Abstract
Adeno-associated virus type 5 (AAV5) has a linear, single-stranded DNA genome of ca. 5 kb and an overlapping transcription profile featuring multiple promoters and a single intron in the center of the genome. Unlike the situation for the prototype AAV2, AAV5 RNAs transcribed from upstream promoters at map units 7 (P7) and 19 (P19), which encode the viral Rep proteins, are predominantly polyadenylated at a site within the intron [(pA)p]. RNAs generated from the AAV5 capsid gene promoter P41, which is only 78 nucleotides (nt) upstream of the intron donor, and 281 nt upstream of (pA)p, primarily readthrough (pA)p, are polyadenylated at a more distal site at the 3′ end of the genome [(pA)d] and ultimately spliced. The intron contains the core sequences sufficient for polyadenylation at (pA)p, which is governed by a G/U-rich downstream element that overlaps with the intron 3′ A2 splice acceptor. In addition, polyadenylation of P7- and P19-generated RNAs at (pA)p is influenced by an upstream element that lies 5′ to the start of the P41 transcript. Our results also suggest that splicing and polyadenylation of P41-generated RNA can compete for the same pool of precursor pre-mRNA molecules. The cis-acting signals within the A2 3′ splice site that govern polyadenylation and splicing of AAV5 RNAs must be optimized to program both (i) the levels of polyadenylation of P7- and P19-generated RNA at (pA)p required to generate the proper levels of the essential Rep proteins and (ii) the splicing of P41-generated RNAs to generate the proper ratio of capsid proteins during AAV5 infection.
Adeno-associated viruses (AAVs) are a group of small, nonenveloped, single-stranded linear DNA viruses that replicate in mammalian cells best in the presence of larger helper DNA viruses, e.g., adenoviruses or herpesviruses (6, 35). Six AAV serotypes (types 1 to 6) have been isolated from either human or simian tissues (4, 5, 7, 16, 19, 23), and the genomes of two additional isolates (proposed as AAV7 and -8) have recently been identified in simian hosts (15). AAV2, which has been studied in the most detail, has an overlapping transcription map that utilizes a polyadenylation site at the 3′ end of the genome. In contrast to RNAs generated from the prototype AAV2, however, which are found to polyadenylate predominantly at the distal polyadenylation site [(pA)d] (26), AAV5 pre-mRNAs also efficiently utilize a more proximal polyadenylation site [(pA)p] within the central intron of the AAV5 genome (see Fig. 1A) (26).
FIG. 1.
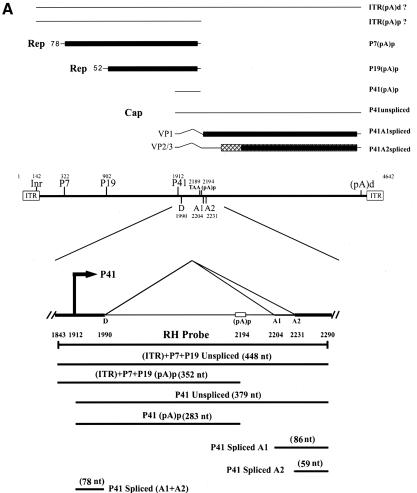
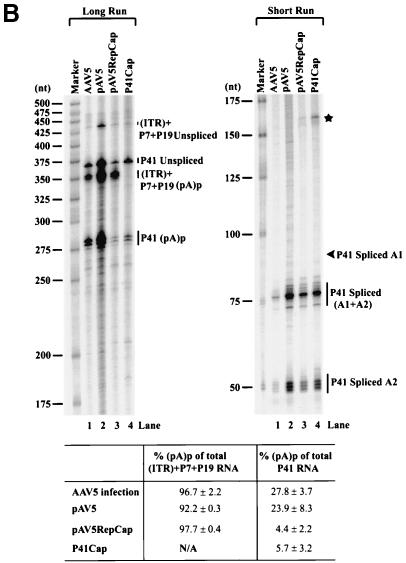
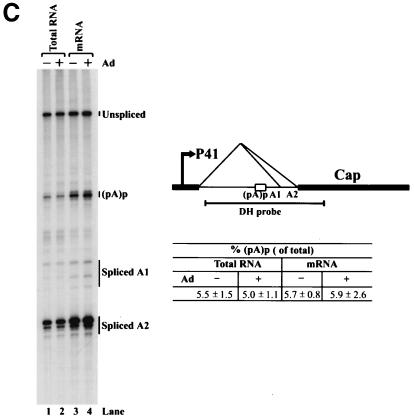
(A) Genetic map of the AAV5 and the RH probe. The 4,642-nt AAV5 genome is shown to scale with the major transcriptional landmarks indicated, including the ITRs, promoters, the initiation sites for the various RNAs, the 5′ splice site (D) and the 3′ splice sites (A1 and A2), and the proximal [(pA)p] and distal [(pA)d] polyadenylation sites. The major transcripts observed in our previous report (26), and in the present study, are listed on the right. Transcripts generated from the ITR have not been fully characterized and so are marked with a question mark. The start site for the ITR-initiated transcripts map to an initiator region (Inr) in the ITR (26). The RH probe (nt 1843 to 2290), which spans the P41 promoter and the intron, is diagrammed on the bottom, and the individual bands protected by RNase protection assay and their sizes are shown as indicated. (B) RNAs generated from the P7 and P19 are polyadenylated at (pA)p at high efficiency, whereas P41-generated RNAs are primary polyadenylated at the (pA)d site. 293 cells were either infected with AAV5 and adenovirus or transfected with an infectious clone (pAV5), an AAV5 RepCap plasmid (pAV5RepCap), or the P41 minimal construct (P41Cap) in the presence of adenovirus coinfection. After 36 to 40 h, cells were harvested and total RNA was isolated. Then, 10 μg of total RNA was protected by the RH probe, and samples were subjected to 6% denaturing polyacrylamide gel electrophoresis for either 3 h 30 min (long run, left panel) or 1 h 30 min (short run, right panel). An RNA marker ladder with their respective sizes is shown on the left. A representative experiment is shown with the identities of the protected bands on the right. Quanitifications of the percentage of the total ITR+P7+P19-generated RNAs polyadenylated at (pA)p or the percentage of the total P41-generated RNAs polyadenylated at (pA)p are shown as averages of the results of at least three individual experiments with standard deviations. P41-generated RNA spliced at A1 is poorly detected by the RH probe. The band marked with an asterisk is most likely a degradation product, and was not seen in other experiments. RNA from unifected cells generated no protection products. (C) The relative ratio of (pA)p is the same in poly(A)-containing RNA, and the DH probe detects relative levels of (pA)p RNAs similar to those detected by the RH probe. Both total and oligo(dT)-selected mRNA were isolated from P41Cap-transfected 293 cells either in the absence or presence of adenovirus coinfection. A total of 10 μg of total RNA or mRNA purified from 20 μg of total RNA were protected by the DH probe as shown in the diagram. A representative experiment is shown with the identities of the protected bands on the right. Quantification of the ratios of RNAs polyadenylated at (pA)p, relative to the total protected RNAs, are shown as averages of the results from at least three individual experiments with standard deviations. RNA from uninfected cells generated no protection products.
