Abstract
Purpose
To evaluate the effect of intravitreal ranibizumab on retinal capillary hemangioblastomas (RCHs) associated with von Hippel-Lindau (VHL) disease that are not amenable or responsive to standard therapy.
Design
Prospective, non-comparative, interventional case series.
Participants
Five patients with VHL associated RCH with exudative changes and visual loss.
Methods
Monthly intravitreal injections of ranibizumab (0.5mg) were given over a course of 6 months for a total of 7 injections, with additional injections considered until week 52. The final study visit was designated as 8 weeks after the final study injection.
Main Outcome Measures
The primary outcome was the change in best-corrected visual acuity (BCVA) of ≥ 15 letters at the final visit compared to baseline. Secondary outcomes included change in lesion size, exudation as assessed clinically and by fluorescein angiography, change in retinal thickness as evaluated by optical coherence tomography, and adverse event assessments.
Results
Patients received an average of 10.0±3.1 injections over an average period of 47±14 weeks including follow-up. Mean change in BCVA was a decrease of 9±20 letters, with 1 patient gaining ≥ 15 letters, and 2 patients losing ≥ 15 letters. Changes in both lesion size and exudation were variable.
Conclusions
Intravitreal ranibizumab, delivered as monotherapy every 4 weeks had minimal beneficial effects on most VHL-related RCHs. Possible treatment efficacy was demonstrated in the patient with the smallest lesion with less exudation. Future prospective studies are needed to determine the potential role of an anti-angiogenic agent possibly in combination with other therapies for the treatment of such advanced ocular tumors associated with VHL.
Introduction
Von Hippel-Lindau (VHL) disease is a multi-systemic, dominantly inherited cancer syndrome associated with germ-line mutations in the VHL gene.1 The hallmark ocular lesion associated with VHL disease is the retinal capillary hemangioblastomas (RCH), present in about 37% of patients with clinically definite and genetically confirmed VHL.2 Of these patients, about 3 in 5 patients have RCHs bilaterally.3 RCHs are found typically either in the peripheral retina or in the juxtapapillary location, and can progress to vision loss with retinal edema, lipid deposition, and exudative retinal detachments.4 Exudative peripheral RCHs are most commonly treated with laser photocoagulation or cryotherapy, often with success. Photodynamic therapy (PDT) has also been used. However, a small subset of peripheral tumors may also be resistant to multiple modes of standard therapy. Juxtapapillary RCHs, owing to their proximity to the optic nerve, are considerably more difficult to treat without adverse effects on the optic nerve or concurrent vision loss.5-7 In these situations, tumor exudation disrupts the surrounding retina, often in the macula, resulting in losses in visual field and central acuity.
Basic science and clinical evidence have indicated that the pathogenesis and growth of VHL-associated tumors, such as the hemangioblastomas found in the central nervous system and retina, are likely to involve vascular endothelial growth factor (VEGF).8 As an inherited disease, tumorigenesis in VHL disease begins with the somatic mutation of the second functional VHL allele and loss of VHL protein function. Cells that lack VHL function are unable to degrade an important transcription factor, called hypoxia-inducible factor −1α (HIF-1 α). In these cells, HIF-1α accumulates and up-regulates the expression of downstream target proteins such as VEGF. Constitutively high levels of VEGF subsequently contribute to hemangioblastoma formation; in animal models where VEGF is overexpressed9, or where VHL is inactivated10, visceral lesions resembling hemangioblastomas form as a consequence, similar to what is found in human disease.
Recent studies have indicated that anti-VEGF strategies can be effective in the treatment of tumors associated with loss of VHL function, especially in the case of renal cell carcinoma.11 For RCHs, recent case reports have also suggested that anti-VEGF agents, delivered either systemically12, 13 or via intravitreal injection14, may be useful in the treatment of RCHs. However, other studies did not find evidence of definitive tumor regression with anti-VEGF treatment, although there may be some reduction in the retinal exudation.15, 16 These case reports are suggestive and indicate a need for prospective pilot studies in investigating the potential of VEGF as a therapeutic target in the treatment of VHL-related disease.
