenyl Moiety bound to p38 and their resolution
Molecular docking and pose generation
A docking study was performed using SYBYL8.1 (Tripos Inc., St Louis, MO 63144 USA) molecular modeling package. Protein structure was prepared by using biopolymer module of SYBYL 8.1. Hydrogen atoms were added to the structure, atom types and charges were assigned using AMBER7 FF99 force field and side chain amides were modified. Magnolol was sketched using SYBYL 8.1 sketch program and minimized by using Tripos force field and Powell method with termination gradient set to 0.05 kcal/mol. The molecule was fully minimized with Gasteiger-Hückel charges. Docking study was performed using Surflex-Dock module of SYBYL 8.1, which uses empirical scoring function using protomol [21].
In vitro pull-down assay
Cell lysates overexpressed p38 were incubated with magnolol-Sepharose 4B (or Sepharose 4B only as a control) beads (50 µl, 50% slurry) in reaction buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP40, 2 µg/ml bovine serum albumin, 0.02 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml of aprotinin, and 1 µg/ml of leupeptin]. After incubation and with gentle rocking overnight at 4℃, the beads were washed five times with buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP40, 0.02 mM PMSF], and proteins bound to the beads were analyzed by Western blotting.
Statistical analysis
The mean±SD was determined for each treatment group in a given experiment. For significant differences observed among treatment groups, they were compared to the vehicle using a Dunnett's two-tailed t test [22].
RESULTS
Effect of magnolol on macrophage activation
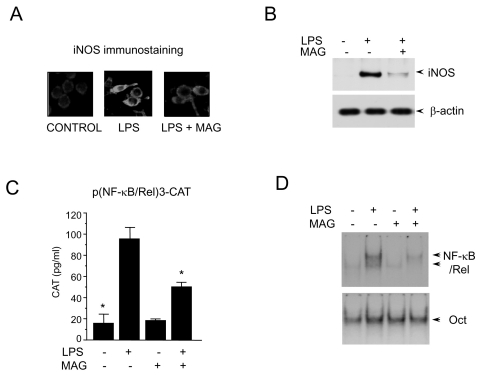
To investigate the effects of magnolol on iNOS production, the expression of iNOS by immunohisto-chemical staining of iNOS was measured. RAW 264.7 cells (5×105 cells/ml) were incubated with magnolol (50 µM) in the presence of LPS (200 ng/ml) for 24 hr on cover slide in 12 well plates. Cells were subjected to immunohistochemical staining using an antibody specific for murine iNOS. Immunohisto-chemical staining of iNOS showed that magnolol inhibited iNOS production (Fig. 1A). No effect on cell viability was observed in any of the treatment groups and it always exceeded 90%, as determined by trypan blue staining (data not shown). After the RAW 264.7 cells were exposed to magnolol in the presence of LPS, the expression level of iNOS gene was monitored by Western immunoblot analysis. As shown in Fig. 1B, iNOS protein production was inhibited by magnolol treatment. Control β-actin was constitutively expressed and was not affected by the treatment of magnolol. These results indicate that magnolol decreases the gene expression of iNOS, which is involved in inflammation [11]. Since it has been reported that protein binding at the κB binding site is necessary to confer inducibility by LPS of iNOS [13], we assessed the effect of magnolol on NF-κB/Rel using a transient transfection assay. When RAW 264.7 cells were transiently transfected with p(NF-κB/Rel)3-CAT, the CAT gene expressions were found to be inhibited by magnolol in the presence of LPS (Fig. 1C). CAT expression by RAW 264.7 cells significantly increased by LPS and LPS-induced CAT expression was inhibited by magnolol treatment. The transcriptional activation of the NF-κB/Rel transcription factor is preceded by the DNA binding of NF-κB/Rel. We further assessed the effect of magnolol on the NF-κB/Rel whose binding motif is in the promoter of iNOS gene using EMSA. LPS treatment of RAW 264.7 cells induced a marked increase in NF-κB/Rel binding to its cognate site. And the induction of NF-κB/Rel binding was inhibited by magnolol (Fig. 1D). Oct had moderate basal binding activity and was not influenced by either LPS or magnolol treatment. The specificity of the retarded bands was confirmed by the addition of an excess of 32P-unlabeled double-stranded κB, or oct that competed for protein binding (data not shown). These results indicate that magnolol decreases the DNA binding and transcriptional activation of NF-κB/Rel, which is important in the regulation of iNOS gene expression.