The AAV5 genome is transcribed by using four promoters: one within the upstream inverted terminal repeat (ITR), and three others at map units 7, 19, and 41, the last of which (P41) is only 78 nucleotide (nt) upstream of the intron donor and 281 nt upstream of (pA)p (see Fig. 1A) (10, 26). AAV5 RNAs generated from P7 and P19, which encode the large and small Rep proteins, respectively, from the large open reading frame in the 5′ half of the genome, utilize (pA)p at high efficiency (26). RNAs generated from P41 promoter, however, which encode the virus capsid proteins from the large open reading frame in the 3′ half of the genome, utilize this nearby site with significantly reduced efficiency; they primarily readthrough to the distal site (pA)d (26). The intron region of AAV5 contains two relatively consensus AAUAAA signals at nt 2177 and 2191, which are immediately upstream of the first intron acceptor A1 at nt 2204 (see Fig. 1A). RNA cleavage and polyadenylation occurs 11 to 14 nt downstream of the first AAUAAA motif (26). AAV2 also contains a single AAUAAA polyadenylation signal in this region; however, its use can only be detected by sensitive PCR techniques (26).
There are numerous examples in which the use of alternative polyadenylation sites governs alternative gene expression. In a number of cases, the determinants that govern the choice of alternate polyadenylation sites have been studied (41). Similar to AAV5, both the calcitonin/CGRP and immunoglobulin M (IgM) heavy chain genes contain a functional polyadenylation site within a functional intron (41). However, in these cases, the regulation of alternative polyadenylation varies either with cell type or differentiation state (1, 12, 30). The AAV5 system is unusual in that different relative levels of polyadenylation are constitutively seen for transcripts generated by different promoters.
We show here that polyadenylation of AAV5 RNAs at (pA)p is governed by a G/U-rich downstream element (DSE) that overlaps, but is not congruous with, the intron 3′ A2 splice acceptor. In addition, polyadenylation of P7- and P19-generated RNAs at (pA)p is influenced by an upstream element (USE) that lies 5′ to the start of the P41 transcript. Our results also suggest that splicing and polyadenylation of P41-generated RNA can compete for the same pool of precursor pre-mRNA molecules. Since cis-acting signals within the A2 3′ splice site govern both polyadenylation and splicing of AAV5 RNAs, they must be optimized to program both the proper levels of polyadenylation of P7- and P19-generated RNAs at (pA)p and splicing of P41-generated RNAs which are required during AAV5 infection.
MATERIALS AND METHODS
Cell and virus.
AAV5 virus was a gift from Jay Chiorini at the National Institutes of Health. For viral RNA isolation, 293 cells were infected with AAV5 and AAV5 (dl309) at multiplicities of infection of 15 and 5, respectively.
Plasmid constructions.
An AAV5 infectious clone (pAV5), AAV5 RepCap plasmid (pAV5RepCap) and P41 minimal construct (P41Cap) were described previously (26). Nucleotide numbers refer to AAV5 and AAV2 sequences with GenBank accession numbers AF085716 and AF043303, respectively.
(i) Minimal plasmids for (pA)p signal mapping.
A P7 minimal construct (P7JCap plasmid) was made by replacement of the Rep sequence (nt 359 to 1974) with procaryotic sequence (nt 35 to 1648) from pBR322. To create P7JCapAV2pAd, the native AAV5 (pA)d (nt 4382 to 4475) of P7JCap was replaced with AAV2 (pA)d (nt 4322 to 4490). P7JCap+GUpAd was constructed by introducing a highly consensus G/U DSE sequence (GUGUGUGUUGUGUAUGUUG) (39), which is the GU-rich DSE sequence from the human papillomavirus type 31 late polyadenylation signal, into the putative DSE region (nt 4437 to 4450) of AAV5 (pA)d. P7JCap-GUpAd was constructed by mutation of the this putative DSE region to a heterologous sequence (GGAGAACACCAGTAA).
P7JAV5IJtpA was constructed by replacement of the AAV5 cap gene in P7JCap (nt 2236 to 4379) with a procaryotic sequence (nt 35 to 2178) from pBR322. P7JAV5IJSVpA and P7JAV5IJbGHpA were made by changing the native AAV5 (pA)d sequences (nt 4382 to 4475) in P7AV5IJtpA to either simian virus 40 pA sequences (nt 3120 to 3250) or bGHpA sequences (nt 1018 to 1249) on the pcDNA3.1 plasmid (Invitrogen).
A series of constructs to evaluate the proximal cis-acting signal of (pA)p were made based on P7JAV5IJtpA as follows. P7JAV5I(mpApI)JtpA, P7JAV5I(mpApII)JtpA and P7JAV5I(mpAp)JtpA were made by mutation of the pApI signal (AAUAAA to AACAAG) at nt 2177, pApII (AAUAAA to GAGAAA) at nt 2191, and mutation of both signals, respectively. P7JAV5I(AV2A1)JtpA was constructed by replacement of the A1 sequence at nt 2177 to 2204 to AAV2 A1 sequence (nt 2182 to 2201) in the intron. P7JAV5I(mG/U)JtpA was constructed by mutation of a putative G/U-rich regulation region (nt 2155 to 2167 from TTTTGGGATTTT to AGAAGGCGCGCCA) upstream of (pA)p. A schematic diagram of those plasmids is also shown in Fig. 2B.
FIG. 2.
