Abstract
Thioredoxin 1 (Trx1) is a small molecule with reactive cysteines that reduces proteins with disulfide bonds through a thiol disulfide exchange reaction. Accumulating lines of evidence suggest that Trx1 protects the heart from ischemia/reperfusion injury, pathological hypertrophy and inflammation, induces preconditioning effects and angiogenesis, and upregulates mitochondrial genes. Exogenously-given recombinant Trx1 (r-Trx1) may protect the heart through its actions in both extracellular and intracellular spaces. In this brief review, the potential of Trx1 therapy for heart disease is discussed.
Keywords: thioredoxin1, disulfide bond, ischemia/reperfusion, pathological cardiac hypertrophy, recombinant, small molecule
Almost 20 million people suffer from ischemic heart disease in the US, and one million patients develop myocardial infarction (MI) every year. Even if patients with acute coronary occlusion are treated with primary percutaneous coronary intervention (PCI), many of them suffer from myocardial damage caused by ischemia/reperfusion (I/R) injury. The development of a new class of medicine minimizing I/R injury would have a significant impact on medical therapy for acute MI patients. Unfortunately, numerous attempts to identify a modality to effectively prevent I/R injury in humans have not been successful, thus far [1].
Thioredoxin1 (Trx1) plays a key role in maintaining the reducing environment in cells [2]. Trx1 has two redox-active cysteine residues (a dithiol) within the conserved active site sequence Cys-Gly-Pro-Cys. This Trx1 dithiol is reduced by receiving electrons from NADPH in the presence of thioredoxin reductase (TR). The reduced Trx1 in turn reduces proteins with disulfide bonds by transferring electrons from its reactive cysteines through thiol disulfide exchange reactions. Thus, NADPH, TR and Trx1 are collectively termed the Trx system, and the electrons initially provided by the Trx system are used to reduce cellular proteins with disulfide bonds. Trx1 also modifies s-nitrosylation of cysteine residues by removing or adding NO. Increasing lines of evidence suggest that posttranslational modification of cysteine residues affects the structure and function of proteins. Disulfide bonds contribute to a protein’s tertiary and/or quaternary structure by forming strong covalent bonds between different peptide chains. Thus, Trx1 affects the structure and function of proteins by modifying cysteine residues.
Trx1 is involved in a wide array of cellular functions (reviewed in [2]). For example, Trx1 reduces peroxiredoxin, which in turn reduces H2O2, thereby alleviating oxidative stress. Trx1 also interacts with transcription factors and intracellular signaling molecules, thereby regulating their activities and subcellular localization [3]. Trx1 is expressed in the heart at baseline and expression of Trx1 is upregulated in response to stress, including ischemia and reperfusion. Studies conducted with in vivo animal models have suggested that Trx1 has many beneficial functions in the heart, including protection against I/R, without obvious side effects [4–6] (Figure 1). Trx1 is a small molecule which functions in both extracellular and intracellular spaces [2], and accumulating lines of evidence suggest that recombinant Trx1 (r-Trx1) can be taken up by cells [7]. These unique properties of Trx1 support the idea that r-Trx1 may be useful for treatment of I/R and other pathological conditions in the heart. In this brief review, we discuss 1) beneficial effects of Trx1 in I/R, 2) beneficial effects of Trx1 during pathological hypertrophy, and 3) potential applications of r-Trx1 for treatment of heart disease (Figure 2).
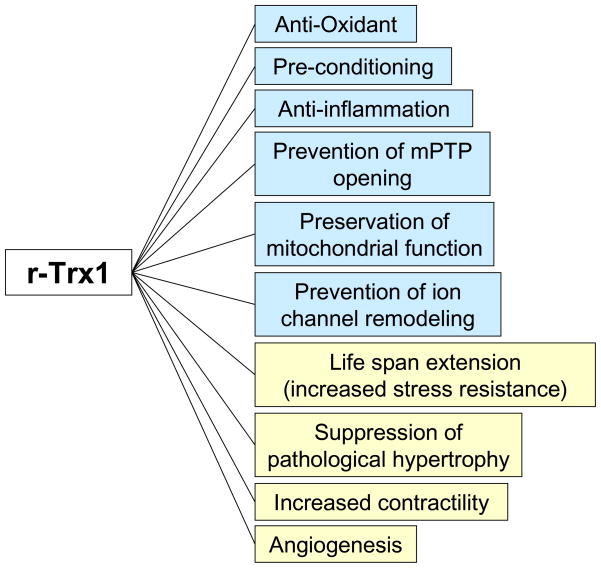
Figure 1.