Fig. 1.
Inhibition of macrophage activation by magnolol. (A) RAW 264.7 cells (5×105 cells/ml) incubated with magnolol (50 µM) in the presence of LPS (200 ng/ml) for 24 hr on cover slide in 12 well plates. Cells were subjected to immunohistochemical staining using an antibody specific for murine iNOS. Immunoreactivity of iNOS was localized along the margins of the cytoplasm in control group. (B) Cells were treated with magnolol in the presence of LPS (200 ng/ml) for 24 hr. Cell lysates were then prepared and subjected to Western immunoblotting. (C) RAW 264.7 cells were transfected with p(NF-κB/Rel)3-CAT by DEAE dextran method. Twenty-four hours after transfection, cells were treated with the magnolol in the presence or absence of LPS (200 ng/ml) for 18 hr. Cell extracts were then prepared and analyzed for the expression of CAT using CAT ELISA kit. (D) Cells (5×105 cells/ml) were incubated with magnolol (50 µM) in the presence or absence of LPS (200 ng/ml) for 2 hr. Nuclear extracts (5 µg/ml) were then isolated and analyzed for the activity of NF-κB/Rel and Oct.
Direct binding of magnolol with p38 kinase and molecular docking
Since p38 is important in the regulation of iNOS gene expression, we investigated the possibility of magnolol in targeting p38 for its anti-inflammatory effects. To propose binding poses of magnolol for p38 kinase inhibitor, we searched p38 co-crystal structures in the PDB. There were 127 co-crystal structures. In an effort to select the best receptor for docking study, we considered the similarity of ligands. Since magnolol has a biphenyl moiety, we collected the p38 kinase co-crystal structures with ligands which have biphenyl moiety. They are 6 structures (3GC7, 1OVE, 3GC8, 3GC9, 1M7Q, 2ZB0). In Table 1, the six ligand structures and their corresponding PDB codes are listed. Out of these six x-ray crystal structures, the structure 3GC7 was selected based on the resolution of the x-ray structure (1.80Å). A docking study was performed using SYBYL8.1 (REF1) molecular modeling package. Protein structure was prepared by using biopolymer module of SYBYL 8.1. Hydrogen atoms were added to structure, atom types and charges were assigned using AMBER7 FF99 force field and side chain amides were modified. Magnolol was sketched by using SYBYL 8.1 sketch program and minimized by using Tripos force field and Powell method with termination gradient set to 0.05 kcal/mol. The molecule was fully minimized with Gasteiger-Hückel charges. Docking study was performed by using Surflex-Dock module of SYBYL 8.1, which uses empirical scoring function using protomol [21].
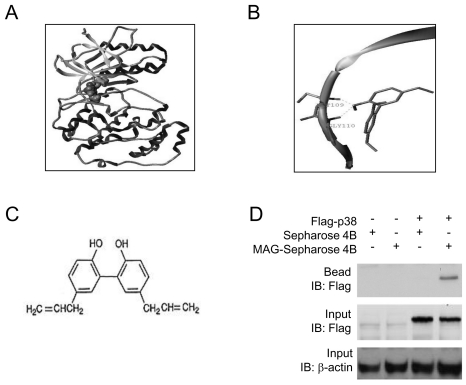
Since majority of the available inhibitors interact in the hinge region, the binding pose for magnolol were selected on the basis of docking score and binding site (hinge contact). It's interesting to note that the hinge residues (M109 and G110) are conserved throughout the p38 group of kinase. Fig. 2A shows the natural product magnolol has been docked with p38 kinase structure (PDB code: 3GC7). The ligand is represented in space fill model and the macromolecule is in ribbon and tube. Fig. 2B shows the proposed binding pose of magnolol's interaction with the backbone of hinge residues M109 and G110. The chemical structure of magnolol is shown in Fig. 2C.