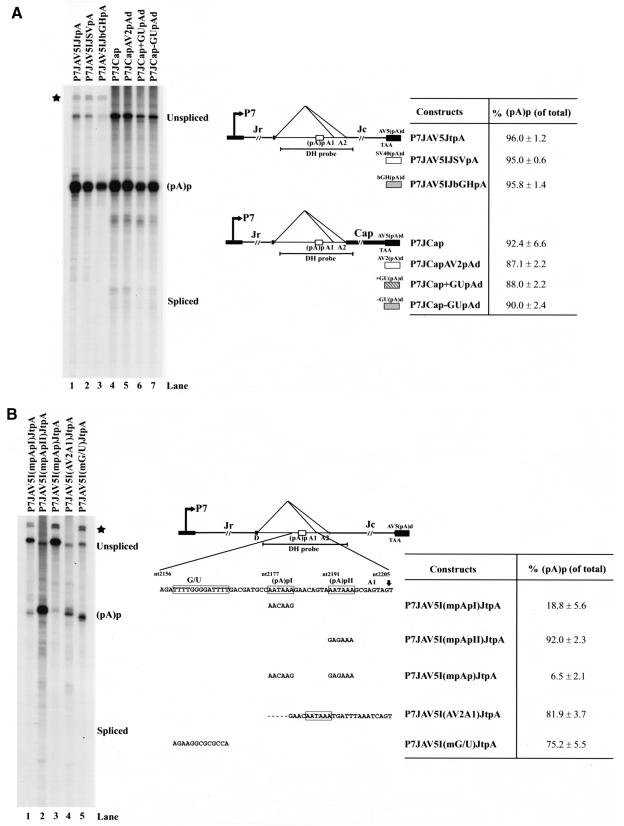
Mapping of the minimal cis-acting sequences within the intron required for AAV5 RNA polyadenylation at (pA)p. (A) 293 cells were transfected with various P7 minimal plasmids based on P7JAV5IJtpA or P7JCap that had alterations to their 3′ distal polyadenylation sites (diagrammed on the right). The sequence denoted as Jr between the P7 promoter and the intron (AAV5 nt 359 to 1920) has been replaced withDNA from pBR322 (nt 35 to 1648), and the sequence denoted as Jc between the intron and the (pA)d site (AAV5 nt 2236 to 4379) has been replaced with DNA from pBR322 (nt 35 to 2178). RNA from uninfected cells generated no protection products. (B) P7 minimal construct-based plasmids (P7JAV5IJtpA [diagrammed on the right]), which contained various mutations near the AAUAAA signal in the intron shown on the right, were transfected into 293 cells. At 36 to 40 h after transfection, total RNA was isolated. Total RNA (10 μg) was protected by the DH probe. A representative experiment is shown with the identities of the protected bands on the right. Quanitification of the ratios of RNAs polyadenylated at (pA)p relative to the total protected RNAs are shown as averages of the results of at least three individual experiments with the standard deviations. Bands marked with an asterisk are undigested probe. RNA from uninfected cells generated no protection products.
(ii) A2 mutation minimal constructs.
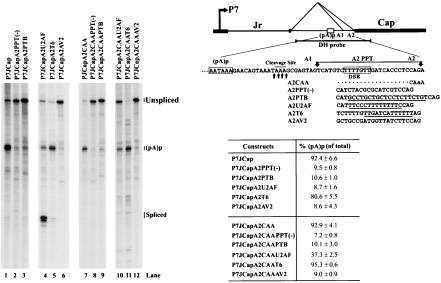
To generate P7JCapA2CAA and P41CapA2CAA, the acceptor 2 (A2) CAG was mutated to be CAA in P7JCap and P41Cap, respectively. Furthermore, a group of mutations [A2PPT(−), A2PTB, A2U2AF, A2T6, and A2AV2] in the AAV5 intron A2 acceptor region (nt 2210 to 2231) were made based on the P7JCap, P7JCapA2CAA, P41Cap, and P41CapA2CAA minimal constructs. Detailed mutated sequences are listed in Fig. 3.
FIG. 3.
Efficient polyadenylation of P7-driven transcripts at (pA)p requires a DSE that overlaps with the A2 3′ splice acceptor site. 293 cells were transfected with the P7JCap (diagrammed on the right, top) and the P7JCapA2CAA-based plasmids, which contain various mutations of the A2 3′ splice site, listed on the right. At the top right of the figure, the consensus PTB- and U2AF-binding sites and the conserved DSE are underlined. The G/U-rich DSE of AAV5 is boxed and overlaps with the A2 polypyrimidine tract (A2 PPT). The four potential cleavage sites of RNAs polyadenylated at (pA)p, determined previously (26), are indicated with arrows. RNA from uninfected cells generated no protection products.
(iii) Constructs for definition of the USE in the rep region.
P7RepCapA2CAA was made by mutation of acceptor A2 (from CAG to CAA) in pAV5RepCap. P7RepCapA2CAAPPT(−) was constructed by replacement of the A2 region in P7RepCapA2CAA with the PPT(−) mutation. To create P7Rep(Jn1266-1637)CapA2CAAPPT(−), a procaryotic pBR322 sequence (nt 369 to 738) was used to replace nt 1266 to 1637 of the P7RepCapA2CAAPPT(−) plasmid. P7Rep(n1266J)CapA2CAAPPT(−) and P7Rep(n1637J)CapA2CAAPPT(−) were made by replacing sequences nt 1266 to 1974 and nt 1637 to 1974 with sequences from pBR322 (nt 35 to 738 and nt 35 to 369, respectively). P7J(Repn1266-1637)CapA2CAAPPT(−) was constructed replacing the rep region (nt 359 to 1266) of P7Rep(n1637J)CapA2CAAPPT(−) with the pBR322 sequence from nt 745 to 1645.
(iv) Probe constructs.
All probes used for RNase protection assays were homologous to the reporter construct being tested. The RH probe and the RH homologous probe clones, which are across the whole intron, were constructed by ligation of a PCR fragment (nt 1843 to 290) into pGEM3Z by BamHI and HindIII linkers.
The DH probe, which covers the (pA)p site and intron acceptors, was described previously (26). All of the homologous DH probe clones were created by ligation of PCR DNA from the constructs that contained the mutations in the DH probe region (nt 1994 to 2341) into pGEM3Z through BamHI and HindIII linkers.
All of the constructs were sequenced to confirm the sequence in the mutated region at the DNA Core Facility, University of Missouri—Columbia.
RNase protection size marker.
Makers for RNase protections were in vitro transcribed from PCR DNAs with a T7 promoter at different sizes by incorporation of [32P]uridine, which were described in details previously (26).
RNase protection assay.
Plasmid DNA (2 μg/60-mm plate) was transiently transfected into ca. 60 to 80% confluent 293 cells, using Lipofectamine Plus reagent (Invitrogen) as previously described (27). When indicated, adenovirus type 5 was coinfected at a multiplicity of infection of 5 after transfection. Total RNA was isolated ca. 36 to 40 h later by using guanidine isothiocyanate as previously described (33). In some circumstances, poly(A)-selected mRNA was purified by oligo(dT) magnetic beads (Dynal Biotech, Oslo, Norway) as previously described (26). RNase protection assays were performed as described before (21, 22, 33), and RH and DH probe templates were generated by either BamHI or EcoRI linearization of the pGEM3ZRH and -DH plasmids. Probes were produced by in vitro transcription by using Sp6 polymerase. The RNase protection assay signal was quantified with the Molecular Image FX machine and Quantity One (version 4.2.2) images software (Bio-Rad, Hercules, Calif.). Relative molecular ratios of individual species of RNAs were determined after they were adjusted for the number of 32P-labeled uridine in each protected band as previously described (33). The percentage of total RNA polyadenylated at (pA)p [i.e., the percent (pA)p of the total] was calculated by dividing the adjusted signal from the (pA)p band with the adjusted signals from all of the bands.
