Abstract
Many therapeutic roles have been proposed for sigma-1 receptor (Sig-1R), but the involvement of Sig-1R in neuropathic pain has currently not been well explored. The present study aimed to evaluate the anti-nociceptive effect of Sig-1R antagonist (BD1047) in a rat model of chronic compression of the dorsal root ganglion (CCD), which is a model of human foraminal stenosis and radicular pain. When stainless steel rods were inserted into the intervertebral foramen of lumbar vertebrae 4 and 5, the CCD developed reliable mechanical (from 3 day) and cold allodynia (from 1 day) as compared with the sham operation group. The spinal expressions of Sig-1R and phosphorylation of extracellular signal-regulated kinase (pERK) were significantly increased from day 3 to day 14 after CCD surgery, as is consistent with the manifestation of allodynia. The BD 1047 (10, 30, 100 mg/kg) administered on postoperative days 0~5 dose-dependently suppressed both the induction of allodynia and the elevation of the spinal pERK expression in a manner comparable with that of gabapentin (100 mg/kg). At 7 days post-CCD surgery, BD1047 (10, 30, 100 mg/kg) administration also produced anti-nociceptive effects on the mechanical and cold allodynia similar with those of gabapentin (100 mg/kg). Therefore, this data suggested that Sig-1R may play an important role in both the development and maintenance of CCD-induced neuropathy.
Keywords: Allodynia, Dorsal root ganglion, Extracellular signal-regulated kinase, Neuropathic pain, Sigma-1 receptor
INTRODUCTION
The accumulated data has revealed that sigma-1 receptors (Sig-1R) are a modulator of a variety of receptors and ion channels, and they act as amplifiers in signal transduction cascades [1]. It has been shown in Sig-1R knockout mice that both phases of formalin-induced paw licking/biting behavior are reduced by approximately 55% as compared to that of wild-type animals [2]. We observe that the Sig-1R antagonist BD1047 has an anti-nociceptive effect in several pain models, including formalin-induced pain behaviors and capsaicin-induced headache [3-5]. Moreover, studies with selective Sig-1R ligands and Sig-1R knockout mice have suggested that Sig-1R is essential for capsaicin-induced mechanical hypersensitivity [6]. Taken together, the accumulated data from these pain models provides evidence to consider using selective Sig-1R antagonists as an innovative approach for treating nociceptive pain.
Peripheral neuropathic pain, which results from damage or dysfunction of peripheral nerves, is one of the most challenging chronic pain conditions to treat as compared with treating nociceptive pain. Major intractable pain symptoms caused by neuropathic pain are known as allodynia evoked by thermal or mechanical stimuli. Our recent study reveals that dehydroepiandrosterone (DHEA) sulphate a proposed endogenous Sig-1R ligand, dose-dependently produces mechanical allodynia in naïve animals with reversible manner by BD1047 [5]. Moreover, DHEA faciliates the induction of mechanical allodynia in the sciatic nerve injury induced neuropathic pain model in rats, which is blocked BD1047 [6]. These overall study shows that the activation of Sig-1R may evoke mechanical allodynia, thus its selective blockage has a therapeutical potential for neuropathic pain. Supporting to this assumption, cold and mechanical allodynia did not develop in Sig-1R null mice exposed to partial sciatic nerve injury [7]. In addition, a recent study from our laboratories reported that BD1047 administered intrathecally during the induction phase, but not the maintenance phase, significantly attenuated mechanical allodynia following chronic constriction injury of the right sciatic nerve in rats [8]. All this data indicates that spinal activation of Sig-1R is also involved in the sciatic nerve injury-induced pain sensation.