Fig. 2.
Molecular docking and pose generation. (A) A docking study was performed using SYBYL8.1 molecular modeling package as described in Materials and methods. Magnolol was docked with p38 kinase structure (PDB code: 3GC7). The ligand is represented in space fill model and the macromolecule is in ribbon and tube. (B) The proposed binding pose of Magnolol showed the interaction with the backbone of hinge residues M109 and G110. (C) Chemical structure of magnolol is shown. (D) Flag-p38 expression vectror was transiently transfected to 293 cells. Cell lysates were then prepared, used for pull-down assay using magnolol-Sepharose 4B beads and subjected to Western blot analysis.
To confirm this prediction, we performed an in vitro pull-down assay using magnolol-conjugated to Sephasore 4B beads. When we transiently transfected Flag-p38 expression vectror into 293 cells, we found p38 binds with magnolol-conjugated Sepharose beads, but not with Sepharose beads alone (Fig. 2D). These data clearly confirmed the direct interaction of magnolol and p38 and support our hypothesis that p38 kinase is a possible target for magnolol.
Inhibition of NF-κB/Rel by p38 kinase inhibitor, SB203580, in LPS-stumulated macrophages
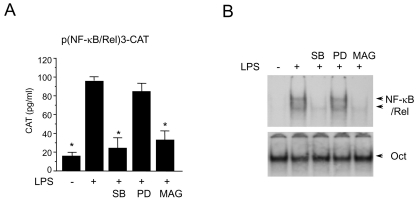
Since p38 kinase was chosen is a possible target for magnolol, further investigation was taken forward to determine if p38 kinase pathway is involved in LPS-induced NF-κB/Rel activation. The p38 kinase pathway was specifically blocked and NF-κB/Rel activation was monitored when RAW 264.7 cells were challenged with LPS. SB203580, a bicyclic imidazole compound, is a specific inhibitor of p38 [23]. PD98059 is a specific inhibitor of MEK-1, mitogen activated protein kinase/extracellular signal-regulated kinase 1, which is responsible for ERK1/2 activation [24]. SB203580 inhibited LPS-induced activation of NF-κB/Rel, while PD98059 did not inhibit (Fig. 3A). The LPS-induced NF-κB/Rel DNA binding activity was also specifically inhibited by SB203580 but not by PD98059 (Fig. 3B). These results suggest that p38 kinase pathway is important in the regulation of NF-κB/Rel activation by LPS.
Fig. 3.
Effects of SB203580 and PD98059 on NF-κB/Rel activation in LPS-stimulated RAW 264.7 cells. (A) RAW 264.7 cells were transfected with p(NF-κB/Rel)3-CAT by DEAE dextran method. Twenty-four hours after transfection, cells were treated with SB203580 (30 µM), PD98059 (50 µM), or magnolol (50 µM) in the presence of LPS (200 ng/ml) for 18 hr. Cell extracts were then prepared and analyzed for the expression of CAT using CAT ELISA kit. (B) RAW 264.7 cells were pretreated with SB203580 (30 µM), PD98059 (50 µM), or magnolol (50 µM) for 30 min before incubation with LPS (200 ng/ml) for 2 hr. Nuclear extracts were then isolated and analyzed for the activity of NF-κB/Rel and Oct.
