Abstract
Osteomyelitis, or bone infection, is a major worldwide cause of morbidity. Treatment is frequently unsatisfactory, yet little is known about pathogenesis of infection. Plasma tumor necrosis factor (TNF), interleukin (IL)-6, and IL-8 concentrations were measured before and after lipopolysaccharide stimulation of whole blood from patients with bacterial and tuberculous osteomyelitis and from controls. Patients with bacterial and tuberculous osteomyelitis mounted an acute-phase response and were anemic and febrile. However, plasma IL-6 concentrations were significantly elevated in only tuberculous osteomyelitis patients (vs. controls, P < .05). IL-6 concentrations correlated with erythrocyte sedimentation rate, C-reactive protein level, and plasma albumin concentration, all acute-phase markers. There were no other correlations between cytokine concentrations and clinical data. Following ex vivo stimulation, TNF, IL-6, and IL-8 were secreted equally by patients and controls. In summary, tuberculous osteomyelitis is characterized by elevated systemic IL-6 concentrations associated with an acute-phase response. For further insight into immunopathology of osteomyelitis, studies on infected bone are required.
Osteomyelitis, or bone infection, is a common clinical problem throughout the world, causing major economic loss and personal morbidity, although exact figures on incidence have not been collected. The majority of cases occur in children, and osteomyelitis usually arises following hematogenous spread of organisms, although infection may also result following trauma [1]. Most patients have mild systemic symptoms, such as fever and malaise, but the intensity of local symptoms, such as pain and tenderness, can range from minimal to severe. Disease may become chronic despite combined surgical and medical therapy [2]. The most common bacterial causes of osteomyelitis are Staphylococcus aureus and Staphylococcus epidermidis. The other major pathogen that causes osteomyelitis is Mycobacterium tuberculosis.
The World Health Organization estimates that ~1.6 billion people are infected with M. tuberculosis worldwide [3], and tuberculosis kills more people than any other single infectious disease [4]. Approximately 2% of cases of tuberculosis affect the skeletal system, and of these, >50% localize to the vertebral column [5]. The worldwide increase in cases of tuberculosis, due in part to the human immunodeficiency virus (HIV) epidemic, has led to increased numbers of patients with tuberculous osteomyelitis in all countries, including the United States [6]. However, the treatments for both bacterial and tuberculous osteomyelitis are frequently unsatisfactory, requiring long-term antimicrobial chemotherapy often combined with surgery, and disease may recur many years after apparent cure [1]. Surprisingly, data on the pathogenesis of osteomyelitis are very limited, and immune responses in this infection are poorly characterized.
In most bacterial infections, proinflammatory cytokines have a central role in host defense to infection (reviewed in [7]). Many in vivo studies of systemic bacterial infection have demonstrated that tumor necrosis factor (TNF) [8, 9], interleukin (IL)-6 [10], and the chemokine IL-8 [11-13] are all important components of the proinflammatory response. However, data from osteomyelitis patients are few. In the one published study from Germany, proinflammatory mediators were measured in patients with posttraumatic osteomyelitis; acute but not chronic infection was associated with elevated plasma concentrations of TNF, IL-6, and IL-8 [14]. Patients with hematogenous osteomyelitis were not investigated, and comparative data are not available.
The principal tissue immune response in tuberculous osteomyelitis is granuloma formation, classically with caseation. TNF has a pivotal role in the development of such antimycobacterial granulomas [15]. Tuberculous osteomyelitis is frequently an occult disease with few localizing signs; its main clinical features are attributable to an active acute-phase response. Secretion of acute-phase proteins is driven by IL-6 [16], and it is clear that M. tuberculosis may activate transcription of the IL-6 gene [17]. IL-8 is also an important component of the immune response in tuberculosis and appears to be involved in the recruitment of antigen-specific T lymphocytes to the granuloma both in vitro [18, 19] and in vivo [20-22]. However, there are no data on the role of these cytokines in tuberculous osteomyelitis.
