Abstract
Glycyrrhizae radix (GR) is an herbal medicine that is commonly used in the East Asia for treating a variety of diseases, including stomach disorders. The objective of the present study was to examine the anti-stress effects of GR on repeated stress-induced alterations of anxiety, learning and memory in rats. Restraint stress was administered for 14 days (2 h/day) to the rats in the Control and GR groups (400 mg/kg/day, PO). Starting on the eighth day, the rats were tested for spatial memory on the Morris water maze test (MW) and for anxiety on the elevated plus maze (EPM). We studied the changes of the expressions of cholineacetyl transferase (ChAT) and tyrosine hydroxylase (TH) in the locus coerleus (LC) using immunohistochemistry. The results showed that the rats treated with GR had significantly reduced stress-induced deficits on their learning and memory on the spatial memory tasks. In addition, the ChAT immunoreactivities were increased. Gor the EPM, treatment with GR increased the time spent in the open arms (p<0.001) as compared to that of the control group. Moreover, GR treatment also normalized the increases of the TH expression in the LC (p<0.001). In conclusion, administration of GR improved spatial learning and memory and reduced stress-induced anxiety. Thus, the present results suggest that GR has the potential to attenuate the behavioral and neurochemical impairments caused by stress.
Keywords: Glycyrrhizae radix (GR), Morris water maze (WM), Elevated plus maze (EPM)
INTRODUCTION
Restraint stress is an easy and well-known method to induce chronic physical and emotional stress [1]. The emotional and physiological responses to repeated restraint stress are initiated by the activation of the hypothalamic-pituitary adrenal axis (HPA), which results in the release of catecholamines and stress hormones such as glucocorticoids from the adrenal glands [1]. Elevated levels of glucocorticoids exert both short and long-term negative effects on the brain and behavior [1,2]. At the behavioral level, exposure to stress has been reported to increase anxiety-related behavior and to impair learning and memory [3,4]. Many animal studies have demonstrated that chronic stress, as well as chronic corticosterone treatment, results in learning and memory deficits on several behavioral tasks, including the water-maze and passive-avoidance tests, and this impairment was accompanied by hippocampal damage [5-10]. In addition, Ader and Cohen et al and McEwen and Stellar et al reported that repeated stress is a risk factor for psychosomatic psychiatric illness, such as anxiety [11,12].
Glycyrrhizae radix (GR) is an herbal medicine that is most frequently prescribed for the treatment of a variety of diseases, including stomach disorders [13]. It has also been described in classical Asian medicine as an agent with the ability to 'improve the tone of the "middle-jiao" and replenish "qi", to remove "heat" and toxic substances and arrest coughing as well as the relief of spasms and pain [14]. In addition, it is widely used as a flavoring adjuvant in drug preparations and an ingredient of cigarettes for its taste and its properties that reduce irritation [14,15].
The aim of the present study was to investigate the effects of GR on repeated restraint stress-induced anxiety and memory loss in rats by evaluating their performance on the elevated plus maze and the Morris water maze. In addition, the changes in the ChAT and TH expressions in the brain were evaluated in order to elucidate the possible mechanisms involved in the identified anti-stress effects.
METHODS
Subjects and stress procedures
Male Sprague Dawley rats at the age of 8 weeks (Orient, Inc. Korea) were used for the study. The rats were housed under controlled temperature (22~24℃) conditions with a 12 h light/dark cycle. The lights were on from 8:00 to 20:00. Food and water were made available ad libitum. All the experiments were approved by the Catholic University Institutional Animal Care and Use Committee. The rats were allowed at least 1 week to adapt to their environment before the experiments. The male rats were randomly divided into three groups (n=7 per group): the nonstressed group (Normal), the stressed group (Control), and the stressed and Glycyrrhizae radix treatment group (GR). The rats were stressed daily for two weeks using a restraint cone. The GR group was treated daily with GR extract (400 mg/kg, p.o.) for two weeks, and the other groups were given sterile saline. Immobilization was started 30 min after the treatments.