Ranibizumab is a recombinant, humanized, monoclonal Fab fragment of a full-length antibody that binds and neutralizes all active forms of VEGF.17, 18 Delivered as an intravitreal injection, it is approved for and effective in the treatment of neovascular age-related macular degeneration19 and has the potential to treat other retinal diseases in which VEGF is up-regulated, such as diabetic retinopathy 20. The use of ranibizumab however has not been previously evaluated in the treatment of VHL disease-related RCHs. In this pilot study, we investigate the potential efficacy of intravitreal ranibizumab in the treatment of RCHs related to VHL disease in patients with advanced disease or disease not amenable to other forms of therapy.
Patients and Methods
This study was an open-label, non-randomized, prospective, pilot study of intravitreal injections of ranibizumab in patients with RCHs associated with VHL disease with either severe ocular disease or disease not amenable to standard therapy. The diagnosis of VHL disease was performed both clinically according to the criteria of Melmon and Rosen21 and genetically by the detection of a disease-causing mutation in the germline sequence of the VHL gene. Inclusion criteria included having best-corrected visual acuity (BCVA) of 20/25 or worse, clear ocular media, and adequate pupillary dilation. Patients were older than 18 years of age and gave written consent. Women of childbearing potential were required to have a negative urine pregnancy test at baseline and agree to urine pregnancy testing monthly before each treatment and for 2 months following the final treatment. Patients of reproductive capacity had to agree to use 2 forms of birth control during the study. Patients with severe cardiac disease or stroke were excluded, as were patients who previously received anti-angiogenic agents, intravitreal injection, or have undergone vitrectomy, glaucoma surgery, or corneal transplant surgery. Only one eye in each patient was treated with ranibizumab in all cases. Informed consent was obtained for all subjects. The study was conducted in the National Institutes of Health Clinical Center, a Health Insurance Portability and Accountability Act (HIPAA)-compliant facility. Institutional Review Board (IRB) approval was obtained for the study protocol and consent, and all procedures conformed to the tenets of the Declaration of Helsinki. This study is registered at www.clinicaltrials.gov (accessed March 11, 2008)
The study design consisted of intravitreal injections of 0.5mg of ranibizumab in the study eye given every 4 weeks for a total of 7 injections over the initial 24-week period. Patients were evaluated at each visit with BCVA, intraocular pressure (IOP) measurements, slit lamp examination, dilated fundus exam and photography, fluorescein angiography, and macular optical coherence tomography (OCT) before each injection. At 24 weeks, patients who demonstrated a decrease in tumor size, reduction in retinal thickening by OCT, or reduction in the extent of fluorescein leakage compared to baseline, were eligible to be considered for repeat injections. Patients were monitored with monthly evaluations until the 52-week time point or at least 8 weeks after their final injection. If a patient experienced a drop in BCVA of more than 30 letters from baseline, progression of the disease, or a serious adverse event attributed to the study treatment, the study therapy was discontinued.
The study medication ranibizumab was formulated as a sterile solution aseptically filled in a 3-ml glass vial, each containing 0.5ml of 10mg/ml ranibizumab aqueous solution (pH 5.5). It was delivered as an intravitreal injection of 0.5mg in a volume of 50 μl into the study eye using the usual injection procedures. Briefly, the periorbital area, lids, and lashes were thoroughly cleansed with betadine, a surgical drape applied, and a lid speculum inserted. Topical anesthesia was applied to the surface of the eye and the study medication was injected into the vitreous using a 30-gauge, ½ inch needle, through the anesthetized conjunctiva and sclera 3.5-4.0mm posterior to the limbus under sterile conditions. Antimicrobial drops were applied after the injection, and patients were evaluated 15 minutes post-procedure with a retinal fundus examination and IOP measurement. Antimicrobial drops were continued for 3 days following injection.