RESULTS
RNAs generated by the AAV5 P7 and P19 promoters are polyadenylated at (pA)p at high frequency, while P41-generated RNAs primarily read through and are polyadenylated downstream at (pA)d.
Although we have previously shown that a significant portion of AAV5 P7- and P19-generated RNAs were polyadenylated internally at (pA)p, the relative extent to which AAV5 P41-generated RNAs used (pA)p was not determined. To address this question, an RNase protection probe (RH; see Fig. 1A) that spanned the intron region was used to assess the levels at which AAV RNAs generated by either the upstream ITR plus P7 plus P19 promoters (which are taken together, since this probe cannot distinguish between them) or RNAs generated by P41 utilized (pA)p. For the experiments described here, we chose to evaluate the usage of (pA)p relative to the total RNA. As can be seen in Fig. 1B, >90% of the RNAs generated from the upstream promoters, either during AAV5 infection (AAV5, lanes 1) after transfection of an AAV5 infectious clone (pAV5, lanes 2) or after transfection of a nonreplicating, ITR-lacking, construct containing the viral rep and cap genes (pAV5RepCap, lanes 3) were polyadenylated at (pA)p. As previously described (26), RNAs generated by these upstream promoters did not accumulate as spliced products to significant levels.
In contrast, only ca. 24 to 28% of RNAs generated by P41 after either infection by AAV5 or transfection of replicating pAV5 utilized (pA)p (Fig. 1B), and only 5% of P41-generated RNAs produced after transfection of pAV5RepCap or a minimal construct containing only the P41 transcription unit (P41Cap, lanes 4) utilized (pA)p. A large portion of the P41-generated transcripts produced by each of these constructs accumulated as spliced RNAs (Fig. 1B). The difference in (pA)p usage between replicating and nonreplicating constructs was not due merely to the presence in cis of the viral ITRs because P41-generated RNAs produced by a nonreplicating, full-length rep mutant construct that retained functional ITRs utilized (pA)p to the same levels as seen for the ITR(−) RepCap constructs (26; J. Qiu and D. J. Pintel, unpublished results).
These results demonstrated that, although the majority of RNAs generated from the upstream ITR, P7, and P19 promoters utilized (pA)p, a much smaller percentage of P41-generated RNAs were polyadenylated at (pA)p. Interestingly, the percentage of P41-generated molecules polyadenylated at (pA)p was increased when the expressing molecule was replication competent.
Using the smaller DH probe, similar relative levels of (pA)p usage were detected in total and poly(A)-selected RNA generated after transfection of P41Cap into 293 cells either in the presence or absence of coinfecting adenovirus (Fig. 1C). This demonstrated that helper adenovirus infection did not effect AAV5 polyadenylation at (pA)p in 293 cells and thus allowed the further characterization of (pA)p usage in 293 cells in the absence of adenovirus by using the DH probe.
Sequences within the AAV5 intron are sufficient to direct cleavage and polyadenylation of P7-generated transcripts.
P7-generated transcripts produced from a construct in which the rep and cap genes of pAV5RepCap were replaced by heterologous sequences from a bacterial plasmid (P7JAV5IJtpA) were polyadenylated at (pA)p to the same levels as seen for wild-type virus and the complete AAV5 constructs, with spliced products remaining barely detectable (Fig. 2A; compare with Fig. 1B). Efficient usage of (pA)p was also seen when the normal AAV5 distal polyadenylation signal (pA)d present in P7JAV5IJtpA, was replaced with either the simian virus 40 polyadenylation (polyA) site (P7JAV5IJSVpA) or the poly(A) site from the bovine growth hormone gene (P7JAV5IJbGHpA) (Fig. 2A). These results suggested that the AAV5 intron contained sufficient information to program cleavage and polyadenylation at (pA)p and that (pA)p usage can occur efficiently when various heterologous poly(A) signals were present downstream. Similar results were also seen when the AAV5 capsid gene was kept intact (Fig. 2A, P7JCap). Substitution of the P7JCap AAV5 distal (pA)d with the AAV2 distal poly(A) site (P7JCapAV2pAd), shown to be highly efficient in in vitro polyadenylation assays (9), or with synthetic poly(A) sites either containing or lacking a highly consensus G/U-rich DSE (P7JCap+GUpAd, P7JCap-GUpAd, respectively), had essentially no effect on the levels of (pA)p usage (Fig. 2A). These results further suggested that (pA)p functions independently of downstream poly(A) signals.
There are two consensus AAUAAA signals within the AAV5 intron. Disruption of the first of these [P7JAV5I(mpApI)JtpA], but not the second [P7JAV5I(mpApII)JtpA], dramatically reduced polyadenylation at (pA)p (Fig. 2B). Disruption of the two together effectively prevented polyadenylation at (pA)p (Fig. 2B). These results demonstrated that the upstream AAUAAA predominantly directed cleavage and polyadenylation at (pA)p and are consistent with previous mapping studies on the site of poly(A) addition (26). When the AAV5 intronic region upstream of A1 containing the two polyadenylation motifs, was replaced with the analogous region of AAV2, the AAV2 AAUAAA signal, cryptic in its normal position within AAV2, was now efficiently utilized [P7JAV5I(AV2A1)JtpA; Fig. 2B, lane 4]. These results suggested that the context of the AAV5 intron was supportive of cleavage and polyadenylation of heterologous AAUAAA motifs. There is a G/U-rich region 10 nt upstream of the AAV5 AAUAAA motifs with similarities to other USEs (11, 20, 29, 32) (see Fig. 2B); however, mutation of this sequence only slightly reduced cleavage and polyadenylation at (pA)p [P7JAV5I(mG/U)JtpA; Fig. 2B, lane 5].
Efficient polyadenylation of P7-driven transcripts at (pA)p requires a downstream element that overlaps with the A2 3′ splice acceptor site.
Greater than 90% of the P7-generated transcripts from P7JCap, in which most of the rep gene has been replaced by heterologous sequences from a bacterial plasmid, was polyadenylated at (pA)p, and very few spliced products accumulated (Fig. 2A, lane 4, and Fig. 3, lane 1). This was similar to wild-type AAV5 and a nonreplicating rep and cap expression plasmid (Fig. 1B). Abolition of splicing of the AAV5 intron to either A1 or A2 can be accomplished by mutation of the A2 splice cleavage site from CAGA to CAAA (J. Qiu, C. Ye, and D. J. Pintel, unpublished data), and this mutation had little effect on polyadenylation of P7-generated transcripts (Fig. 3, compare lanes 1 and 7).