In the present study, to determine whether or not localized osteomyelitis stimulated a significant systemic cytokine response, we first measured the plasma concentrations of TNF, IL-6, and IL-8 in bacterial and mycobacterial osteomyelitis patients and in control subjects. Second, we investigated the ex vivo lipopolysaccharide-stimulated secretion of such cytokines, using a whole blood culture system [23, 24]. The advantage of this system is that it requires small volumes of blood, is clinically robust, does not require purification of leukocytes (which may be associated with cytokine gene activation [25]), and uses a physiologic milieu. The relative drawback of the whole blood method is that it is not easy to determine which leukocyte types are secreting cytokines, and results are influenced by the leukocyte differential count. However, the whole blood system has provided useful data in the study of immunopathogenesis of bacterial [13, 26, 27], mycobacterial [21], and viral [28] infections.
Patients and Methods
Study population
Twenty-five consecutive patients admitted to the orthopedic wards of the University Teaching Hospital, Lusaka, Zambia, with bone infection were entered into this study. The study was performed over 2 months, during which 61% of orthopedic inpatients were hospitalized because of bone or joint infections. Patients were stratified on the basis of standard clinical, laboratory, and radiologic assessment as having either bacterial or tuberculous osteomyelitis. Entering the study (or choosing not to) did not affect clinical management protocols, and no patient declined to participate. However, 2 patients were excluded from this study because of diagnostic uncertainty. All patients included in the study had no other acute medical problems.
In addition, 13 control subjects were recruited. The original intention had been to recruit all controls from healthy relatives of patients, but few were willing to volunteer. Therefore, controls were either patients with chronic mechanical, noninflammatory disorders such as contractures following childhood polio (n = 8) or entirely healthy relatives of patients (n = 5). All controls were asymptomatic and appeared to be in good health on screening by questionnaire.
Clinical evaluation
Patients were admitted, and a routine clinical history and examination were performed. Patients had samples taken for microbiologic analysis and underwent plain radiology (computed tomography scanning and magnetic resonance imaging were not available). In addition, a full blood cell count and erythrocyte sedimentation rate (ESR) were obtained in line with local practice. An additional 5-mL blood sample was taken from the patients’ antecubital vein, anticoagulated with potassium-EDTA (Sigma, Poole, UK), and immediately centrifuged at 1000 g for 5 min. Plasma was stored and subsequently analyzed in the UK for a full biochemical profile, which included renal and liver function tests and measurement of the acute-phase reactant C-reactive protein (CRP). Anonymous testing for the presence of either HIV-1 or -2 antibody was performed by VIDAS ELISA (bioMérieux, Basingstoke, UK).
Experimental protocols
At the same venesection was performed to obtain a sample for biochemical profile, a further 10 mL of blood was collected, anticoagulated with potassium-EDTA, and split into 2 5-mL aliquots, each in polypropylene endotoxin-free tubes. The first aliquot was immediately centrifuged at 1000 g for 5 min, and the plasma was stored at −30°C for later cytokine analysis. The second 5-mL aliquot of blood was stimulated ex vivo with lipopolysaccharide (LPS) from Escherichia coli (serotype O127:B8; Sigma) added to a final concentration of 1 μg/mL ex vivo. After incubation at 37°C for 24 h, the plasma was separated by centrifugation and stored as above. Subsequently, samples were taken to the United Kingdom on dry ice and thereafter kept at ~80°C. We have previously shown that there is no loss of cytokine bioactivity when samples are stored in this manner [11].
Cytokine assays
Plasma TNF concentrations were assayed using the highly sensitive WEHI 164 cell line, subclone 13 (gift of A. Waage, University of Trondheim, Trondheim, Norway) as previously described [29]. Plasma IL-6 concentrations were measured using the B9 cell proliferation assay [30], which we have previously shown to be specific for this cytokine [31]. Plasma IL-8 concentrations were measured using a validated in-house ELISA [32]. All samples were handled as potentially infectious, using appropriate containment facilities. Measurements were done in duplicate with appropriate controls and carried out by a laboratory blinded to the clinical details.