Case Reports
Patient 1
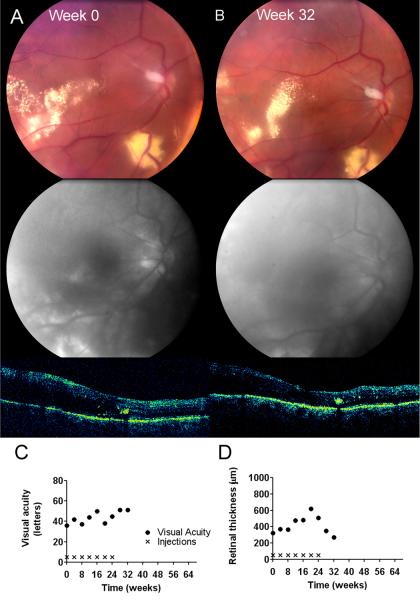
A 33-year-old male with systemic VHL disease with renal, pancreatic, cerebellar, and endolymphatic sac involvement, also had severe bilateral ocular disease related to RCHs. Extensive RCH involvement in his left eye had resulted in enucleation 4 years prior to study enrollment. The right eye had a large juxtapapillary lesion that had been treated with argon laser photocoagulation 12 years prior, resulting in a small centrocecal scotomatous field defect. Visual acuity immediately post-treatment was however preserved at 20/25. Subsequent to laser treatment, the RCH had continued to grow progressively with aggressive exudation. At baseline, BCVA was 20/500 (18 letters). The juxtapapillary RCH was associated with significant circinate lipid, intraretinal hemorrhage, and marked retinal edema extending into the macula (Figure 1A). Fluorescein angiography showed extensive late leakage into the subretinal space in the macula. OCT imaging demonstrated an extensive serous retinal detachment with central elevation of 1802 microns from the retinal pigment epithelium profile (Figure 1C). After the initial 3 injections of ranibizumab, vision improved to the 20/250 – 20/320 (25-28 letters) range, however over the next 4 injections, visual acuity steadily deteriorated to 20/500 (15 letters). The size of the lesion expanded from baseline, with increasing exudation and lipid accumulation in the macula. New RCHs also emerged in the posterior pole (Figure 1B). Retinal thickness also increased after 7 injections, extending beyond that measurable by OCT imaging. Further injections were discontinued due to the lack of treatment efficacy. On the final study visit, 8 weeks after the final injection, visual acuity further decreased to 20/640 (11 letters) (Figure 1D). No adverse events related to the study intervention were detected.
figure 1.

Patient 2
A 41-year old woman with a clinical and genetic diagnosis of VHL disease with a history of pheochromocytoma had unilateral RCH involvement that had not been previously treated. At baseline, visual acuity was 20/50 (66 letters). A large, endophytic, juxtapapillary lesion, surrounded by fluid and circinate lipid exudate extending into the fovea, was observed (Figure 2A) (available at http://aaojournal.org) in her left eye (study eye). OCT showed subretinal fluid extending into the macula with a central foveal thickness of 483 μm. After 7 injections, visual acuity and the exudative nature of the lesion remained relatively stable, and 5 further injections were given once every 4 weeks until the 44-week time point (a total of 12 injections). Over the course of the first 10 injections, vision fluctuated between 20/40 and 20/63 (73-56 letters). Vision however declined to 20/100 – 20/125 (49-45 letters) over the course of the last 2 injections. Further injections were withheld at this point. On the final study visit 8 weeks later, there had been an interval increase in fluorescein leakage, central macular edema, and retinal thickness (Figure 2B) compared to baseline. Central foveal thickness had increased to 762 μm (Figures 2B and D), and visual acuity in the study eye declined to 20/250 (Figure 2C). No adverse events related to the study intervention were detected.
figure 2.