When the sequence in the A2 3′ splice acceptor region was altered, however, polyadenylation at (pA)p was affected. This region contains a modest polypyrimidine tract that is required for splicing of the AAV5 intron from P41-generated pre- mRNAs. When mutations were made that reduced the uridine content of this region, which should reduce the binding of U2AF [P7JCapA2PPT(−)] (36), introduced a consensus polypyrimidine tract-binding protein (PTB) binding site (18) (P7JCapA2PTB), or substituted the A2 region of AAV2 (P7JCapA2AV2), the percentage of P7-generated RNAs utilizing (pA)p was significantly decreased (Fig. 3, lanes 2, 3, and 6). These mutations, however, also decreased the relative usage of (pA)p when they were combined with the A2 cleavage site mutation that eliminated any residual splicing of the intron from these RNAs (the A2CAA mutation set, Fig. 3, lanes 8, 9, and 12), confirming that they affected polyadenylation directly. Taken together, these results suggest that the A2 3′ splice acceptor region contains a cis-acting element required for efficient polyadenylation at (pA)p.
A decrease in the relative usage of (pA)p was also seen when a mutation predicted to enhance splicing by introducing a sequence predicted to be a strong binding site for U2AF (36), was introduced into this region (P7JCapA2U2AF). As expected, the reduction in relative usage of (pA)p for this mutation was associated with an increase in splicing of the P7-generated transcripts (Fig. 3, lane 4). However, in contrast to the A2 mutations described above, when this mutation was introduced into the A2 cleavage site mutant background that eliminated splicing of the intron (A2CAA), the reduction in the relative usage of (pA)p was not as pronounced as when the A2 cleavage site was unaltered: for example, P7JCapA2U2AF [ca. 9% (pA)p] versus P7JCapA2CAAU2AF [ca. 37% (pA)p] (Fig. 3, lanes 4 and 10). Although under normal circumstances P7-generated RNAs are not spliced, these results suggested that when the AAV5 intron was strengthened, splicing of P7-generated RNAs occurred and was likely competing with polyadenylation for the same pool of precursor pre-mRNAs.
The A2 3′ splice acceptor region contains a G/U-rich motif (TTTTGTT) that could potentially function as a DSE (boxed in Fig. 3), and all of the mutations described above that affected polyadenylation altered that sequence. Introduction of a very strong consensus DSE [TTGANNNTTTTTT (28, 40), in P7JCapA2T6], that did not alter the resident G/U-rich motif, neither decreased nor increased polyadenylation of P7-generated transcripts at (pA)p significantly compared to levels detected for P7JCap. Taken together, these results indicate that the TTTTGTT motif in the AAV5 A2 3′ splice site region functions as a DSE, and the activity of this element is not subject to further enhancement. (We did see, however, that this consensus sequence functioned more strongly as a DSE in P41-generated RNA produced by P41CapA2CAAT6 [described below]). Interestingly, the DSE-altering U2AF-binding site mutation (P7JCapA2CAAU2AF; Fig. 3, lane 10) dramatically reduced the relative usage of (pA)p, even though it introduced a stretch of eight uridines downstream of the original DSE. This highlighted the importance of the TTTGTT motif for polyadenylation at (pA)p and suggested that merely a stretch of uridines is not sufficient for DSE activity.
Efficient polyadenylation of P41-driven transcripts at (pA)p also requires a downstream element that overlaps with the A2 3′ splice acceptor site and competes with the splicing process.
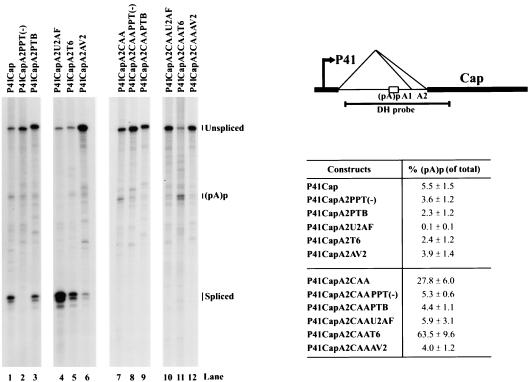
Unlike the P7- and P19-generated transcripts, which efficiently use the proximal polyadenylaton site (pA)p, most P41-generated transcripts read through (pA)p and are polyadenylated at (pA)d and ultimately spliced instead (Fig. 1B). From a nonreplicating P41 minimal construct (P41Cap), only 5 to 6% of the RNA was polyadenylated at (pA)p (Fig. 4, see also Fig. 1B), whereas ca. 10 to 15% of the RNA persisted as unspliced RNA that is presumably polyadenylated at the distal polyadenylation site, (pA)d (Fig. 4). When splicing was abolished in P41Cap by mutation of the A2 cleavage site (P41CapA2CAA), usage of (pA)p increased fivefold (from 5.5 to 27.8%, Fig. 4, lanes 1 and 7). This suggested that, in contrast to P7-generated transcripts (Fig. 3), even under normal conditions, splicing and polyadenylation factors likely compete for the same pool of P41-generated pre-mRNA under normal conditions.
FIG. 4.
Efficient polyadenylation of P41-driven transcripts at (pA)p requires a downstream element that overlaps with the A2 3′ splice acceptor site and competes with the splicing process. 293 cells were transfected with the P41Cap (diagrammed on the right) and the P41CapA2CAA-based plasmids, which contain various mutations (diagrammed in Fig. 3) of the A2 3′ splice acceptor site. Total RNA was protected by homologous DH probes, and a representative experiment is shown with the identities of the protected bands on the right. Quanitification of the ratios of RNAs polyadenylated at (pA)p relative to the total protected RNAs are shown as averages with the standard deviations and are results of at least three individual experiments. RNA from uninfected cells generated no protection products.
Mutation, in the P41Cap constructs, of the putative DSE and polypyrimidine tract as described above resulted in a modest decrease in the percentage of P41-generated transcripts that used (pA)p (Fig. 4). The greatest effect was seen with the mutation that introduced a U2AF-binding site into this region and which improved splicing most dramatically (P41CapA2U2AF; Fig. 4, lane 4). Similar to the P7-driven constructs, when the U2AF mutation was introduced into the A2 cleavage site mutant background (A2CAA), the reduction in relative levels of P41-generated RNA polyadenylated at (pA)p was reversed (Fig. 4, compare lanes 4 and 10). The relative levels of polyadenylation at (pA)p of transcripts generated by P41CapAT6, which is predicted to introduce both a very strong DSE (TTGANNNTTTTTT) and an improved polypyrimidine tract, while leaving the resident TTTTGTT intact, was also decreased at the expense of increased splicing. However, in contrast to results obtained with this mutation in the P7-expressing construct described above (Fig. 3), (pA)p usage was increased dramatically when this mutation was introduced into the background that abolished splicing [compare P41CapA2T6, with ∼2.5% (pA)p (Fig. 4, lane 5), to P41CapA2CAAT6 with ∼64% (pA)p (Fig. 4, lane 11)]. These results further suggested that splicing competes with polyadenylation for molecules from the same pool of P41-generated pre-mRNAs. Furthermore, the finding that, within the A2 cleavage site background, A2 region mutations reduced (pA)p usage five- to sixfold less than the parental construct P41CapA2CAA (Fig. 4, compare lane 7 with lanes 8, 9, 10, and 12) is also consistent with these mutations disrupting a cis-acting DSE. These results demonstrated that the region between A1 and A2 serves an important role in both polyadenylation of P7-, P19-, and P41-generated transcripts at (pA)p, as well as splicing of P41-generated pre-mRNAs.