On the other hand, activation and sensitization of nociceptive dorsal root ganglion (DRG) neurons can lead to chronic low back pain, sciatica, allodynia and other manifestations of lumbar radiculopathy. This may occur in humans when chronic compression on the DRG (CCD) is evoked by a herniated lumbar disk, or when the DGR is exposed to the herniated nucleus pulposus, but there is minimal morphological abnormality. It is notable that CCD produces profound effects on tetrodotoxin-resistant and tetrodotoxin-sensitive sodium currents, and these effects are different from those caused by sciatic nerve injury [9]. Because the pathological impact of each type of injury is different (soma vs axon), animal models generate distinct or controversial results that need to be specifically analyzed within the context of each experimental condition. Thus, the present study aimed to investigate whether Sig-1R was involved in the CCD induced neuronal excitability during both the induction and maintenance phases.
A large number of studies have provided evidence that the mitogen activated protein kinases (MAPKs) pathways contribute to pain sensitization after tissue and nerve injury via distinct molecular and cellular mechanisms. Activation (phosphorylation) of MAPKs under different persistent pain conditions results in the induction and maintenance of pain hypersensitivity via non-transcriptional and transcriptional regulation [10]. In particular, phosphorylated extracellular signal-regulated kinase (pERK) in the spinal cord dorsal horn neurons plays an important role in the induction and maintenance of pain hypersensitivity caused by partial sciatic nerve injury [7,11]. For this reason, we further examined whether pERK is changed in the spinal cord after CCD and we explored the role of pERK in the modulation of neuronal excitability by Sig-1R.
METHODS
Animals
Male Sprague-Dawley rats (Dae Han Biolink Co., Eumsung, South Korea) were housed in colony cages with free access to food and water and maintained in temperature and light controlled rooms (23±2℃, 12/12 h light/dark cycle with lights on at 08:00). All of the methods used in the present study were approved by the Institute of Animal Care and Use Committee at Chonbuk National University and conform to NIH guidelines (NIH publication No. 86-23, revised in 1985).
Neuropathic surgery (CCD)
Under anesthesia by an intraperitoneal injection mixture of ketamine (90 mg/kg) and xylazine (9 mg/kg), the transverse process and intervertebral foramina of L4 and L5 were exposed unilaterally as previously described [12]. A stainless steel L shaped rod (0.63 mm in diameter and 4 mm in length) was inserted into each foramen, one at L4 and the other at the L5 ganglion.
Drug treatment
BD1047 dihydrobromide (Tocris, Avonmouth, United Kingdom) is a selective antagonist for Sig-1R and it has a greater affinity for the Sig-1R than for the Sig-2R. Because gabapentin (Sigma Chemical, St. Louis, MO, USA) is an anticonvulsant that successfully treats many neuropathic pain syndromes, it was used for a positive drug control as previously described [13]. Both BD1047 and gabapentin were dissolved in saline and injected orally (0.5 ml/100 g body weight) into the CCD-induced neuropathic rats. Drug treatments were performed twice a day, either on postoperative days 0~5 or on postoperative day 7. The control animals were injected with the same volume of saline.
Allodynia test
Behavioral tests were performed 1 day before CCD surgery on all the animals to obtain the normal baseline values of the withdrawal response to mechanical and cold stimuli. The animals were randomly assigned to each drug-treatment group, and all the subsequent behavioral testing was performed with the evaluators being 'blind' to the animals' group assignment.
The number of paw withdrawal responses to normally innocuous mechanical stimuli was measured by using a von Frey filament with a force of 2.0 g (North Coast Medical, Morgan Hill, CA). Rats were placed on a metal mesh grid under a plastic chamber, and the von Frey filament was applied from underneath the metal mesh flooring to each hind paw. The von Frey filament was applied 10 times to each hind paw, and the number of paw withdrawal responses out of 10 was then counted. The results of the mechanical behavioral testing for each experimental animal were expressed as a percent of the withdrawal response frequency, which represented the percentage of paw withdrawals out of the maximum of 10 as previously described [8].
Cold stimulation of the hind paw was carried out as previously described [14]. A jet of 100 µl of acetone was applied to the middle of the plantar surface of the hind paw, with the aid of an insulin syringe and from a short distance (5 mm), through the wire mesh floor of the observation chamber. The shakes and/or licking of the hind paw, due to the cooling evoked by acetone's evaporation, were observed over the first 2 min following application and this was used as an index of the nociceptive responsiveness to cold stimulation. The frequency of nociceptive responsiveness during 5 time trials with 5-min intervals was calculated.