Preparation of herbal extracts
The Glycyrrhizae radix was purchased from an oriental drug store (Jungdo Inc. Seoul, Korea). The specimens were deposited at the herbarium located in the College of Oriental Medicine, Kyung Hee University. The dried Glycyrrhizae radix samples (200 g) were immersed in a 10-fold volume of dH2O, boiled at 80℃ for 1 h and then the water extract was collected. The process was repeated once, and the extracts were combined and concentrated with a rotary evaporator and then vacuum-dried to yield 9.1% pure Glycyrrhizae radix (w/w) extract.
Elevated plus maze
The plus-maze apparatus was constructed with black wood. It consisted of two open-arms (the arms extended from a central 50×10 cm space) and two enclosed arms (50×10×40 cm). The arms extended from a central platform (10×10 cm). The apparatus was elevated 50 cm above the floor. The animals were transported to the testing room at least 1 hr prior to starting the experiment.
After all of the stress sessions, the rats were individually placed in the central platform facing a closed arm and they were allowed to explore the maze for a 5-min test period. The duration of time spent in the open arms and closed arms were the behavioral measures that were recorded for each rat. The apparatus was wiped clean with a damp sponge and dried with paper towels between tests.
Morris water maze test
After 7 days of immobilized stress, all the animals started training on the MWM task in a swimming pool (1.8 m diameter and 0.5 m high, filled with milky water at a temperature in the 22±2℃ range) for 7 days. A 12 cm diameter round platform was hidden in a constant location (the quadrant center) within the pool with its top surface submerged 1.5 cm below the water level. The rats were trained to locate the hidden island during four trials per day for 6 days. After the 6th day, they were started in the quadrant opposite to the target and they were forced to swim for 60s in the pool without a platform. The spatial memory of the rats was assessed as the latency time. The time spent searching for the platform in the training quadrant, i.e., the previous location of the platform, was recorded and this was used as a measure of memory retention. A video camera was mounted on the ceiling above the pool and it was connected to a video-recorder and tracking device (S-MART; Pan-Lab, Barcelona, Spain), which permitted on-line and off-line automated tracking of the path taken by the animal [16].
Immunohistochemistry
After the behavioral tests were completed, the animals were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and then perfused transcardially with 100 ml of saline followed by 500 ml of a 4% solution of formaldehyde prepared in phosphate buffer. The brains were then removed, post-fixed in the same fixative for two to three hours at 4℃ and then the brains were placed overnight at 4℃ in PBS containing 20% sucrose. The following day, the brain was cut into coronal sections that were sliced to 30 µm-thicknesses. The sections were processed for the ChAT immunoreactivity using sheep ChAT polyclonal antibodies (ChAT, concentration 1:2,000; Chemicon international, Temecula, CA.,USA) and the tyrosine hydroxylase (TH) immunoreactivity using mouse-TH monoclonal antibodies (TH, concentration 1:2,000; Zymed Laboratories INC. San Francisco, CA., USA). The sections were then processed by a conventional avidin-biotin-peroxidase method (Vector Laboratories, Burlingame, CA, U.S.A.). The tissue immunoreactivity was developed using diaminobenzadine (Sigma U.S.A.) as the chromogen. The sections were mounted on gelatine-coated slides, air-dried and coverslipped for microscopic analysis. A microrectangular grid (200×200 µm) was placed according to the atlas of Paxinos and Watson [17] under a light microscope (×100 magnification) to measure the cells.
Statistical analysis
Statistical comparisons were performed on the results of the behavioral and histochemical studies using the one-way ANOVA, repeated measures of the two-way ANOVA and the LSD post hoc test. All of the results are presented as means±S.E.M. SPSS 15.0 software for Windows was used for the statistical analysis. The significance level was set at a p values<0.05.