Efficient polyadenylation of P7-driven transcripts at (pA)p requires an upstream element that lies prior to the initiation of the P41 transcripts.
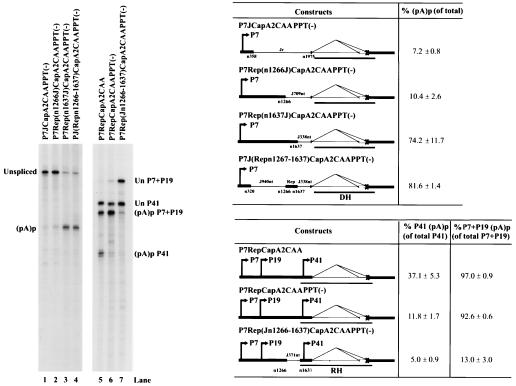
Surprisingly, mutations within the DSE and polypyrimidine tract region that significantly reduced (pA)p usage in P7JCap [PPT(−) and PTB; Fig. 3 and 5, lane 1], had little effect on (pA)p usage in P7- and P19-generated transcripts in a Rep-Cap background [P7RepCapA2CAAPPT(−) is shown in Fig. 5 (lane 6) as an example]. This construct also contains the A2 cleavage site mutation, which inhibits splicing. Although the PPT(−) DSE mutation did not reduce (pA)p usage for P7 and P19 RNA generated from this construct, it still inhibited polyadenylation at (pA)p of P41-generated transcripts (compare Fig. 5, lanes 5 and 6). This suggested that, although the DSE was necessary to direct efficient polyadenylation at (pA)p in the absence of the rep gene (P7JCap), there must also be an element within these RNAs upstream of P41 that was functionally equivalent.
FIG. 5.
Efficient polyadenylation of P7-and P19-generated RNAs at (pA)p requires an USE that lies upstream of the P41 promoter. 293 cells were transfected with the following plasmids, which are diagrammed on the right: P7JCapA2CAAPPT(−) (lane 1), P7Rep(n1266J)CapA2CAAPPT(−) (lane 2), P7Rep(n1637J)CapA2CAAPPT(−) (lane 3), P7Rep(n1267-1636J)CapA2CAAPPT(−) (lane 4), P7RepCapA2CAA (lane 5), P7RepCapA2CAAPPT(−) (lane 6), and P7Rep(Jn1266-1637)CapA2CAAPPT(−) (lane 7). Total RNAs were protected either with the DH probe (lanes 1 to 4) or the RH probe (lanes 5 to 7). A representative experiment is shown, with the identities of the protected bands on the right. Quantification of the ratios of RNAs polyadenylated at (pA)p relative to the total protected RNAs are shown as averages with the standard deviations and are the results from at least three individual experiments. For lanes 5, 6, and 7, the percent P41 (pA)p is the ratio of P41 RNA polyadenylated at (pA)p relative to the total P41-generated RNA, whereas the percent P7+P19 (pA)p is the ratio of P7+P19 RNA polyadenylated at (pA)p relative to the total P7+P19-generated RNAs. RNA from uninfected cells generated no protection products.
To further map where this upstream element resided, we replaced the heterologous DNA in P7JCapA2CAAPPT(−) with increasing amounts of rep gene sequences. The DSE mutation retained a debilitating effect on (pA)p usage within constructs that included rep sequences that extended downstream through nt 1266 [P7Rep(n1266J)CapA2CAAPPT(−); Fig. 5]. The DSE mutation was suppressed, however, in a background of rep sequences that extended further, through nt 1637 [P7Rep(n1637J)CapA2CAAPPT(−); Fig. 5]. This suggested that the region between nt 1266 to 1637 contained an element that could overcome the effect of debilitating mutations in the (pA)p DSE. Insertion of this region by itself into the P7JCapA2CAAPPT(−) background was shown to have a similar compensating effect [P7J(Repn1267-1637J)CapA2CAAPPT(−); Fig. 5, lane 4]. In a reciprocal experiment, mutations of the A2 DSE region that had no effect on (pA)p usage in the RepCap background, regained a debilitating effect when the nt 1266 to 1637 region of rep gene sequences was replaced by heterologous sequences [P7Rep(Jn1266-1637)CapA2CAAPPT(−); Fig. 5, lane 7]. The effect on P41-driven RNA was only modestly reduced when the nt 1266 to 1637 region was replaced with plasmid sequences (12 to 5%), compared to the effect on P7-driven RNA (92 to 13%) (Fig. 5, compare lanes 6 and 7). These results suggested that polyadenylation of P7- and P19-driven transcripts were also influenced by a USE positioned prior to the start of the P41 transcripts, which was at least partially redundant with the downstream element between A1 and A2.
DISCUSSION
In contrast to RNA generated by prototypical AAV2, AAV5 RNA is cleaved and polyadenylated at a site, (pA)p, within the intron in the center of the AAV5 genome. The presence of a polyadenylation site within a functional small intron in viral systems is unusual: well-studied examples of internal cleavage and polyadenylation in retroviruses (2, 3) and papillomaviruses (14) have quite different configurations. Polyadenylation of the IgM heavy-chain genes (25, 37, 38) and the calcitonin gene cluster (41), which occurs at a polyadenylation site within a functional intron, varies with cell type or differentiation state (1, 12, 30). The AAV5 system is unusual in that different relative levels of polyadenylation are constitutively seen for transcripts generated by different promoters.
For the experiments described, we have chosen to evaluate the usage of (pA)p relative to the total RNA. This unbiased approach revealed that competition between splicing and polyadenylation can occur for molecules from the same pool of precursor AAV5 pre-mRNAs. It should be noted, however, that such measurements may overlook the loss of other species due to their specific degradation.
The majority of RNAs generated from the AAV5 P7 and P19 upstream promoters utilize (pA)p; however, the majority of RNAs generated from the proximal P41 promoter are not polyadenylated at (pA)p but rather extend to the distal site (pA)d and are ultimately spliced. The same relative ratio of the various RNA species was seen both in 293 and HeLa cells coinfected with helper adenovirus. Whether the same ratios are produced after coinfection with other helper viruses has not yet been determined.