Statistical analysis
Normally distributed (clinical) data are shown as means (±SE); groups were compared by the unpaired t test. Nonparametric (cytokine) data are shown as medians (ranges), and groups were compared using the Mann-Whitney U test. Assessment of whether two variables were independent of each other was by calculation of Spearman’s rank correlation coefficient (ρ). All analyses were performed using Statview version 4.0 for the Macintosh (Abacus Concepts, Berkeley, CA). P < .05 was considered a significant difference between groups.
Results
Thirteen control subjects, 13 bacterial osteomyelitis patients, and 12 tuberculous osteomyelitis patients were recruited to the study. Two patients had bacterial osteomyelitis affecting the upper limb, 3 had the hip affected, and 8 had the lower limb affected. In 9 cases, a joint was affected as well as a long bone. There were 3 cases of posttraumatic osteomyelitis, and 10 resulted from hematogenous infection. Tuberculous osteomyelitis patients had M. tuberculosis infection that affected the spine in 8 cases, the hip in 3, and the knee in 1. The mean (±SE) ages of the patients were 31.1 years (±2.7) for the controls, 24.2 years (±4.7) for the bacterial osteomyelitis patients and 30.6 years, (± 5.7) for the tuberculous osteomyelitis patients. There were 5 males in the control group, 5 in the bacterial osteomyelitis group, and 3 in the tuberculous osteomyelitis group. Fifty-four percent of the bacterial osteomyelitis patients were febrile, as were 42% of the tuberculous osteomyelitis patients; none of the controls was febrile.
The hematologic, biochemical, and serologic characteristics of the study population and control subjects are shown in table 1. Controls with noninflammatory disorders and relatives of healthy patients were similar except that 5 of 8 hospital controls but only 2 of 5 patient relatives were HIV-positive. Both bacterial and tuberculous osteomyelitis patients were anemic compared with controls. In addition, bacterial osteomyelitis patients had total leukocyte counts significantly higher than those of controls and a significant thrombocytosis compared with either controls or tuberculous osteomyelitis patients. Bacterial osteomyelitis patients had a significant acute-phase protein response with elevated ESRs, raised CRP levels, and reduced plasma albumin levels compared with controls. Tuberculous osteomyelitis patients also had elevated ESRs and lower albumin levels than did control subjects. Bacterial osteomyelitis patients had significantly lower plasma sodium concentrations than controls. Study patients with bacterial osteomyelitis or tuberculous osteomyelitis also had lower plasma creatinine concentrations than controls, but all values were within the normal range. There were significantly more HIV-seropositive patients in both the control and tuberculous osteomyelitis groups than in the bacterial osteomyelitis group.
Table 1.