RESULTS
Effects of GR on the elevated plus maze
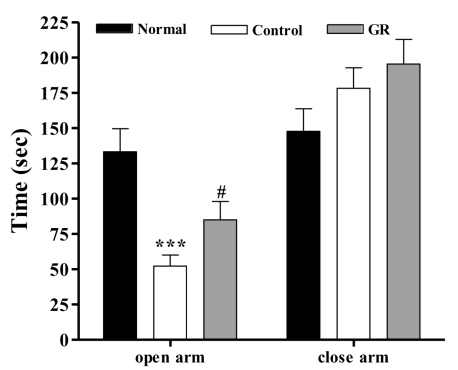
As shown in Fig. 1, the one-way ANOVA performed for assessing the time spent in the closed arms and the time spent in the open arms of the elevated maze revealed significant group differences. The LSD test indicated that the control group spent significantly less time in the open arms [F2,20=12.717, p<0.001] and increased time in the closed arms [F2,20=1.680] compared to that of the normal group. After administration of GR, the time spent in the open arms was significantly increased in the GR group compared to that of the control group (p<0.001).
Fig. 1.
Time spent in the open and closed arms in the elevated plus maze maze. The GR group was daily treated with the GR extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of elevated plus maze maze were analyzed by performing separate one-way ANOVA among the groups were followed by LSD test. Each value represents the mean±S.E.M. ***p<0.001 compared to normal group and & #p<0.05 compared to control group, respectively.
Effects of GR on the morris water maze test
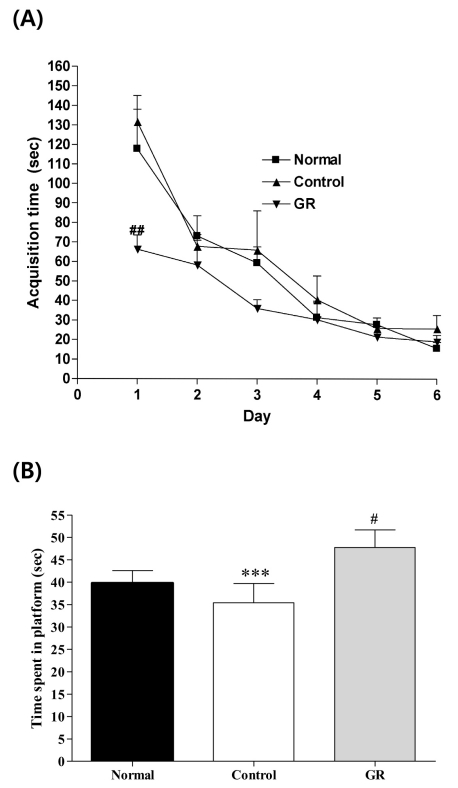
Fig. 2A shows the recorded mean group latencies to reach the hidden platform in the MWM for all the groups over 6 days [F2,18=35.574, p<0.05]. On the first day, the GR group had a significant decrease in the time spent searching for the platform compared to that of the control group (p<0.01). However, after the second day, there were no statistically significant differences among the groups. There was a slight trend for a significant interaction effect on the distance traveled to reach the platform.
Fig. 2.
(A) Changes of the latency time during 6d of the acquisition test in the Morris water maze test. The AM group was daily treated with the GR extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. Repeated measures of two-way ANOVA of swimming time among the groups following by LSD test. Each value represents the mean±S.E.M. ##p<0.01 compared to control group. (B) The latency time on the 7th day of the retention test in the Morris water maze test. The GR group was daily treated with the GR extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments.The results of retention test were analyzed by performing separate measures of one-way ANOVA of swimming time among the groups were followed by LSD test. Each value represents the mean±S.E.M. ***p<0.001 compared to normal group and & #p<0.05 compared to control group, respectively.
To examine the spatial memory, on the seventh day of retention testing, the time spent swimming to the platform was compared among the groups, as is shown in Fig. 2B. The times spent around the platform were significantly different among the groups [F2,20=28.226, p<0.001]; the GR group spent more time around the platform than did the control group (p<0.001).