DISCUSSION
We demonstrate that magnolol treatment significantly attenuates LPS-induced iNOS production through the blocking of NF-κB/Rel activation in the macrophage line RAW 264.7. A molecular modeling study was performed to propose the binding poses for magnolol targeting the ATP binding stite of p38 kinase (3GC7). Magnolol was found to occupy the ATP binding pocket of p38 while interacting with the hinge residues (GLY110, Met 109) which are conserved throughout the p38 kinase family. We also showed that magnolol binds to the p38 kinase. The p38 kinase is an important mediator of stress-induced gene expression [21]. In particular, the p38 kinase is known to play a key role in LPS-induced signal transduction pathways leading to cytokine synthesis [15]. It was demonstrated that p38 MAPK activation is involved in iNOS expression in TNF-α and IL-1-stimulated mouse astrocytes, as well as in LPS-stimulated mouse macrophages [16,17]. A previous study [25] conducted by us also showed that the p38 MAPK pathway is specifically involved in LPS-induced iNOS expression because iNOS mRNA production in the presence of a specific inhibitor of p38 MAPK, SB203580, was dramatically diminished. In contrast, PD98059, a specific inhibitor of MEK1 had no effect on iNOS expression. Thus, magnolol, like SB203580, inhibits the iNOS gene expression by blocking the p38 kinase pathway. The p38 MAPK also regulates LPS-induced TNF-α, IL-1, and IL-10 production in monocytes and TNF-induced IL-6 production in fibroblasts [26-28]. These findings are consistent with the idea that p38 MAPK can be predominantly activated by LPS and inflammatory cytokines such as TNF and IL-1, and can play an important role in the expression of a number of proinflammatory molecules [15].
The present study showed that NF-κB/Rel is positively regulated by LPS for iNOS gene expression, and magnolol treatment of RAW 264.7 cell had significantly inhibited LPS-induced NF-κB/Rel activity. The NF-κB/Rel is a pleiotropic regulator of many genes involved in immune and inflammatory responses, including iNOS [13]. NF-κB/Rel exists in the cytoplasm of unstimulated cells in a quiescent form bound to its inhibitor, IκB. Macrophage activation by certain external stimuli results in the phosphorylation of IκB, thus releasing the active DNA-binding form of NF-κB/Rel to translocate to the nucleus to bind κB motifs in the regulatory region of a variety of genes. Reporter gene assay showed strong induction by LPS of NF-κB/Rel transcriptional activation. Magnolol inhibited the induction of NF-κB/Rel (Fig. 1C). The inhibition of DNA binding of NF-κB/Rel by magnolol was further confirmed by eltrophoretic mobility shift assay.
In summary, these experiments demonstrate that magnolol, a hydroxylated biphenyl compound isolated from Magnolia officinalis, inhibits the LPS-induced expression of iNOS gene in RAW 264.7 cells. Based on the findings of this study, the most likely mechanism that can account for this biological effect involves the inhibition of NF-κ/Rel through negative regulation of p38 kinase pathway. At least two significant findings are brought out by these studies. Firstly, these experiments further cement the criticality of the role played by p38 kinase pathway and NF-κ/Rel in the regulation of iNOS. Secondly, due to the critical role that NO release plays in mediating inflammatory responses, the inhibitory effects of magnolol on iNOS suggest that magnolol may represent a useful anti-inflammatory agent.
ACKNOWLEDGEMENTS
This research was supported by research funds from Chosun University, 2005.