Laboratory characteristics of patients with bacterial (OM) and tuberculous (TB) osteomyelitis and control subjects
| Controls | OM patients | TB patients | |
|---|---|---|---|
| Hematology | |||
| Hemoglobin (g/dL) | 13.1 (0.84) | 9.47 (0.75)* | 10.2 (0.90)* |
| Mean corpuscular volume (fL) | 83.8 (2.6) | 80.4 (4.0) | 82.3 (1.6) |
| Total leukocyte count (×109/L) | 5.7 (0.68) | 9.4 (1.17)* | 7.0 (0.63) |
| Lymphocyte count (×109/L) | 2.7 (0.3) | 3.3 (0.5) | 3.1 (0.4) |
| Platelet count (×109/L) | 235 (24) | 525 (84)* | 299 (46)† |
| Erythrocyte sedimentation rate (mm/1 h) | 33.4 (11) | 96.8 (11)* | 81.7 (11.4)* |
| Biochemistry | |||
| Sodium (mmol/L) | 139 (0.84) | 135 (1.1)* | 138 (1.5) |
| Bicarbonate (mmol/L) | 19.5 (0.50) | 19.5 (0.67) | 20.7 (0.60) |
| Urea (mmol/L) | 3.61 (0.24) | 3.05 (0.31) | 3.41 (0.21) |
| Creatinine (μmol/L) | 79.2 (3.3) | 64.0 (4.7) | 68.5 (3.3) |
| Total protein (g/L) | 78.2 (1.9) | 75.3 (2.4) | 81.4 (2.5) |
| Albumin (g/dL) | 40.6 (0.88) | 32.4 (1.7)* | 34.5 (2.5)* |
| Phosphate (mmol/L) | 1.17 (0.065) | 1.30 (0.06) | 1.22 (0.06) |
| Bilirubin (μmol/L) | 9.92 (0.73) | 6.69 (1.8) | 7.50 (1.5) |
| Aspartate transaminase (IU/L) | 46.9 (3.6) | 56.3 (6.7) | 49.0 (4.8) |
| Urate (mmol/L) | 0.31 (0.02) | 0.28 (0.02) | 0.33 (0.02) |
| C-reactive protein (mg/L) | 1.85 (0.88) | 44.2 (15)* | 35.8 (18) |
| Serology, % human immunodeficiency virus– positive |
53.8 | 15.4* | 58.3† |
NOTE. Data are mean (±SE); P determined by unpaired t test.
P < .05 vs. controls.
P <.05 for OM vs. TB patients.
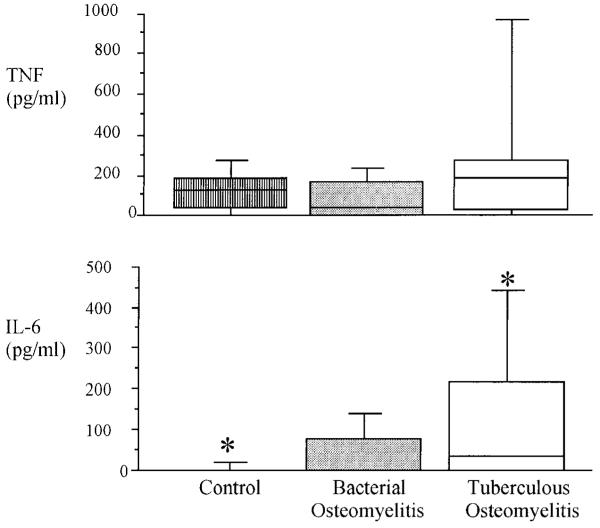
The greatest differences in plasma cytokine concentration on admission to the study were in IL-6 concentrations (figure 1). Plasma IL-6 concentrations of tuberculous osteomyelitis patients were significantly elevated over those of controls. Control subjects with noninflammatory disorders and those who were healthy relatives of patients were similar in terms of all aspects of proinflammatory cytokine secretion. IL-6 concentrations in bacterial osteomyelitis patients were intermediate between those of tuberculous osteomyelitis patients and controls, but in this small study, the difference was not statistically significant. In contrast, there were no significant differences in plasma TNF concentrations between the 3 study groups. IL-8 was detected in the plasma of only 1 person, a healthy control (data not shown). Following ex vivo LPS stimulation, significantly increased amounts of TNF, IL-6, and IL-8 were released into plasma compared with unstimulated samples, but there were no significant differences in LPS-stimulated cytokine concentrations between the 3 study groups (table 2).
Figure 1.
Circulating plasma TNF and IL-6 concentrations in patients with tuberculous osteomyelitis, patients with bacterial osteomyelitis, and control subjects at study entry. Box represents middle 2 quartiles divided by line at median point; horizontal lines show 10th and 90th centiles. IL-8 data are not shown, as only 1 subject had detectable IL-8 in circulation. * Groups between which there is statistically significant (P < .05) difference in IL-6 concentrations.
Table 2.