Effects of GR on the cholinergic system
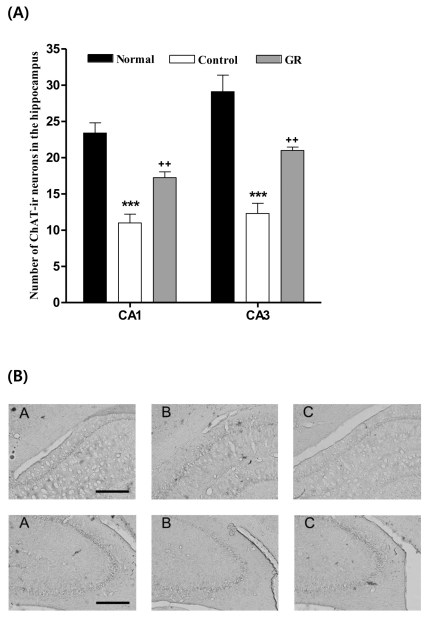
The results of the evaluations of the ChAT immunoreactive cells per section, from different hippocampal formations, are shown in Fig. 3. The number of ChAT neurons in the CA1 area was 23.4±1.4 in the normal group, 11.0±1.2 in the control group and 22.9±2.5 in the GR group [F2,23=28.2, p<0.001]; these results show a two-fold increase in the number of ChAT neurons in the GR group compared to that in the controls (p<0.001). The ChAT immunoreactive cells in the CA3 area were 29.1±2.3 in the normal group, 12.3±1.4 in the control group and 27.8±1.6 in the GR group [F2,23=27.0, p<0.001]. Thus, the number of ChAT positive neurons in the GR group was increased by 226% compared to that in the control group (p<0.001).
Fig. 3.
(A) Number of choline acetyltransferase (ChAT) immunostained nuclei in the different hippocampal areas of the experimental groups after 8d of the behavior test. The GR group was daily treated with the GR extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments.The results of ChAT-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. ***p<0.001 compared to normal group and ++p<0.01 compared to control group. (B) Photographs showing the distribution of ChAT-immunoreactive cells in hippocampus of Normal group (A), Control group (B), GR group (C). Sections were cut coronally at 30 µm and the scale bar represents 200 µm (200×200).
Effects of GR on the catecholaminergic system
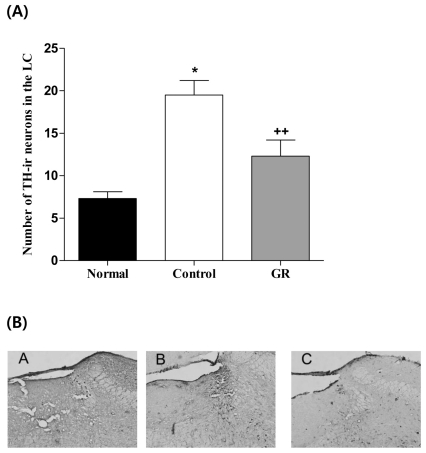
The results of the TH immunoreactive cells per section, from the locus coeruleus, are shown in Fig. 4. The TH immunoreactive cells in the LC area were 7.3±0.8 in the normal group, 19.5±1.7 in the control group and 8.8±0.9 in the GR group [F2,28=30.6, p<0.001]. Thus, the number of TH positive neurons in the GR group was decreased by 45.0% compared to that of the control group (p<0.001).
Fig. 4.
Number of Tyrosine hydroxylase (TH) immunostained nuclei in the locus coerleus areas of the experimental groups. The AM group was daily treated with the GR extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of TH-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. *p<0.05 compared to normal group and ++p<0.01 compared to control group. (B) Photographs showing the distribution of TH-immunoreactive cells in LC of Normal group (A), Control group (B), GR group (C). Sections were cut coronally at 30 µm and the scale bar represents 200 µm (200×200).
DISCUSSION
The main findings of this study were that treatments with the crude extract of GR reduced the repeated stress-induced anxiety and memory loss. Moreover, treatment with GR significantly suppressed the increases of the TH expression and it enhanced the ChAT expression in the stressed rats.