Western blotting
The right dorsal quadrants of the L4-L6 spinal cords were extracted at different time points (at 0, 1, 3, 5 and 7 days; n=5/each time point) and stored in liquid nitrogen. The spinal dorsal horn segments were homogenized in buffer containing 1 M Tris (pH 7.5), 1% NP-40, 0.5 M EDTA (pH 7.5), 50 mM EGTA, 1 M dithiothreitol, 1 M benzanidine and 0.1 M PMSF. The total amount of protein in each sample was determined using the Bradford assay before loading the proteins on to polyacrylamide gels. The spinal cord homogenates (50 µg protein) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and they were transferred to nitrocellulose membranes. After the blots had been washed with TBST (10 mM Tris-HCl [pH 7.6], 150 mM NaCl and 0.05% Tween-20), the membranes were blocked with 5% skim milk for 1 h and then they were incubated with the appropriate primary antibody for Sig-1R (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA), p44/42 MAPK (1:1,000, Cell Signaling Technologies, MA, USA), phospho-p44/42 MAPK (1:1,000, Cell Signaling Technologies, MA, USA) and β-actin (loading control; Sigma, St. Louis, MO). The membranes were then washed, the primary antibodies were detected using the appropriate secondary immunoglobulin G conjugated to horseradish peroxidase, and the bands were subsequently visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, Uppsala, Sweden). The positive pixel area of the specific bands was then measured with a computer-assisted imaging analysis system (Metamorph; Universal Imaging Co., West Chester, PA) and this was normalized against the corresponding loading control bands. Normalization was performed by calculating the percent of the positive pixel area of the Sig-1R as compared to that of the loading control in each experiment. The normalized data from before nerve injury (0 day) was set as 100%, and the results are shown as the percent change from the pre-CCD condition at each time point.
Data and statistical analysis
Data values were expressed as the mean±SEM. All data were analyzed using the commercially available software GraphPad Prizm 5.0 (Graphpad Software, San Diego, CA, USA). Statistical analysis was carried out using One-way analysis of variance (ANOVA) for repeated measures followed by Post hoc Newman-Keuls Multiple Comparison test.
RESULTS
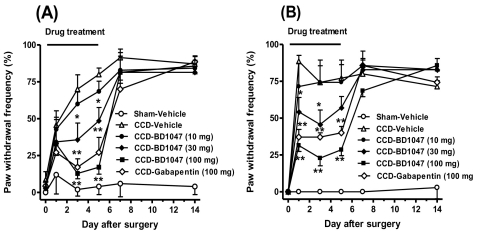
The animal in the CCD group developed characteristic neuropathic pain behaviors, including mechanical and cold allodynia. The paw withdrawal frequency by the von Frey filament (2 g) was significantly increased from 3 day following CCD surgery as compared with that of the sham operative animals and this pain behavior persistently remained until 2 week after surgery (Fig. 1A). Similarly, the paw withdrawal frequency by acetone was significantly increased in the CCD group, which began on postoperative day 1 and it lasted up to 14 days after CCD (Fig. 1B).
Fig. 1.
Repeated daily (from days 0 to 5) oral administration of BD1047 (10, 30, 100 mg/kg) or gabapentin (100 mg/kg) prevented mechanical allodynia (A) and cold allodynia (B) following chronic compression of dorsal root ganglion (CCD). *p<0.05, **p<0.01 compared to CCD-Vehicle group. Each group contains 7 animals.
Repeated daily (from days 0 to 5) oral treatment with BD1047 (10, 30, 100 mg/kg) dose-dependently reduced the increase of paw withdrawal frequency by either mechanical (Fig. 1A) or cold stimuli (Fig. 1B) as compared with that of the vehicle-treated CCD rats, and the anti-nociceptive potency of BD1047 was comparable with gabapentin (100 mg/kg). After termination of repeated BD1047 injection on day 5, this suppressive effect of BD1047 on both mechanical and cold allodynia completely disappeared at next test time point (7 day) (Fig. 1).