Polyadenylation of both P7- and P41-generated RNAs at (pA)p is influenced by a G/U-rich DSE that lies within the 3′ splice site of the intron. The role of the DSE for polyadenylation of P41-driven RNA was directly apparent for P41 minimal constructs. For P7-driven constructs, however, the role of the DSE only became apparent when DSE mutations were made in the P7JCap background, in which the rep gene region was replaced with heterologous sequences from a bacterial plasmid. This effect was abolished in the rep background because of the suppressing effect of a USE between AAV5 nt 1266 and 1637. These results suggested that polyadenylation of P7-generated RNAs was influenced by both an USE in addition to the DSE and that these elements are at least partially redundant. The USE lies approximately 500 nt upstream of (pA)p, farther than many previously described upstream elements that influence downstream polyadenylation (8, 11, 20, 29, 31, 32). The USE lies upstream of the start of the P41-generated RNAs, which are, therefore, not subject to the additional level of control this element imposes on P7-driven RNAs. The USE is not required if the DSE is intact; however, it can compensate for the loss of DSE function. Although the relationship between these two elements is not yet clear, the AAVs have an overlapping transcription profile, and perhaps the redundant function of the USE, lacking in P41-generated RNAs, specifically ensures that the AAV5 P7- and P19-generated RNAs are polyadenylated at (pA)p at high efficiency.
We have observed that polyadenylation of P41-generated RNAs at (pA)p is significantly increased when it is produced from a replication-competent molecule. Whether this effect is due to genome replication directly, as has been shown for the polyadenylation of adenovirus (34) and B19-generated RNAs (17), or perhaps to other reasons, such as increased localization to regions rich in polyadenylation factors, has not yet been determined. Whether P41 RNAs polyadenylated at (pA)p, which can comprise 25 to 28% of P41-generated RNA during AAV5 coinfection with Ad5, have any role during AAV5 infection will also require further investigation.
There have been a number of examples that have been described, in which splicing and polyadenylation have been shown to compete for the same pool of pre-mRNA molecules. For instance, alternative processing of IgM heavy-chain pre-mRNAs is regulated during B-cell differentiation, and the appropriate levels of mRNAs are achieved by competition between splicing and polyadenylation at a poly(A) site within an intron (24). Although the polyadenylation and splicing of AAV5 P41-generated RNAs compete for the same pool of pre-mRNA molecules, it also seems that splicing may be more dominant in this regard. When the polypyrimidine tract mutation which introduced six uridines downstream of the DSE was introduced into the P41 minimal construct in which splicing was abolished by the A2 acceptor mutation (P41CapA2CAAT6), (pA)p usage was dramatically increased, suggesting that the six-uridine substitution mutation improved the polyadenylation-enhancing effect of the DSE. The same mutation in a splicing-competent background (P41CapA2T6), however, did not increase the relative amounts of product polyadenylated at (pA)p (Fig. 4, compare lanes 6 and 13), suggesting that splicing may be inherently stronger than polyadenylation in this construct. It is possible, therefore, that the propensity to splice AAV5 P41-generated pre-mRNAs [a choice that precludes polyadenylation at (pA)p], is greater than the propensity to polyadenylate at (pA)p, which ultimately diminishes the pool of unspliced molecules. This is consistent with the requirement to generate spliced P41 transcripts to produce the appropriate amount of capsid proteins during infection. There are a number of examples in which U1 snRNP binding to pre-mRNAs influences polyadenylation at nearby sites (2, 3, 13, 14), and the presence of (pA)p within an intron suggests that perhaps this may also be so for AAV5.
For AAV5, the DSE-containing A2 3′ splice site plays a dual role in both polyadenylation and splicing, and there is clearly a complicated regulation between the two. The DSE overlaps, but is not congruent with, the 3′ acceptor of the AAV5 intron. The relative strengths of these two signals must allow both for levels of polyadenylation of the upstream promoter-generated RNAs that generate the proper levels of the essential Rep proteins and for efficient splicing to generate the correct ratio of spliced P41-generated RNAs to produce the individual capsid proteins at the appropriate stoichiometry. The relative efficiency of these processes is controlled to a significant degree by the strength of the cis-acting signals governing them, and they likely have evolved to program the optimal amounts of viral gene products required for the AAV5 life cycle. Interestingly, the 3′ splice site region of the A2 acceptor of AAV2 does not contain a G/U-rich DSE-like element, and this region of AAV2 is unable to support polyadenylation at the AAV5 (pA)p site. Conversely, either when (pA)p is replaced with the internal AAUAAA signal and surrounding region from AAV2 (Fig. 2B) or when the internal region of the AAV2 intron from the donor to A1, which contains the internal AAUAAA signal normally cryptic in its original position, is inserted into AAV5 and linked to the AAV5 DSE-containing A2 3′ splice site (data not shown), polyadenylation at the AAV2 AAUAAA site is highly efficient. This suggests that at least one element that differentiates the use of this site between the two viruses is the presence of the DSE. We have also observed that the Rep-encoding RNAs generated by the P9 and P19 promoters of the autonomous goose parvovirus also utilize an internal polyadenylation site that has a DSE located at a similar position within its A2 3′ splice site (Qiu and Pintel, unpublished). An understanding of why AAV5 and goose parvovirus have evolved to use an internal polyadenylation mechanism, rather than utilize the transcript profile seen for those AAVs represented by the prototype AAV2, will likely reveal an important feature of parvovirus biology.
Acknowledgments
We thank Jay Chiorini (National Institutes of Health) for AAV5 virus. We thank Lisa Burger for excellent technical assistance and Greg Tullis for helpful discussions and critical reading of the manuscript.
R.N. was supported by the MU Molecular Biology Program. This study was supported by PHS grants RO1 AI46458 and RO1 AI21302 from the National Institute of Allergy and Infectious Disease to D.J.P.