The restraint stress used in this study is widely used in studies on the molecular and behavioral effects of chronic stress, and it has been very helpful in improving our understanding of stress-related brain pathology and the changes in cognition [1]. Restraint elicits a variety of physiological stress responses; the magnitude of these responses can be decreased or increased by a prior stress history [1,11,18]. To establish a practical animal model for chronic stress, we examined the behavioral traits of anxiety and memory loss as stress-assessment parameters. We found that 2 hours for 14 days of restraint stress significantly induced anxiety and memory loss, and that repeated restraint stress changed the expression of neurotransmitters in the brain.
The degeneration of the cholinergic innervations from the basal forebrain to the hippocampal formation in the temporal lobe is thought to be one of the factors involved in determining the progression of memory decay, both during normal aging and in AD [16]. The best available marker for cholinergic neurons in the basal forebrain is the ChAT activity. ChAT is the biosynthetic enzyme for Ach, and ChAT is presently the most specific cholinergic marker for checking the nervous system [19]. According to the cholinergic hypothesis, memory impairments in patients with senile dementia are due to a selective and irreversible deficiency in the cholinergic functions in the brain (the basal forebrain, cortex, hippocampus and amygdala) [20]. Therefore, ChAT activators may compensate for the reduced Ach levels in the brains with AD disease. A significant reduction in ChAT activity has been reported in the postmortem brains of mentally impaired patients. In addition, Gottesfeld et al. showed a decrease in ChAT activity in the hippocampus after repeated immobilization stress [21,22]. However, in the present study, treatment with GR prevented stress-induced loss of ChAT-immunoreactive neurons, suggesting that GR exerts beneficial effects on cholinergic neurotransmission in the brain by increasing ChAT activity in the hippocampus. The behavioral and neurochemical results of this study indicate that the memory-enhancing effects of GR may be mediated by cholinergic mechanisms in the rat brain.
Most norepinephrine (NE)-containing neurons in the brain are concentrated in the nucleus locus coeruleus (LC) [23,24]. These neurons are part of a widespread network with a broad range of functions, and this network extends throughout the neuroaxis and accounts for about 70% of all brain NE in primates [23]. Activation of the LC produces intense anxiety, hypervigilance and an inhibition of exploratory behavior [23,26,27]. Based on this data, it has been proposed that clinical anxiety or depression may be the result of alterations in the activity of the LC noradrenergic system. The LC is considered a crucial site for the CNS stress response. An abundance of data collected using different techniques has shown that corticotropin releasing hormone can modulate the rate of discharge of NE and TH in LC neurons [28-30]. In this study, the repeated restraint stress caused a 35.0% increase in the TH-ir cells in the LC. However, treatment with GR significantly reduced the expected increases of the TH-ir cells in the LC. These results suggest that treatment with GR effectively decreased the over-expressed TH-ir neurons in the LC.
The present study investigated the effects of GR on repeated restraint stress-induced anxiety and memory loss in rats by evaluating their performance on the elevated plus maze and the Morris water maze. In addition, the changes in the ChAT and TH expression in the LC were determined to be the possible mechanisms involved in the identified anti-stress effects.
In conclusion, the results of this study show that extract of GR possesses anti-stress properties in rats. However, further studies are necessary to confirm and extend these results. These findings provide evidence that GR is useful for reducing stress under certain circumstances.
ACKNOWLEDGEMENTS
This study was supported by Hi Seoul Science (Humanities) Fellowship from Seoul Scholarship Foundation and the Korea Healthcate Technology R&D Project, Ministry for Health, Welfare & Family Affairs (grant no. A091037).