Patient 3
A 35-year-old woman with a clinical and genetic diagnosis of VHL disease and a history of spinal cord hemangioblastoma had a unilateral, juxtapapillary RCH in her right eye (study eye). No previous treatment had been rendered. Baseline visual acuity was 20/40 (70 letters). The lesion was surrounded with intraretinal fluid and dense circinate lipid that extended into the fovea. OCT demonstrated cystic changes in the macula with a foveal thickness of 589 μm (Figures 3A and D) (available at http://aaojournal.org). During the first 28 weeks of study treatment, visual acuity fluctuated between 20/32 and 20/63 (75-61 letters), while OCT measurements indicated a slight trend towards decreasing central foveal thickness (Figures 3C and D). The patient continued on treatment, receiving a total of 14 injections over 52 weeks during the study. On her final visit at 60 weeks, lesion size was stable compared to baseline (Figure 3B). Fluorescein angiography revealed stable late leakage, however, new telangiectatic vessels developed in the macula. There had been some redistribution of hard lipid exudate in the macula, but the amount of exudation was relatively unchanged. Central macular OCT thickness was significantly decreased, measuring at 276 μm (decrease of 179 μm compared to baseline). Visual acuity however declined to 20/100 (49 letters). Mild adverse effects possibly related to treatment are transient erythema and edema in the upper lid of one study eye that spontaneously resolved.
figure 3.
Patient 4
A 62-year-old female with a clinical and genetic diagnosis of VHL disease and a history of bilateral renal, spinal cord, and cerebellar VHL involvement, as well as bilateral RCHs was enrolled. The right eye, which was the study eye, had extensive peripheral RCHs in the inferior and superotemporal quadrants that had been previously treated with cryotherapy and a scleral buckle 11 years prior to enrollment, followed by cataract extraction a few years later. These peripheral tumors were treated with laser photocoagulation, most recently 2 years ago, but this had failed to control the exudation. Baseline vision was 20/160 in the study eye. Exudative changes in the posterior pole were seen originating from the peripheral lesions and extending into the macula; OCT showed diffuse macular thickening and a foveal thickness of 323 μm (Figures 4A and D) (available at http://aaojournal.org). After 7 consecutive injections, the exudative changes increased, with increased leakage on angiography and increased foveal thickness on OCT (505 μm). Ranibizumab treatment was stopped and the lesions treated with argon laser photocoagulation at week 28. At her final visit, 8 weeks after the last injection, there was less angiographic leakage in the macula, decreased exudation, macular thickness had also decreased to 269 μm, and visual acuity slightly improved to 20/100 (51 letters - Figures 4B, C, and D). No adverse events related to the study intervention were detected.
figure 4.
Patient 5
A 51-year-old man presenting with bilateral RCHs had a documented germline VHL mutation and systemic VHL disease involving his spinal cord. At baseline, the left eye (study eye) contained an endophytic, juxtapapillary RCH with prominent surface vascularization about 1 disk diameter in size. Although central vision was good (20/25 or 79 letters), and OCT showed a normal foveal thickness, there was exudative activity with leakage on fluorescein angiography and thickening of the retina in the nasal macula. This was evident on both clinical exam and on OCT (389 μm on the outer nasal quadrant on the Stratus OCT grid) (Figures 5A and E). The patient reported symptoms of a pericentral scotoma; ancillary microperimetry testing also revealed decreased retinal sensitivity in the nasal macula that approached fixation. He was enrolled and treated with an initial course of 7 injections, resulting in a decrease in the prominence of the RCH and surface neovascularization. The amount of exudation and nasal macula thickness also clinically improved (Figure 5B). Retinal thickness in the nasal quadrant on OCT decreased to 297 μm (decrease of 92 μm). Visual acuity increased to 20/16 (89 letters) and study treatment was suspended. Sixteen weeks after the last injection, at week 44, the RCH was noted to again increase in prominence, with an increase in OCT thickness in the nasal macula to 445 μm (Figures 5C and E). Injections of ranibizumab resumed and continued until week 52 (3 additional injections, for a total of 10 injections). A similar improvement in the same parameters was again observed. At the final visit, visual acuity was slightly improved (by 6 letters), overall exudation was decreased, and nasal macula thickness OCT was lower (320 μm) compared to baseline. No adverse events related to the study intervention were detected.
figure 5.
Results
Demographics and ocular characteristics of the 5 patients in this prospective treatment study are given in Table 1.
Table 1.