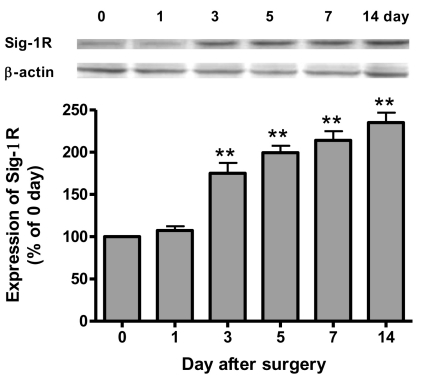
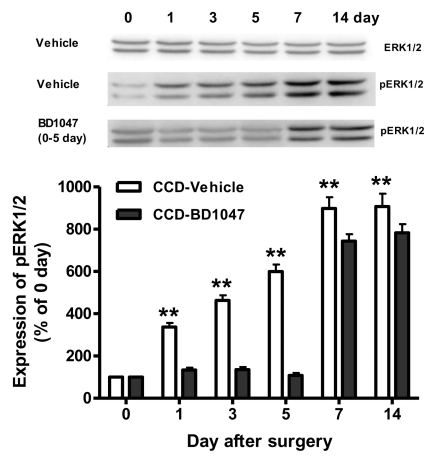
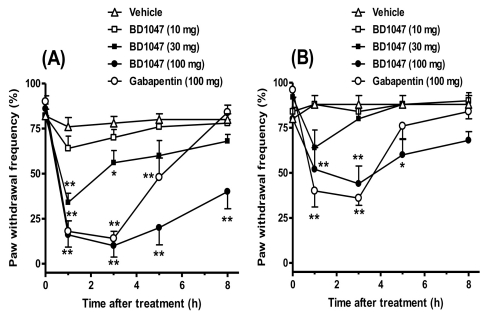
After CCD injury, the expression of Sig-1R in the ipsilateral spinal cord dorsal horn was time-dependently increased from 3 days and this was sustained throughout the 14 day experimental period after CCD surgery (Fig. 2). Although the expression of ERK1/2 in the ipsilateral spinal dorsal horn sample was not changed following CCD surgery (Fig. 3), phosphorylation of ERK1/2 (pERK) was significantly increased from 1 day and this was sustained through 14 day after CCD surgery (Fig. 3). More importantly, the increase of pERK was completely inhibited by the repeated daily (from days 0 to 5) oral treatment with BD1047 (100 mg/kg), whereas this suppressive effect of BD1047 against pERK elevation disappeared after the termination of drug treatment (Fig. 3). On the 7th day at the maintenance phase of the mechanical and cold allodynia established in the CCD rats, a single oral BD1047 treatment (10, 30, 100 mg/kg) dose-dependently reduced the mechanical (Fig. 4A) and cold allodynia (Fig. 4B) as compared with that of the vehicle-treated CCD rats.
Fig. 2.
Western blotting analysis illustrating the change of sigma-1 receptor (Sig-1R) expression in the ipsilateral dorsal quadrant of L4-L6 spinal cords after following chronic compression of dorsal root ganglion (n=5 at each time point). **p<0.01 compared to control group (0 day).
Fig. 3.
Western blotting analysis of the effect of daily BD1047 treatment (100 mg/kg, 0~5 day) on chronic compression of dorsal root ganglion (CCD) induced ERK1/2 expression and phosphorylated ERK1/2 (pERK) in the ipsilateral dorsal quadrant of L4-L6 spinal cords (n=5 at each time point in each group). **p<0.01 compared to control group (0 day).
Fig. 4.
Oral administration of BD1047 (10, 30, 100 mg/kg) or gabapentin (100 mg/kg) suppressed mechanical allodynia (A) and cold allodynia (B) at 7 day after chronic compression of dorsal root ganglion (CCD). *p<0.05, **p<0.01 compared to Vehicle group. Each group contains 7 animals.