REFERENCES
- 1.Amara, S. G., V. Jonas, M. G. Rosenfeld, E. S. Ong, and R. M. Evans. 1982. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240-244. [DOI] [PubMed] [Google Scholar]
- 2.Ashe, M. P., P. Griffin, W. James, and N. J. Proudfoot. 1995. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 9:3008-3025. [DOI] [PubMed] [Google Scholar]
- 3.Ashe, M. P., L. H. Pearson, and N. J. Proudfoot. 1997. The HIV-1 5′ LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J. 16:5752-5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atchison, R. W., B. C. Casto, and W. M. Hammon. 1965. Adenovirus-associated defective virus particles. Science 149:754-756. [DOI] [PubMed] [Google Scholar]
- 5.Bantel-Schaal, U., and H. zur Hausen. 1984. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology 134:52-63. [DOI] [PubMed] [Google Scholar]
- 6.Berns, K. 1996. Parvoviridae: the viruses and their replication, p. 1017-1041. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 7.Blacklow, N. R., M. D. Hoggan, A. Z. Kapikian, J. B. Austin, and W. P. Rowe. 1968. Epidemiology of adenovirus-associated virus infection in a nursery population. Am. J. Epidemiol. 88:368-378. [DOI] [PubMed] [Google Scholar]
- 8.Brown, P. H., L. S. Tiley, and B. R. Cullen. 1991. Efficient polyadenylation within the human immunodeficiency virus type 1 long terminal repeat requires flanking U3-specific sequences. J. Virol. 65:3340-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, F., and J. Wilusz. 1998. Auxiliary downstream elements are required for efficient polyadenylation of mammalian pre-mRNAs. Nucleic Acids Res. 26:2891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeZazzo, J. D., and M. J. Imperiale. 1989. Sequences upstream of AAUAAA influence poly(A) site selection in a complex transcription unit. Mol. Cell. Biol. 9:4951-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwalds-Gilbert, G., K. L. Veraldi, and C. Milcarek. 1997. Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res. 25:2547-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortes, P., Y. Cuevas, F. Guan, P. Liu, S. Pentlicky, S. P. Jung, M. I. Martinez-Chantar, J. Prieto, D. Rowe, and S. I. Gunderson. 2003. Inhibiting expression of specific genes in mammalian cells with 5′ end-mutated U1 small nuclear RNAs targeted to terminal exons of pre-mRNA. Proc. Natl. Acad. Sci. USA 100:8264-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furth, P. A., W. T. Choe, J. H. Rex, J. C. Byrne, and C. C. Baker. 1994. Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol. Cell. Biol. 14:5278-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoggan, M. D., N. R. Blacklow, and W. P. Rowe. 1966. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. USA 55:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, J. M., S. W. Green, T. Shimada, and N. S. Young. 1992. A block in full-length transcript maturation in cells nonpermissive for B19 parvovirus. J. Virol. 66:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou, H., D. M. Helfman, R. F. Gagel, and S. M. Berget. 1999. Polypyrimidine tract-binding protein positively regulates inclusion of an alternative 3′-terminal exon. Mol. Cell. Biol. 19:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayor, H. D., and J. L. Melnick. 1966. Small deoxyribonucleic acid-containing viruses (picodnavirus group). Nature 210:331-332. [DOI] [PubMed] [Google Scholar]
- 20.Moreira, A., M. Wollerton, J. Monks, and N. J. Proudfoot. 1995. Upstream sequence elements enhance poly(A) site efficiency of the C2 complement gene and are phylogenetically conserved. EMBO J. 14:3809-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouw, M., and D. J. Pintel. 2000. Adeno-associated virus RNAs appear in a temporal order and their splicing is stimulated during coinfection with adenovirus. J. Virol. 74:9878-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naeger, L. K., R. V. Schoborg, Q. Zhao, G. E. Tullis, and D. J. Pintel. 1992. Nonsense mutations inhibit splicing of MVM RNA in cis when they interrupt the reading frame of either exon of the final spliced product. Genes Dev. 6:1107-1111. [DOI] [PubMed] [Google Scholar]
- 23.Parks, W. P., J. L. Melnick, R. Rongey, and H. D. Mayor. 1967. Physical assay and growth cycle studies of a defective adeno-satellite virus. J. Virol. 1:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson, M. L. 1994. Regulated immunoglobulin (Ig) RNA processing does not require specific cis-acting sequences: non-Ig RNA can be alternatively processed in B cells and plasma cells. Mol. Cell. Biol. 14:7891-7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips, C., S. Jung, and S. I. Gunderson. 2001. Regulation of nuclear poly(A) addition controls the expression of immunoglobulin M secretory mRNA. EMBO J. 20:6443-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu, J., R. Nayak, G. E. Tullis, and D. J. Pintel. 2002. Characterization of the transcription profile of AAV5 reveals a number of unique features compared to previously characterized adeno-associated viruses. J. Virol. 76:12435-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu, J., and D. J. Pintel. 2002. The adeno-associated virus type 2 Rep protein regulates RNA processing via interaction with the transcription template. Mol. Cell. Biol. 22:3639-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renan, M. J. 1987. Conserved 12-bp element downstream from mRNA polyadenylation sites. Gene 60:245-254. [DOI] [PubMed] [Google Scholar]
- 29.Russnak, R., and D. Ganem. 1990. Sequences 5′ to the polyadenylation signal mediate differential poly(A) site use in hepatitis B viruses. Genes Dev. 4:764-776. [DOI] [PubMed] [Google Scholar]
- 30.Sabate, M. I., L. S. Stolarsky, M. M. Polak, S. R. Bloom, I. M. Varndell, M. A. Ghatei, R. M. Evans, and M. G. Rosenfeld. 1985. Regulation of neuroendocrine gene expression by alternative RNA processing. Colocalization of calcitonin and calcitonin gene-related peptide in thyroid C-cells. J. Biol. Chem. 260:2589-2592. [PubMed] [Google Scholar]
- 31.Sanfacon, H., P. Brodmann, and T. Hohn. 1991. A dissection of the cauliflower mosaic virus polyadenylation signal. Genes Dev. 5:141-149. [DOI] [PubMed] [Google Scholar]
- 32.Schek, N., C. Cooke, and J. C. Alwine. 1992. Definition of the upstream efficiency element of the simian virus 40 late polyadenylation signal by using in vitro analyses. Mol. Cell. Biol. 12:5386-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoborg, R. V., and D. J. Pintel. 1991. Accumulation of MVM gene products is differentially regulated by transcription initiation, RNA processing, and protein stability. Virology 181:22-34. [DOI] [PubMed] [Google Scholar]
- 34.Shenk, T. 2001. Adenoviridae: the viruses and their replication, p. 2283-2284. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 35.Siegl, G., R. C. Bates, K. I. Berns, B. J. Carter, D. C. Kelly, E. Kurstak, and P. Tattersall. 1985. Characteristics and taxonomy of Parvoviridae. Intervirology 23:61-73. [DOI] [PubMed] [Google Scholar]
- 36.Singh, R., J. Valcárcel, and M. R. Green. 1995. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268:1173-1176. [DOI] [PubMed] [Google Scholar]
- 37.Takagaki, Y., and J. L. Manley. 1998. Levels of polyadenylation factor CstF-64 control IgM heavy chain mRNA accumulation and other events associated with B-cell differentiation. Mol. Cell 2:761-771. [DOI] [PubMed] [Google Scholar]
- 38.Takagaki, Y., R. L. Seipelt, M. L. Peterson, and J. L. Manley. 1996. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B-cell differentiation. Cell 87:941-952. [DOI] [PubMed] [Google Scholar]
- 39.Terhune, S. S., C. Milcarek, and L. A. Laimins. 1999. Regulation of human papillomavirus type 31 polyadenylation during the differentiation-dependent life cycle. J. Virol. 73:7185-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarudnaya, M. I., I. M. Kolomiets, A. L. Potyahaylo, and D. M. Hovorun. 2003. Downstream elements of mammalian pre-mRNA polyadenylation signals: primary, secondary and higher-order structures. Nucleic Acids Res. 31:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, J., L. Hyman, and C. Moore. 1999. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNa synthesis. Microbiol. Mol. Biol. Rev. 63:405-445. [DOI] [PMC free article] [PubMed] [Google Scholar]