Patient demographics, lesion characteristics, study parameters and changes in anatomy and visual function of 5 patients in study.
| Patient | Sex | Age | Lesion Location |
Visual Acuity at baseline |
Total number of Injections |
Additional treatment during study |
Length of follow up (weeks) |
Visual Acuity at final visit |
Change in visual acuity (letters) |
Change in lesion size* |
Change in clinical exudation and angiographic leakage* |
Change in retinal thickness on OCT (μm)* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 33 | Juxtapapillary | 20/500 | 7 | None | 32 | 20/640 | −7 | ↑ | ↑ | NA; increased by clinical exam |
| 2 | F | 41 | Juxtapapillary | 20/50 | 12 | None | 52 | 20/250 | −36 | Stable | ↑ | +279 |
| 3 | F | 35 | Juxtapapillary | 20/40 | 14 | None | 60 | 20/100 | −21 | Stable | Stable | −179 |
| 4 | F | 63 | Peripheral | 20/160 | 7 | Argon laser photocoagulation × 1 |
32 | 20/100 | +15 | Stable | ↓ | −54 |
| 5 | M | 51 | Juxtapapillary | 20/25 | 10 | None | 60 | 20/20 | +6 | ↓ | ↓ | −67** |
= final visit compared to baseline visit
= measured in the outer nasal macular quadrant
NA = not available, M = male, F = female, OCT = Optical coherence tomography
Active exudative activity related to the RCHs was evident in all study eyes at baseline. Visual acuities at baseline ranged from 20/25 to 20/500 (79 to 18 letters, average = 54±26 letters). After 7 initial injections, patients were assessed for treatment benefit. In 2 patients (Patients #1 and #4), treatment benefit was not apparent and injections were stopped. In the remaining patients, further injections were given on the discretion of the treating physician, based on evaluation of possible benefit. The average number of injections for the 5 patients was 10.0±3.1. Patients were followed up until 8 weeks after their final injection; the average length of follow-up was 47±14 weeks.
Lesion size was examined during study treatment. Lesion expansion was observed in 1 out of 5 cases. This case consisted of a large, aggressively expanding and exudative, juxtapapillary RCH (Patient #1), lesion growth had continued while on treatment. Additional RCHs were also noted to arise de novo in the treated eye during this time. Lesion regression was observed in only 1 case of smaller, less exudative, juxtapapillary RCH (Patient #5). In this case, lesion size and associated retinal neovascularization decreased during the initial 7 injections, but returned when injections were temporarily suspended. Lesion size was stable in the remaining 3 cases.
Lesion exudation during the study was evaluated by clinical examination and by extent of leakage on late phases on fluorescein angiography. Comparison of exudative changes at the final visit compared to baseline showed that 2 treated eyes had evidence of increased exudative changes, 2 eyes had evidence of decreased exudation, while 1 eye was stable.
Macular edema was evaluated at final visit and baseline by clinical examination and OCT imaging. Two eyes (Patients #1 and #2) showed definite increases in macular edema while 3 eyes (Patients #3, #4, and #5) showed decreases in macular edema associated with lesion exudation. In one case (Patient #4), this decrease was questionably associated with intravitreal ranibizumab as laser argon photocoagulation was also given when the lesion appeared unresponsive to the study treatment. In the 2 remaining cases, decreases in macular thickness were associated with improved vision in 1 case (Patient #5) but not in the other (Patient #3).
Central visual acuity improved at final visit compared to baseline in only 2 cases (Patient #4 and #5). The 3 remaining cases experienced a decrease in visual acuity during study follow up. The overall mean change in vision was a decrease of 9±20 letters (range = −36 to +15 letters). Only 1 out of 5 patients (Patient #4) experienced an improvement in visual acuity of equal or greater than 15 letters.
No severe or life-threatening adverse events were recorded during this study. Only one patient experienced a related adverse event that was related to study treatment in the form of transient lid edema and erythema immediately following intravitreal injection.
Discussion
In this study, we evaluated the effects of intravitreal ranibizumab (0.5mg) in 5 patients in treating advanced retinal disease associated with VHL-related RCHs. To our knowledge, this is the first report of the use of ranibizumab and the second prospective study on the use of anti-VEGF therapy for this indication. Aberrantly high levels of VEGF secreted by tumor cells lacking the VHL protein are thought to promote the formation and growth of hemangioblastomas.8 Originally termed vascular permeability factor (VPF)22, VEGF is also likely to promote increased vascular permeability of the RCHs and contribute to their exudative behavior. The rationale of anti-VEGF treatment is to achieve disease control by decreasing the growth and exudation of RCHs through limiting the contribution of VEGF to these processes.