r-Trx1 has multiple beneficial effects upon I/R injury in the heart. The functions indicated by blue are relevant to acute I/R injury whereas those indicated by yellow are relevant to chronic development of cardiac remodeling and heart failure.
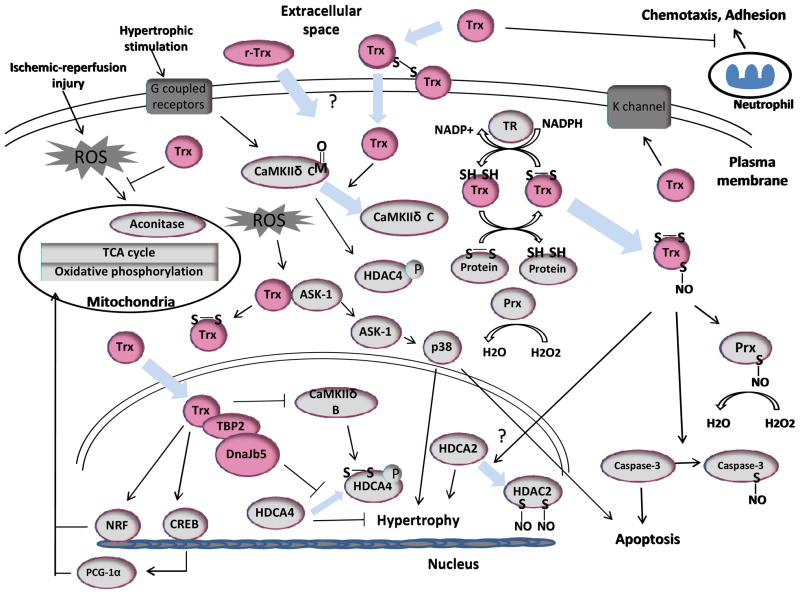
Figure 2.
Proposed signaling mechanisms through which r-Trx1 protects the heart from ischemia/reperfusion and pathological hypertrophy.
ROS reactive oxygen species; Prx peroxiredoxin; HDAC histone deacetylases; TR thioredoxin reductase; CaMKII Ca2+ calmodulin dependent protein kinase; NRF, nuclear respiratory factor; CREB cAMP Responsive Element Binding Protein; TBP-2, thioredoxin binding protein-2; PGC-1α, Peroxisome proliferator-activated receptor-gamma coactivator-1α.
Trx1 reduces I/R injury
Studies conducted with a genetically altered mouse model [4] and adenovirus mediated gene delivery [6] suggested that overexpression of Trx1 protects the heart from I/R injury, prevents sudden death, and improves post-MI cardiac function. Trx1 is upregulated by ischemic preconditioning, and inhibition of Trx1 by RNA interference attenuates the cardioprotective effect of preconditioning [8], suggesting that endogenous Trx1 mediates this potent mechanism of cardioprotection. These studies have provided proof of concept for the idea that upregulation of Trx1 can be used as a therapeutic intervention to reduce I/R injury.