Proinflammatory cytokine concentrations following ex vivo stimulation of whole blood leukocytes by lipopolysaccharide
| Cytokine (pg/mL) | Control | Osteomyelitis | Bone TB |
|---|---|---|---|
| TNF | 189 (40–950) |
123 (19–3874) |
146 (6–873) |
| IL-6 | 17,145 (6723–108,183) |
17,641 (1801–150,346) |
23,781 (4672–76541) |
| IL-8 | 25 (25–500,000) |
463 (25–1668) |
25 (25–4898) |
NOTE. Data are median (range) values. P values were not significant for differences in cytokine concentrations between patients with bacterial osteomyelitis (OM) vs. control subjects, patients with tuberculosis (TB) vs. osteomyelitis (TB) control subjects, or OM vs. TB patients.
Cytokine concentrations on admission to the study correlated only poorly with hematologic and biochemical indices. Plasma IL-6 levels correlated with ESR (P = .05, ρ = .41), CRP (P = 0.5, ρ = .32), and albumin (P .05, ρ = −.34; Spearman’s correlation) concentrations, which are all markers of the acute-phase protein response. The only other parameter with which IL-6 had any correlation was plasma sodium concentration (P = .05, ρ = .41; Spearman’s correlation). Plasma IL-6 did not correlate with any measured clinical variable, such as the presence of fever or HIV seropositivity. Circulating plasma TNF and IL-8 concentrations did not correlate with any clinical, hematologic, or biochemical parameter. In addition, plasma concentrations of any one cytokine did not correlate with the concentration of any other cytokine.
Following ex vivo stimulation of whole blood by LPS, some additional correlations between cytokine concentrations and laboratory data were noted, although all ρ values remained <.45. TNF correlated with plasma urea, and IL-8 correlated with plasma creatinine concentrations. Both urea and creatinine concentrations are elevated in renal failure. Stimulated IL-8 concentrations also correlated with leukocyte and platelet counts. IL-6 concentrations following ex vivo stimulation did not correlate with laboratory indices. Once again, the concentration of any specific measured cytokine following ex vivo stimulation did not correlate with any other cytokine. There were also no correlations between stimulated cytokine concentrations and clinical parameters, including HIV infection status.
Discussion
Osteomyelitis frequently presents to the clinician with many nonspecific symptoms, such as fever or malaise, rather than accurate localizing signs, particularly in patients with tuberculous bone infection. The clinical, hematologic, and biochemical characteristics of our study population demonstrated a systemic acute-phase response in both tuberculous and bacterial osteomyelitis patients. However, plasma IL-6 concentrations were only significantly raised in tuberculous osteomyelitis patients over controls but only elevated to a lesser nonsignificant extent in bacterial osteomyelitis patients. IL-6 is likely to have several actions in tuberculous osteomyelitis, and this cytokine is known to have an important role in the acute-phase response [33, 34]. Because the acute-phase response in bacterial osteomyelitis patients was at least as vigorous as that observed in tuberculous osteomyelitis patients, IL-6 cannot be the only mediator involved. IL-6 may have a complex role, altering both osteoclast and osteoblast function during bone remodeling [35], and such repair activity will be critical following the tissue destruction that is characteristic of tuberculous osteomyelitis. It has also been suggested that at least in murine models of tuberculosis, IL-6 may have some direct antimycobacterial activity [36], although this is more controversial in humans. In previous studies involving patients with pulmonary tuberculosis, IL-6 mRNA was detectable only infrequently in the peripheral leukocytes by using the reverse transcriptase–polymerase chain reaction [37]. However, IL-6 was present in low levels in plasma of patients with nonfatal disease and at significantly raised concentrations in patients who died [21]. The source of such circulating IL-6 is likely to be infected tissues, and in samples collected by bronchoalveolar lavage of patients with pulmonary disease, IL-6 was readily detected [38]. IL-6 concentrations at sites of osteomyelitis are not known.