All patients enrolled in the study had active exudative disease associated with vision loss. All study eyes contained RCHs that have either failed previous conventional therapy, or had lesions in locations not amenable by standard ablative therapy (e.g.. juxtapapillary lesions). We evaluated both anatomical and functional responses to study therapy in treated eyes, comparing parameters at the final visit with those at baseline. Our results indicate that therapeutic response to intravitreal ranibizumab is limited. In general, changes in exudation and retinal thickness correlated with changes in visual acuity, except in one case (Patient #3) in which retinal thickness improved but visual acuity decreased, possibly as a result of long-standing macular edema. In addition, in one case (Patient #4), improvements were only seen after intravitreal ranibizumab was supplemented by laser photocoagulation. It is possible that the vision improvement was due to solely to the laser photocoagulation. There was also a likely effect of baseline lesion size and exudation on the success of treatment; in the smallest and least exudative of lesions (Patient #5), ranibizumab monotherapy had the largest beneficial anatomical effect. These effects are closely correlated with the institution and cessation of treatment, and are very likely a primary result of treatment.
The mixed nature of the effect of intravitreal ranibizumab is similar to the results of previous case-reports of anti-VEGF therapies used for ocular lesions of VHL. SU5416, an intravenously administered inhibitor of VEGF receptor 2, had been reported to improve visual function in one case12, and to decrease RCH exudation in another13. In both cases, no changes in tumor size were found. Madhusudan et al.23 also reported a case series of 6 patients; of these, only 2 were reported to achieve stability or improvement in their retinal lesions. Systemic bevacizumab has been investigated in a single patient with no significant beneficial therapeutic effect.15 In another patient, intravitreal bevacizumab combined with PDT was reported to result in tumor regression and decreased exudation.14 We previously reported the results of a pilot, prospective study of intravitreal pegaptanib, an anti-VEGF aptamer, for VHL-related RCHs; pegaptanib did not have an effect on RCH regression but can minimally decrease exudation and lipid deposition in some cases.16
Although intravitreal ranibizumab did not demonstrate broad efficacy in this pilot study, and produced disappointing results in individual cases, our findings provide some insight regarding the use of anti-angiogenic therapy for VHL-related disease in particular, and for ocular tumors in general.24 While intravitreal ranibizumab did not exert any significant beneficial effect on the largest and most exudative lesions in our series (Patients #1 and #2), it demonstrated an effect on the smallest lesion in our series (Patient #5). In this last case, beneficial effects on anatomy and visual function were apparent and temporally correlated with treatment. The demonstration that anti-VEGF treatment, as monotherapy, can in some cases exert anti-tumorigenic effects underlines the contribution of VEGF effects to the progression of the lesion and the validity of the approach. However, the overall results also indicate limitations of intravitreal anti-VEGF treatment as monotherapy. In the case of VHL-related lesions, transformed tumor cells are thought to constantly produce VEGF and other growth factors, inducing lesion growth and exudation. In addition to their transformed nature, these lesions are substantially larger in volume than neovascularization complexes seen in age-related macular degeneration or proliferative diabetic retinopathy, and may produce larger and more constant amounts of VEGF. Also, smaller lesions may have a higher rate of cell proliferation, and may be more sensitive to anti-angiogenic inhibition. The limitations of the treatment paradigm used in the present study may have involved the following: (1) an effective dose required for VHL lesions may need to be higher than the dose required for the treatment of choroidal neovascularization; (2) the route of administration through the vitreous may limit the access to tumor cells in the interior of a large endophytic lesion; and (3) more sustained drug levels may be required for VHL lesions. These limitations are intuitively amplified in cases of larger lesions than in smaller ones, consistent with the findings of our case series.