How does Trx1 prevent myocardial injury after I/R? Trx1 reduces proteins with oxidized cysteines through the thiol disulfide exchange reaction. Trx1 also reduces H2O2, a major component of reactive oxygen species (ROS), indirectly through reduction of peroxiredoxin. However, the cellular actions of Trx1 are more diverse than what would be expected of a simple anti-oxidant. Trx1 modulates the functions of a wide variety of signaling molecules and transcription factors through protein-protein interaction and/or thiol disulfide exchange reactions [3]. For example, Trx1 protects mitochondrial proteins with iron-sulfur clusters, such as aconitase, from oxidation and consequent dysfunction [9]. Trx1 also upregulates mitochondrial proteins involved in oxidative phosphorylation and the TCA cycle, which are easily damaged by oxidative stress during I/R [10]. Preservation of the mitochondrial function minimizes mitochondrial oxidative stress and prevents mPTP opening [11], a critical event during I/R which stimulates the death of cardiac myocytes. Trx1 also inhibits expression of proinflammatory cytokines, chemotaxis, complement activation, and neutrophil adhesion. This is important because I/R stimulates cell adhesion molecules and migration of neutrophils into the heart [1]. Subsequent production of inflammatory cytokines and ROS enhances myocardial injury. Another potentially important mechanism is the effect of Trx1 upon ion channel remodeling. Downregulation of the K+ channel by oxidative stress could cause lethal arrhythmia and contractile dysfunction in the heart. Upregulation of Trx1 may prevent downregulation of the Kv4 channel and consequent electrical instability during I/R [12].
Exogenously applied r-Trx1 reduces infarct size in a mouse model of I/R [7], suggesting that either r-Trx1 or small molecules mimicking Trx1 may be useful for treatment of I/R injury in humans. It should be noted, however, that many cardioprotective mechanisms observed in mice are not effective in clinical treatment of I/R injury in humans. Although the species difference could be a major reason for the failures, another important reason may be the fact that patients with heart attack are often treated for the first time at the time of reperfusion, whereas many interventions in animal studies have been conducted without taking the timing of the intervention into serious consideration [1]. In this regard, it would be important to further evaluate the effectiveness of Trx1 based therapy by administering r-Trx1 specifically at the time of reperfusion, preferably in a large animal model of I/R.
Trx1 prevents pathological hypertrophy
Trx1 has additional beneficial effects that would be observed if treatment with r-Trx1 were extended through the subacute phase of MI after I/R. These include suppression of pathological hypertrophy/ion channel remodeling, increased contractility, angiogenesis [13] and improved mitochondrial function [10]. Suppression of endogenous Trx1 by overexpression of Trx1-DN induces cardiac hypertrophy at baseline and exacerbates the hypertrophic response induced by transverse aortic constriction (TAC). On the other hand, overexpression of Trx1 attenuates cardiac hypertrophy induced by TAC [5]. These findings indicate that Trx1 suppresses pathological cardiac hypertrophy.
Pathological hypertrophy caused by a variety of stimuli is commonly mediated through the nucleo-cytoplasmic translocation of class II histone deacetylases (HDACs) and subsequent de-repression of transcription factors mediating cardiac hypertrophy, including MEF2, NFAT, and CAMTA2 [14]. The nucleo-cytoplasmic translocation of class II HDACs is believed to be regulated by phosphorylation-dependent mechanisms. Thus, the HDAC kinases, including CaMKII, PKD and GRK5, could be important targets for treatment of pathological hypertrophy [14]. CaMKII can be activated by methionine oxidation in a Ca2+ calmodulin-independent manner [15]. Trx1 reduces methionine reductase, which in turn negatively regulates CaMKII.
We have shown that HDAC4 is subject to redox modification through intramolecular disulfide bond formation between Cys-667 and Cys-669, which in turn induces nucleo-cytoplasmic translocation of HDAC4 [16]. Trx1 effectively reduces class II HDACs oxidized in response to hypertrophic stimuli, a mechanism which plays an important role in mediating the anti-hypertrophic actions of Trx1 [16]. Importantly, Trx1 induces nuclear translocation of HDAC4 even when HDAC4 is phosphorylated by hypertrophic stimuli, suggesting that Trx1 can reverse cardiac hypertrophy initiated by phosphorylation-dependent mechanisms.