ABBREVIATIONS
- GR
Glycyrrhizae radix
- ChAT
choline acetyltransferase
- TH
tyrosine hydroxylase
- LC
locus coerleus
References
- 1.Chrousos GP. Stressor, stress and neuroendocrinology intergration of the adaptive response. The 1997 Hans Selye Memorial lecture. Ann NY Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 2.Pare WP. The effects of chronic environmental stress and stomach ulceration, adrenal function and consummatory behavior in the rat. J Psychol. 1964;57:143–151. doi: 10.1080/00223980.1964.9916683. [DOI] [PubMed] [Google Scholar]
- 3.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversibal impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 4.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlrodiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 5.Bisagno V, Grillo CA, Piroli GG, Giraldo P, MaEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaprophysin levels in female rats. Pharmacol Biochem Behav. 2004;78:541–550. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticoserone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn-Litvak PS, Tata DA, Gorby HE, McCloskey DP, Richardson G, Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but nor glial volume fraction in hippocampal area CA3. Neuroscience. 2004;124:429–438. doi: 10.1016/j.neuroscience.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Krugers HJ, Goltstein PM, van der Linden S, Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci. 2006;23:3051–3055. doi: 10.1111/j.1460-9568.2006.04842.x. [DOI] [PubMed] [Google Scholar]
- 9.Mclay R, Klinski A. Changes in prescription habits with the introduction of generic fluoxetine. Mil Med. 2008;173:100–104. doi: 10.7205/milmed.173.1.100. [DOI] [PubMed] [Google Scholar]
- 10.Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- 11.Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Stellar E. Stress and the individual:mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 13.Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- 14.Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG. Cytoprotective aeffects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and PARP cleavage. Toxicology. 2004;197:239–251. doi: 10.1016/j.tox.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Kee CH. The pharmacology of Chinese herbs. 2nd ed. Boca Ranton: CRC press; 1999. pp. 363–367. [Google Scholar]
- 16.Morris RG. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C, Pennisi M. Bregma, lamda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang YT, Tan QR, Sun LL, Cao J, Dou KF, Xia B, Wang W. Possible therapeutic effect of a traditional chinese medicine, sinisan, on chronic restraint stress related disorders. Neurosci Lett. 2008;449:215–219. doi: 10.1016/j.neulet.2008.10.100. [DOI] [PubMed] [Google Scholar]
- 19.Oda Y. Choline acetyltransferase: the structurem distribution and pathologic changes in the central nervous system. Pathology International. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 20.Giacobini E. Long term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer's disease. J Neural Transm Suppl. 2002;(62):181–187. doi: 10.1007/978-3-7091-6139-5_17. [DOI] [PubMed] [Google Scholar]
- 21.Gottesfeld Z, Kvetnansky R, Kopin IJ, Jacobowitz DM. Effects of repeated immobilization stress on glutamate decarboxylase and choline acetyltransferase in discrete brain regions. Brain Res. 1978;152:374–378. doi: 10.1016/0006-8993(78)90267-6. [DOI] [PubMed] [Google Scholar]
- 22.Park HJ, Han SM, Yoon WJ, Kim KS, Shim I. The effect of Puerariae Flos on stress-induced deficits of learning and memory in ovariectomized female rats. Korean J Physiol Pharmacol. 2009;13:85–89. doi: 10.4196/kjpp.2009.13.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinan TG, Aston-Jones G. Acute haloperidol increases impulse activity of brain noradrenergic neurons. Brain Res. 1984;307:359–362. doi: 10.1016/0006-8993(84)90495-5. [DOI] [PubMed] [Google Scholar]
- 24.Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 2007;41:61–73. [PubMed] [Google Scholar]
- 25.Foote SL, Berridge CW, Adams LM, Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog Brain Res. 1991;88:521–532. doi: 10.1016/s0079-6123(08)63831-5. [DOI] [PubMed] [Google Scholar]
- 26.Redmond DE, Jr, Huang YH. The primate locus coerleus and effects of clonidine on opiate withdrawal. J Clin Psychiatry. 1982;43:25–29. [PubMed] [Google Scholar]
- 27.Melia KR, Rasmussen RZ, Terwillinger JW, Haycook EJ, Nestler RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase the rat locus coeruleus: Effects of chronic stress and drug treatment. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 28.Valentino RJ, Foote SL. Corticotropin-releasing factor distrups sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- 29.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotrophin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 30.Ma S, Mifflin SW, Cunningham JT, Mortilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coerleus in response to subsequent immobilization stress. Neuroscience. 2008;154:1639–1647. doi: 10.1016/j.neuroscience.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]