Our results suggest the following future directions for anti-angiogenic therapy: the use of systemic anti-VEGF therapy, possibly combined with intravitreal therapy, to increase drug access to a large vascularized lesion; the targeting of multiple angiogenic molecules as suggested by the molecular biology of the disease (e.g. other angiogenic molecules upregulated with the loss of VHL function, such as platelet-derived growth factor (PDGF)); the delivery of higher and more sustained levels of drug as may be affected by sustained-release drug devices and intraocular gene therapy techniques; and the use of multiple modalities in combination such as anti-angiogenic therapy with laser-based ablative approaches (argon laser photocoagulation and PDT). The beneficial anatomical and functional results seen in the one case (Patient #4) receiving both ranibizumab and argon laser photocoagulation may have possibly resulted from this combined treatment. As juxtapapillary and recalcitrant VHL-related RCHs continue to be therapeutic challenges for which comprehensive and effective treatments are still lacking, further prospective, controlled trials building on the present understanding will be important in advancing future anti-angiogenic therapies for ocular tumors.
Acknowledgments
Financial Support: This study was supported by the Intramural Research Program of the National Institutes of Health, Division of Epidemiology and Clinical Research, National Eye Institute.
Footnotes
References
- 1.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 2.Wong WT, Agron E, Coleman HR, et al. Genotype-phenotype correlation in von Hippel-Lindau disease with retinal angiomatosis. Arch Ophthalmol. 2007;125:239–45. doi: 10.1001/archopht.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WT, Agron E, Coleman HR, et al. Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel-Lindau disease. Ophthalmology. 2008;115:181–8. doi: 10.1016/j.ophtha.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–42. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 5.McCabe CM, Flynn HW, Jr., Shields CL, et al. Juxtapapillary capillary hemangiomas. Clinical features and visual acuity outcomes. Ophthalmology. 2000;107:2240–8. doi: 10.1016/s0161-6420(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 6.Singh AD, Nouri M, Shields CL, et al. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Erfurth UM, Kusserow C, Barbazetto IA, Laqua H. Benefits and complications of photodynamic therapy of papillary capillary hemangiomas. Ophthalmology. 2002;109:1256–66. doi: 10.1016/s0161-6420(02)01059-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin WG., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997;94:8761–6. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–8. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–43. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 12.Aiello LP, George DJ, Cahill MT, et al. Rapid and durable recovery of visual function in a patient with von hippel-lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109:1745–51. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- 13.Girmens JF, Erginay A, Massin P, et al. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003;136:194–6. doi: 10.1016/s0002-9394(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Ziemssen F, Voelker M, Inhoffen W, et al. Combined treatment of a juxtapapillary retinal capillary haemangioma with intravitreal bevacizumab and photodynamic therapy. Eye. 2007;21:1125–6. doi: 10.1038/sj.eye.6702896. [DOI] [PubMed] [Google Scholar]
- 15.von Buelow M, Pape S, Hoerauf H. Systemic bevacizumab treatment of a juxtapapillary retinal haemangioma. Acta Ophthalmol Scand. 2007;85:114–6. doi: 10.1111/j.1600-0420.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 16.Dahr SS, Cusick M, Rodriguez-Coleman H, et al. Intravitreal anti-vascular endothelial growth factor therapy with pegaptanib for advanced von Hippel-Lindau disease of the retina. Retina. 2007;27:150–8. doi: 10.1097/IAE.0b013e318030a290. [DOI] [PubMed] [Google Scholar]
- 17.Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 18.Lowe J, Araujo J, Yang J, et al. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85:425–30. doi: 10.1016/j.exer.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 20.Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–12. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Melmon KL, Rosen SW. Lindau’s Disease. Review of the Literature and Study of a Large Kindred. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 22.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–8. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhusudan S, Deplanque G, Braybrooke JP, et al. Antiangiogenic therapy for von Hippel-Lindau disease. Jama. 2004;291:943–4. doi: 10.1001/jama.291.8.943. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblatt MI, Azar DT. Anti-angiogenic therapy: Prospects for treatment of ocular tumors. Semin Ophthalmol. 2006;21:151–60. doi: 10.1080/08820530500350787. [DOI] [PubMed] [Google Scholar]