Recent evidence suggests that cysteine residues (Cys-262 and Cys-274) in HDAC2, a class I HDAC, are subjected to nitrosylation, which in turn induces dissociation of HDAC2 from its nuclear target and negatively regulates dendritic growth and branching in neurons [17]. Since class I HDACs, including HDAC2, suppress transcription of genes negatively regulating hypertrophy [17], nitrosylation of HDAC2 might inhibit cardiac hypertrophy by de-repressing transcription of the anti-hypertrophic genes. Although Cys-32 and Cys-35 in Trx1 play a key role in mediating the thiol disulfide exchange reaction, three other cysteines in Trx1, namely Cys-62, Cys-69, and Cys-73, can also be modified by NO and participate in transnitrosylation. This raises a possibility that Trx1 may also inhibit cardiac hypertrophy through transnitrosylation of HDAC2. Thus, Trx1 affects important proximal signaling mechanisms regulating the development of pathological hypertrophy. Together with the effect of Trx1 upon other signaling mechanisms mediating cardiac hypertrophy [18], stimulation of Trx1 inhibits pathological hypertrophy and prevents the progression of heart failure.
In order to suppress the progression of pathological hypertrophy with Trx1, however, long-term activation of Trx1 is essential. Unfortunately, treatment with intravenous infusion of r-Trx1 is not practical for this purpose. The development of orally active small molecule compounds mimicking the action of Trx1 may eventually allow us to use Trx1 for long-term treatment of pathological hypertrophy and heart failure.
Unsolved questions
Nakamura et al reported that the half life of r-Trx1 in plasma is about 1h in mouse, 2h in rat and 8 h in monkeys [2]. Although a previous study showed that exogenously given r-Trx1 is taken up by the heart and prevents I/R injury [7], the concentration of r-Trx1 in the heart after intravenous injection is low [2]. This raises a question as to whether r-Trx1 protects the heart primarily through its action in the intracellular space. Trx1 in the extracellular space forms an inter-molecular disulfide bond with membrane-bound Trx1 in the lipid raft, which is in turn transported into the cytosol [19]. However, the precise mechanism through which r-Trx1 is taken up is not well understood. If formation of the inter-molecular disulfide bond is an essential step for delivery, an additional step to reduce r-Trx1 would be required to make r-Trx1 with reducing capability available inside cardiac myocytes. Alternatively, attaching a cell permeable amino acid stretch to r-Trx1 may be considered, provided that such modification does not affect the substrate accessibility of r-Trx1. To what extent and how long intravenously applied r-Trx1 remains in the reduced state is currently unknown. If therapeutic effects of r-Trx1 critically rely on its reduced state, a method to enhance/prolong the availability of r-Trx1 in its reduced form must be developed.
It has been shown that preincubation of r-Trx1 with S-nitrosoguanosine induces S-nitrosylation of r-Trx1 and markedly enhances the protective effect of r-Trx1 against I/R injury [7]. We have shown recently that Trx1 is itself nitrosylated at Cys-73 when Cys-32/Cys-35 form a disulfide bond, and that Cys-73-nitrosylated Trx1 transnitrosylates target proteins, such as peroxiredoxin [20]. S-nitrosylation not only enhances the beneficial effects of target proteins but also protects them from further oxidation. Stamler’s group has shown, however, that Trx1 and Trx2 also mediate stimulus-dependent denitrosylation of proteins, including mitochondria associated caspase-3 [21]. Thus, it is possible that the protective effects of r-Trx1 may be mediated through unconventional functions of Trx1 and affected by complex interplay between protein oxidation and nitrosylation. Further investigation is required to elucidate the molecular mechanisms involved in the therapeutic actions of r-Trx1.
An important goal of Trx1 based treatment for cardiovascular disease is to enhance the effective concentration of Trx1 in the heart. This goal may be achieved by either enhancing intracellular synthesis or inhibiting degradation of Trx1. Geranylgeranylacetone, an anti-ulcer drug, and resveratrol, a naturally occurring polyphenol, are known to upregulate Trx1 [13]. Further investigation into either transcription or microRNA regulating expression of Trx1 may allow for identification of a method to selectively upregulate the tissue level of Trx1. Transgenic overexpression of Trx1 protects against I/R injury in the mouse heart [4], but the extent of the increase in the level of transgene-derived Trx1 over endogenous Trx1 in the heart is unknown in this model. Tao et al used 2mg/kg of s-Trx1, ip, to show cardioprotective effects against I/R injury. However, the tissue level of Trx1 achieved by this method was not described [7]. The minimum tissue level of Trx1 required for achieving cardioprotection remains to be elucidated. Along this line, a question arises as to whether increasing the tissue level of r-Trx1 alone without concomitant upregulation of NADPH or TR, the other key components of the Trx system, is really sufficient to achieve the therapeutic benefits of r-Trx1, since the actions of r-Trx1 mediating its therapeutic effects would depend upon persistent increases in the level of reduced cysteines rather than the total amount of Trx1, which could be oxidized rapidly. The effectiveness of an intervention to enhance the whole activity of the Trx system should be evaluated.