The failure to detect any elevated plasma levels of TNF or IL-8 in patients with bacterial osteomyelitis is in sharp contrast to the raised concentrations of these cytokines in patients with systemic bacterial infections [8, 9, 11-13]. Similarly, raised plasma TNF and IL-8 concentrations have been frequently reported in patients with pulmonary or miliary tuberculosis [20-22, 38, 39]. These findings are consistent with the localized nature of osteomyelitis lesions, which are often relatively walled off from the systemic circulation, with the clinical consequence that lesions are poorly penetrated by many antibiotics. Thus, IL-8, which is synthesized by osteoblasts [40], may well be produced at the site of bone infection but not be detectable systemically.
In terms of clinical laboratory markers, both bacterial and tuberculous osteomyelitis patients were more ill than controls and had a significantly elevated acute-phase response, anemia (normocytic and probably secondary to tissue inflammation), and hypoalbuminemia. Bacterial osteomyelitis patients had an elevated total leukocyte count and a reduced plasma sodium concentration compared with controls, although the means were still within the normal range; such findings are typical of bacterial infection. However, the only major correlation between plasma cytokine concentrations and clinical measurements was between plasma IL-6 concentration and markers of the acute-phase response. Plasma TNF and IL-8 did not correlate with each other or with any measured clinical variables. We have recently made a similar observation concerning lack of correlation between plasma cytokine concentrations and clinical data in a much larger group of 251 critically ill patients [41], and it is likely that any relationships between clinical measurements and cytokine concentrations are complex in infection. Thus, for example, although TNF is a pyrogen [42] and fever may coexist with elevated plasma TNF concentrations [43], the development of fever does not necessarily have a simple relationship to plasma TNF concentrations. One interesting clinical observation from this study was that patients who were HIV-seropositive were much more likely to have mycobacterial than bacterial osteomyelitis. This is consistent with the known clinical and immunologic associations between HIV and tuberculosis [44-46]. Both tuberculous osteomyelitis patients and hospital controls had a >50% chance of being HIV-seropositive, a finding consistent with previous data [47]. The 15.4% level of HIV seropositivity in the bacterial osteomyelitis group is nearer the incidence in the general Zambian population [48]. The reason that the control subjects had a higher incidence of HIV seropositivity is likely to be that they will have had multiple blood transfusions because 5 of the 7 HIV-seropositive subjects in this group were patients with chronic mechanical, noninflammatory disorders rather than healthy relatives of patients.
Following ex vivo stimulation of whole blood leukocytes, no major differences between TNF, IL-6, or IL-8 concentrations were observed in the 3 groups. However, because leukocyte counts were raised in bacterial osteomyelitis patients, the cytokine production per cell may be reduced in this group, although the range of cytokine concentrations following LPS stimulation was large in all groups. It is possible that leukocytes from bacterial osteomyelitis patients were in a partial refractory state or that there was an inhibitor of proinflammatory cytokine secretion circulating in these patients. We have previously found evidence of such an inhibitor in patients with pulmonary and miliary tuberculosis in whom ex vivo LPS stimulation of blood leukocytes from patients who died was not associated with IL-8 secretion in contrast to ex vivo IL-8 secretion from stimulated whole blood of survivors [21]. However, in this study, no patient died from osteomyelitis, an observation typical of this infection that is associated with major morbidity rather than mortality. Cytokine concentrations after ex vivo stimulation also correlated poorly with clinical laboratory measurements, although levels of the neutrophil and T cell chemo-attractant IL-8 did relate to leukocyte (and platelet) count.
In summary, this study has demonstrated that plasma concentrations of the acute-phase cytokine IL-6 are significantly elevated in patients with tuberculous osteomyelitis. In addition, plasma IL-6 concentrations correlate with the acute-phase protein response, but HIV seropositivity did not appear to influence these findings. A second significant finding in this study was the generally poor correlation between cytokines and osteomyelitis. This is most likely a result of the fact that focal osteomyelitis is probably associated with a limited cellular inflammatory response. Such a poor inflammatory response is consistent both with the fact that patients frequently present with advanced disease and with the observation that antibiotic treatment alone may often be unsuccessful. Since systemic inflammatory markers correlate poorly with clinical and laboratory parameters, it is imperative that future studies focus on local tissue cytokine production.