ABBREVIATIONS
- iNOS
indusible nitric oxide synthase
- NF-κB/Rel
nuclear factor κB/Rel
- LPS
lipopolysaccharide
References
- 1.Wang JP, Hsu MF, Raung SL, Chen CC, Kuo JS, Teng CM. Anti-inflammatory and analgesic effects of magnolol. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:707–712. doi: 10.1007/BF00168746. [DOI] [PubMed] [Google Scholar]
- 2.Teng CM, Yu SM, Chen CC, Huang YL, Huang TF. EDRF-release and Ca2+-channel blockade by magnolol, an anti-platelet agent isolated from Chinese herb Magnolia officinalis, in rat thoracic aorta. Life Sci. 1990;47:1153–1161. doi: 10.1016/0024-3205(90)90176-r. [DOI] [PubMed] [Google Scholar]
- 3.Fujita S, Taira J. Biphenyl compounds are hydroxy radical scavengers: their effective inhibition for UVinduced mutation in Salmolella typhimurium TA102. Free Radic Biol Med. 1994;17:273–277. doi: 10.1016/0891-5849(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang JP, Lin PL, Hsu MF, Chen CC. Possible involvement of protein kinase C inhibition in the reduction of phorbol ester-induced neutrophil aggregation by magnolol in the rat. J Pharm Pharmacol. 1998;50:1167–1172. doi: 10.1111/j.2042-7158.1998.tb03329.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang JP, Hsu MF, Raung SL, Chang LC, Tsao LT, Lin PL, Chen CC. Inhibition by agnolol of formylmethionyl-leucyl-phenylalanine-induced respiratory burst in rat neutrophils. J Pharm Pharmacol. 1999;51:285–294. doi: 10.1211/0022357991772466. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Lin SJ, Chen JW, Ku HH, Chen YL. Magnolol attenuates VCAM-1 expression in vitro in TNF-a-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br J Pharmacol. 2002;135:37–47. doi: 10.1038/sj.bjp.0704458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda H, Kageura T, Oda M, Morikawa T, Sakamoto Y, Yoshikawa M. Effects of constituents from the bark of Magnolia obovata on nitric oxide production in lipopolysaccharide-activated macrophages. Chem Pharm Bull. 2001;49:716–720. doi: 10.1248/cpb.49.716. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Jung E, Park J, Jung K, Lee S, Hong S, Park J, Park E, Kim J, Park S, Park D. Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-kappaB activation signaling. Planta Med. 2005;71:338–343. doi: 10.1055/s-2005-864100. [DOI] [PubMed] [Google Scholar]
- 9.Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 10.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 11.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for Larginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 12.Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 14.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen- activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva J, Pierrat B, Mary JL, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J Biol Chem. 1997;272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 17.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide snthase induction mediated by lipopolysaccharide in RAW 2647 cells. Mol Pharmacol. 1999;55:481–488. [PubMed] [Google Scholar]
- 18.Jeon YJ, Yang KH, Pulaski JT, Kaminski NE. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor- kappa B/Rel activation. Mol Pharmacol. 1996;50:334–341. [PubMed] [Google Scholar]
- 19.Xie H, Chiles TC, Rothstein TL. Induction of CREB activity via the surface Ig receptor of B cells. J Immunol. 1993;151:880–889. [PubMed] [Google Scholar]
- 20.Jeon YJ, Han SH, Lee YW, Yea SS, Yang KH. Inhibition of NF-kappa B/Rel nuclear translocation by dexamethasone: mechanism for the inhibition of iNOS gene expression. Biochem Mol Biol Int. 1998;45:435–441. doi: 10.1080/15216549800202822. [DOI] [PubMed] [Google Scholar]
- 21.Ruppert J, Welch W, Jain AN. Automatic identification and representation of protein binding sites for molecular docking. Protein Sci. 1997;6:524–533. doi: 10.1002/pro.5560060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 23.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 24.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon YJ, Kim YG, Lee M, Park SM, Han SB, Kim HM. Radicicol suppresses expression of inducible nitric oxide synthase by blocking p38 kinase and nuclear factor-kB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000;294:548–554. [PubMed] [Google Scholar]
- 26.Foey AD, Parry S, Williams LM, Feldmann M, Foxwel lBM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-a: Role of the p38 and p442/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 27.Beyaert F, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 28.Chae HJ, Kim HK, Lee WK, Chae SW. Bolckade of p38 Mitogen-activated protein kinase pathway inhibits interleukin-6 release and expression in primary neonatal cardiomyocytes. Korean J Physiol Pharmacol. 2002;6:319–325. [PubMed] [Google Scholar]