Conclusions
The many beneficial effects of Trx1, together with remarkably little reported evidence of its adverse effects, make Trx1 an attractive molecule for treatment of heart diseases. Clinical application of r-Trx1 has already been considered in the area of infectious disease, where r-Trx1 acts primarily in extracellular spaces. In terms of its cardiac application, however, further investigation is required 1) to achieve more efficient intracellular delivery of r-Trx1 into cardiac myocytes and 2) to elucidate the exact molecular mechanism mediating the beneficial effects of r-Trx1 in the heart. Judging from the protective effect of r-Trx1 against I/R injury in rodents [7], it would be quite interesting to conduct preclinical studies with large animal models. If cardiac therapies with r-Trx1 are successful, they would provide a proof of concept for further development of small molecule compounds mimicking the action of r-Trx1 for use in much wider applications in cardiovascular diseases, including chronic heart failure.
Acknowledgments
Sources of Funding
This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, and AG27211, and the Foundation of Leducq Transatlantic Network of Excellence.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, Hoshino Y, Okuyama H, Matsuo Y, Yodoi J. Thioredoxin 1 delivery as new therapeutics. Adv Drug Deliv Rev. 2009;61:303–9. doi: 10.1016/j.addr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Ago T, Sadoshima J. Thioredoxin and ventricular remodeling. J Mol Cell Cardiol. 2006;41:762–73. doi: 10.1016/j.yjmcc.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turoczi T, Chang VW, Engelman RM, Maulik N, Ho YS, Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. J Mol Cell Cardiol. 2003;35:695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, et al. Inhibition of thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel SM, Thirunavukkarasu M, Penumathsa SV, Koneru S, Zhan L, Maulik G, et al. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–55. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, et al. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected] Proc Natl Acad Sci U S A. 2004;101:11471–6. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das DK. Thioredoxin regulation of ischemic preconditioning. Antioxid Redox Signal. 2004;6:405–12. doi: 10.1089/152308604322899477. [DOI] [PubMed] [Google Scholar]
- 9.Fu C, Hu J, Ago T, Sadoshima J, Li H. Quantitative analysis of redox-sensitive proteome with DIGE and ICAT. J Proteome Res. 2008;7:3789–802. doi: 10.1021/pr800233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ago T, Yeh I, Yamamoto M, Schinke-Braun M, Brown JA, Tian B, et al. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid Redox Signal. 2006;8:1635–50. doi: 10.1089/ars.2006.8.1635. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Nakamura H, Hattori I, Tanito M, Yodoi J. Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett. 2002;321:81–4. doi: 10.1016/s0304-3940(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Tang K, Xie B, Li S, Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol. 2008;295:H416–24. doi: 10.1152/ajpheart.91446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 15.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–93. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–5. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 18.Ago T, Sadoshima J. Thioredoxin1 as a negative regulator of cardiac hypertrophy. Antioxid Redox Signal. 2007;9:679–87. doi: 10.1089/ars.2007.1529. [DOI] [PubMed] [Google Scholar]
- 19.Kondo N, Ishii Y, Kwon YW, Tanito M, Sakakura-Nishiyama J, Mochizuki M, et al. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid Redox Signal. 2007;9:1439–48. doi: 10.1089/ars.2007.1665. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Liu T, Chen W, Oka SI, Fu C, Jain MR, et al. Redox Regulatory Mechanism of Transnitrosylation by Thioredoxin. Mol Cell Proteomics. doi: 10.1074/mcp.M110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–4. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]