Acknowledgments
Grant support: NIH (GM-44918).
Footnotes
Informed consent was obtained from all subjects. Ethical approval was obtained before the study began from the University Teaching Hospital of Lusaka Research and Ethics Committee.
References
- 1.Mader JT, Calhoun J. Osteomyelitis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. Vol. 1. Churchill Livingstone; New York: 1995. pp. 1039–51. 1. [Google Scholar]
- 2.Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 3.Ravilione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 4.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Abramson SB. Infections of the musculoskeletal system by M. tuberculosis. In: Rom WN, Gray S, editors. Tuberculosis. Little Brown; Boston: 1996. pp. 635–44. [Google Scholar]
- 6.Rezai AR, Lee M, Cooper PR, Errico TJ, Koslow M. Modern management of spinal tuberculosis. Neurosurgery. 1995;36:87–98. doi: 10.1227/00006123-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Remick DG, Friedland JS. Cytokines in health and disease. 2nd ed. Marcel Dekker; New York: 1997. [Google Scholar]
- 8.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 9.Mathison JC, Wolfson E, Ulevitch J. Participation of tumor necrosis factor in the mediation of gram-negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988;81:1925–37. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP, The Swiss-Dutch J5 Immunoglobulin Study Group High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. Am J Med. 1991;91:23–9. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- 11.Friedland JS, Suputtamongkol Y, Remick DG, et al. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun. 1992;60:2402–8. doi: 10.1128/iai.60.6.2402-2408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hack CE, Hart M, van Schijndel RJMS, et al. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60:2835–42. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marie C, Fitting C, Cheval C, et al. Presence of high levels of leukocyte-associated interleukin-8 upon cell activation and in patients with sepsis syndrome. Infect Immun. 1997;65:865–71. doi: 10.1128/iai.65.3.865-871.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klosterhalfen B, Peters KM, Tons C, Hauptmann S, Klein CL, Kirkpatrick CJ. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J Trauma. 1996;40:372–8. doi: 10.1097/00005373-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Kindler V, Sappino A, Grau GE, Piguet P, Vassalli P. The inducing role of tumour necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 16.Gauldie J, Richards C, Harnish D, Lansdorf P, Baumann H. Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1990;84:7251–5. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Broser M, Rom WN. Activation of the interleukin-6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-κB. Proc Natl Acad Sci USA. 1994;91:2225–9. doi: 10.1073/pnas.91.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedland JS, Remick DG, Shattock R, Griffin GE. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992;22:1373–8. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson PC, Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 1992;149:2689–94. [PubMed] [Google Scholar]
- 20.Larsen CG, Thomsen MK, Gesser B, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. J Immunol. 1995;155:2151–7. [PubMed] [Google Scholar]
- 21.Friedland JS, Hartley JC, Hartley CGC, Shattock RJ, Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–8. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurashima K, Mukaida N, Fujimura M, et al. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–7. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 23.DeForge LE, Remick DG. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated whole blood. Biochem Biophys Res Comm. 1991;174:18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- 24.Wilson BMG, Severn A, Rapson NT, Chana J, Hopkins P. A convenient human whole blood culture system for studying the regulation of tumour necrosis factor release by bacterial lipopolysaccharide. J Immunol Methods. 1991;139:233–40. doi: 10.1016/0022-1759(91)90193-j. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara K, Strieter RM, Chensue SW, Standiford TJ, Kunkel SL. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leukoc Biol. 1991;50:287–95. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- 26.van Deuren M, van der Ven-Jongekrijg J, Demacker PNM, et al. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Dis. 1994;169:157–61. doi: 10.1093/infdis/169.1.157. [DOI] [PubMed] [Google Scholar]