DISCUSSION
A large number of therapeutic roles have been proposed for Sig-1R, but the involvement of Sig-1R in chronic neuropathic pain has not been well explored. The present study aimed to elucidate the potential role of Sig-1R in a CCD rat model, which is a model of foraminal stenosis and radicular pain in human. Foraminal stenosis encompasses some of the most common and popular reasons that are used to explain acute and chronic sciatica. Under CCD conditions, spontaneous action potentials can originate from multiple locations in both the soma and the axons of the DRG neurons and these spontaneous action potentials may alter the transmission of sensory information from peripheral receptors [12]. According to this etiology, the paw withdrawal frequency by either mechanical or cold stimuli was significantly increased following CCD surgery as compared with that of the sham operative animals in our study. CCD induced pain behavior (allodynia) was dose-dependently attenuated by oral treatment of the Sig-1R antagonist BD1047 during both the induction (0~5 days) and maintenance phases (day 7). Moreover, the spinal expression of Sig-1R was significantly increased from 3 days to 14 days after CCD surgery, and this was consistent with the manifestation of allodynia. This data indicated that systemic blockage of Sig-1R has a potential therapeutic action against foraminal stenosis and radicular pain during both the early and late phases of these disease conditions.
Very little is known about whether there are differences in the somal (DRG) and axonal (sciatic nerve) mechanisms of generating allodynia. Several lines of evidence have suggested that the origin of spontaneous activity in the CCD was most likely the soma rather than the axons or the terminal endings [12]. Differing alterations of sodium currents in small dorsal root ganglion neurons have also been observed after CCD and sciatic nerve injury [9]. It is notable that the level of the Sig-1R expression and the anti-nociceptive potency according to the treatment time point is different between the DRG compression model and our previous sciatic nerve injury model [8]. Following chronic constriction injury of the right sciatic nerve, the Sig-1R expression significantly increased in the ipsilateral spinal cord dorsal horn from day 1 to day 3 and this was restored to the normal level at 7th day. Furthermore, BD1047 administered during the induction phase (0~5 days), but not the maintenance phase (15~20 days), blocked the mechanical allodynia. Thus, we suggest there is differential modulation by Sig-1R of the sensory/nociceptive pathways depending on the type of nerve injury.
MAPK mediates several cellular responses to mitogenic and differentiation signals, and the activation (phosphorylation) of ERK in the horn neurons by noxious stimulation is known to contribute to pain hypersensitivity. In turn, activity of the dorsal horn neurons promotes activation of spinal glia. This neuron-glia interaction involves ERK signaling in the positive feedback that enhances and prolongs pain sensitization in neuropathic conditions [15]. In several nerve injury models, intrathecal ERK inhibitors reduce pain hypersensitivity when administered during both the induction and maintenance phases of neuropathic pain [16]. The present study demonstrated that the spinal expressions of pERK were significantly increased from day 1 to day 14 after CCD surgery, and this is consistent with the manifestation of allodynia. Both allodynia induction and spinal pERK elevation were completely prevented during the time of BD 1047 treatment (0~5 days after CCD), but the expression of pERK and the allodynia were significantly re-increased after termination of BD1047 treatment up to that of the vehicle pretreated group. This data indicated that Sig-1R induced ERK activation (phosphorylation) could contribute to mechanical and cold allodynia after CCD injury. Furthermore, the Sig-1R knockout mice did not show any increase of pERK in the spinal cord after sciatic nerve injury [7], suggesting that Sig-1R is related to the mechanism of induction of ERK activation in the neuropathic condition.