- 27.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJH. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–50. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown CC, Poli G, Lubaki N, et al. Elevated levels of tumor necrosis factor-α in Zairian neonate plasmas: implications for perinatal infection with the human immunodeficiency virus. J Infect Dis. 1994;169:975–80. doi: 10.1093/infdis/169.5.975. [DOI] [PubMed] [Google Scholar]
- 29.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 30.Aarden LA, De Groot DR, Schaap OL, Lansdorp PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–6. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 31.Ulich TR, Guo K, Remick DG, del Castillo J, Yin S. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–23. [PubMed] [Google Scholar]
- 32.DeForge LE, Remick DG. Sandwich ELISA for detection of picogram quantities of interleukin-8. Immunol Invest. 1991;20:89–97. doi: 10.3109/08820139109054928. [DOI] [PubMed] [Google Scholar]
- 33.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6–deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 34.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz MC, Lorenzo JA. Local regulators of bone: IL-1, TNF, lymphotoxin, interferon-γ, IL-8, IL-10, IL-4, the LIF/IL-6 family and additional cytokines. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. Academic Press; San Diego: 1996. pp. 687–700. [Google Scholar]
- 36.Flesch IEA, Kaufmann SHE. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (IL-6) Infect Immun. 1990;58:269–71. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauf V, Rom WN, Smith KA, et al. Cytokine gene activation and modified responsiveness to interleukin-2 in the blood of tuberculosis patients. J Infect Dis. 1993;168:1056–9. doi: 10.1093/infdis/168.4.1056. [DOI] [PubMed] [Google Scholar]
- 38.Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1β, interleukin-6 and tumor necrosis factor-α by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799–804. doi: 10.1164/ajrccm.153.2.8564135. [DOI] [PubMed] [Google Scholar]
- 39.Rydberg J, Miorner H, Chandramuki A, Lantz M. Assessment of a possible imbalance between tumor necrosis factor (TNF) and soluble TNF receptor forms in tuberculous infection of the central nervous system. J Infect Dis. 1995;172:301–4. doi: 10.1093/infdis/172.1.301. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhary LR, Spelsberg TC, Riggs BL. Production of various cytokines by normal human osteoblast-like cells in response to interleukin-1β and tumor necrosis factor-α: lack of regulation by 17β-estradiol. Endocrinology. 1992;130:2528–34. doi: 10.1210/endo.130.5.1572280. [DOI] [PubMed] [Google Scholar]
- 41.Friedland JS, Porter JC, Daryanani S, et al. Prognosis, physiology and plasma proinflammatory cytokine concentrations in profoundly ill patients. Crit Care Med. 1996;24:1775–81. doi: 10.1097/00003246-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 43.Cannon JG, Tompkins RG, Gelfand JA, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Perneger TV, Sudre P, Lundgren JD, Hirschel B, the AIDS in Europe Study Group Does the onset of tuberculosis in AIDS predict shorter survival? Results of a cohort study in 17 European countries over 13 years. BMJ. 1995;311:1468–71. doi: 10.1136/bmj.311.7018.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman LN, Williams MT, Singh TP, Frieden TR. Tuberculosis, AIDS and death amongst substance abusers on welfare in New York City. N Engl J Med. 1996;334:828–33. doi: 10.1056/NEJM199603283341304. [DOI] [PubMed] [Google Scholar]
- 46.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 47.Elliott AM, Halwiindi B, Hayes RJ, et al. Impact of human immunodeficiency virus on mortality of patients treated for tuberculosis in a cohort study in Zambia. Trans R Soc Trop Med Hyg. 1995;89:78–82. doi: 10.1016/0035-9203(95)90668-1. [DOI] [PubMed] [Google Scholar]
- 48.Fylkesnes K, Musonda RM, Kasumba K, et al. The HIV epidemic in Zambia: socio-demographic prevalence patterns and indications of trends among childbearing women. AIDS. 1997;11:339–45. doi: 10.1097/00002030-199703110-00012. [DOI] [PubMed] [Google Scholar]