We found that the level of Sig-1R in the spinal cord is very low in the normal condition, whereas a high density of Sig-1R was observed in the CCD condition. A previous knockout study also showed that the absence of Sig-1R did not influence nociceptive behaviors by themselves in normal conditions [7]. For strengthened Sig-1R activity in the CCD pain state, the endogenous Sig-1R ligands should be increased and this will be accompanied with the increase of the receptor population. The possibility that neurosteroids could be endogenous activators/inactivators of Sig-1R and possibly be even the 'endogenous ligand' for this receptor has generated significant interest in this area [17]. Recent data has demonstrated that the production of neurosteroids such as estradiol and progesterone is up-regulated in DRG neurons after chronic nerve injury [18]. It is further confirmed that intrathecal injection of neurosteroid (i.e. dehydroepiandrosterone sulphate) produces mechanical allodynia and that the development of this allodynia is reproduced by Sig-1R ligands [5-7,19]. It is possible that the CCD-induced persistent pain is produced by the increase of the Sig-1R receptor population as well as production of its endogenous ligands.
In conclusion, the current study demonstrates that (1) the activation of spinal Sig-1R plays a critical role in the induction of mechanical and cold allodynia in CCD neuropathic pain; (2) CCD injury induces persistent up-regulation of Sig-1R in the spinal dorsal horn, and this increased expression is correlated with allodynia induction; (3) the anti-nociceptive effect of Sig-1R antagonist is closely related with ERK activation in the spinal cord. These results suggest the potential therapeutic use of Sig-1R antagonists in the clinical management of neuropathic pain, including foraminal stenosis and radicular pain in human.
ACKNOWLEDGEMENTS
This paper was supported by research funds of Chonbuk National University in 2010.
ABBREVIATIONS
- Sig-1R
sigma-1 receptors
- DRG
dorsal root ganglion
- CCD
chronic compression on DRG
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinase
References
- 1.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006;148:490–498. doi: 10.1038/sj.bjp.0706764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon SY, Roh DH, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of the neurosteroid, DHEAS, produces mechanical allodynia in mice: involvement of spinal sigma-1 and GABA receptors. Br J Pharmacol. 2009;157:666–673. doi: 10.1111/j.1476-5381.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibaly C, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Biochemical and functional evidence for the control of pain mechanisms by dehydroepiandrosterone endogenously synthesized in the spinal cord. FASEB J. 2008;22:93–104. doi: 10.1096/fj.07-8930com. [DOI] [PubMed] [Google Scholar]
- 7.de la Puente B, Nadal X, Portillo-Salido E, Sanchez-Arroyos R, Ovalle S, Palacios G, Muro A, Romero L, Entrena JM, Baeyens JM, Lopez-Garcia JA, Maldonado R, Zamanillo D, Vela JM. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009;145:294–303. doi: 10.1016/j.pain.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, Lee JH. Intrathecal injection of the sigma(1) receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology. 2008;109:879–889. doi: 10.1097/ALN.0b013e3181895a83. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZJ, Song XJ. Differing alterations of sodium currents in small dorsal root ganglion neurons after ganglion compression and peripheral nerve injury. Mol Pain. 2008;4:20. doi: 10.1186/1744-8069-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99:175–184. doi: 10.1016/s0304-3959(02)00097-0. [DOI] [PubMed] [Google Scholar]
- 12.Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–14068. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005;94:1131–1139. doi: 10.1111/j.1471-4159.2005.03263.x. [DOI] [PubMed] [Google Scholar]
- 14.Werner MF, Kassuya CA, Ferreira J, Zampronio AR, Calixto JB, Rae GA. Peripheral kinin B(1) and B(2) receptor-operated mechanisms are implicated in neuropathic nociception induced by spinal nerve ligation in rats. Neuropharmacology. 2007;53:48–57. doi: 10.1016/j.neuropharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- 17.Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neuro-steroidogenesis in sensory neurons. Glia. 2010;58:169–180. doi: 10.1002/glia.20910. [DOI] [PubMed] [Google Scholar]
- 19.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Lee JH. Intrathecal administration of sigma-1 receptor agonists facilitates nociception: involvement of a protein kinase C-dependent pathway. J Neurosci Res. 2008;86:3644–3654. doi: 10.1002/jnr.21802. [DOI] [PubMed] [Google Scholar]