Summary
Transmembrane AMPA receptor regulatory proteins (TARPs) and cornichon proteins (CNIH-2/3) independently modulate AMPA receptor trafficking and gating. However, the potential for interactions of these subunits within an AMPA receptor complex is unknown. Here, we find that TARPs γ-4, γ-7 and γ-8, but not γ-2, γ-3 or γ-5, cause AMPA receptors to “resensitize” upon continued glutamate application. With γ-8, resensitization occurs with all GluA subunit combinations; however, γ-8-containing hippocampal neurons do not display resensitization. In recombinant systems, CNIH-2 abrogates γ-8-mediated resensitization and modifies AMPA receptor pharmacology and gating to match that of hippocampal neurons. In hippocampus, γ-8 and CNIH-2 associate in postsynaptic densities and CNIH-2 protein levels are markedly diminished in γ-8 knockout mice. Manipulating neuronal CNIH-2 levels modulates the electrophysiological properties of extrasynaptic and synaptic γ-8-containing AMPA receptors. Thus, γ-8 and CNIH-2 functionally interact with common hippocampal AMPA receptor complexes to modulate synergistically kinetics and pharmacology.
Introduction
AMPA receptors are glutamate-gated ion channels that transduce most fast excitatory synaptic transmission in mammalian brain. These receptors mediate neuron-to-neuron signaling that controls reflexes, behavior and cognition. The synaptic plasticity that underlies learning and memory often involves activity-dependent recruitment of synaptic AMPA receptors (Kandel, 2001; Malinow et al., 2000; Nicoll and Malenka, 1999). Furthermore, dysregulation of AMPA receptors has been implicated in numerous neurodegenerative and psychiatric disorders (Lipton and Rosenberg, 1994).
AMPA receptors comprise homo- and hetero-tetramers of the principal pore forming subunits GluA1-4 (Collingridge et al., 2009; Dingledine et al., 1999; Hollmann and Heinemann, 1994; Mayer and Armstrong, 2004; Seeburg, 1993). Transmembrane regulatory AMPA receptor proteins (TARPs) are obligatory auxiliary subunits for many, if not all, neuronal and glial AMPA receptor complexes (Cho et al., 2007; Coombs and Cull-Candy, 2009; Nicoll et al., 2006; Osten and Stern-Bach, 2006; Ziff, 2007). TARP subunits regulate AMPA receptor protein biogenesis, trafficking and stability, and also control channel pharmacology and gating. Six transmembrane AMPA receptor regulatory protein (TARP) isoforms, classified as Type I (γ-2, -3, -4, and -8) and Type II (γ-5 and -7), are discretely expressed in specific neuronal and glial populations and differentially regulate synaptic transmission throughout the brain (Cho et al., 2007; Fukaya et al., 2005; Kato et al., 2008; Kato et al., 2007; Milstein et al., 2007; Moss et al., 2003; Soto et al., 2009; Tomita et al., 2003).
Key insights regarding the essential roles for TARPs derive from studies of mutant mice. Cerebellar granule cells from stargazer mice, which have a null mutation in γ-2, are deficient in functional AMPA receptors (Chen et al., 2000; Hashimoto et al., 1999). In γ-8 knockout mice, hippocampal AMPA receptors do not progress through the secretory pathway and do not efficiently traffic to dendrites (Fukaya et al., 2006; Rouach et al., 2005). In γ-4 knockout mice, striatal mEPSC kinetics are faster than those found in wild type mice (Milstein et al., 2007). Taken together, these genetic studies suggest that TARP subunits associate with newly synthesized principal AMPA receptor subunits, mediate their surface trafficking, cluster them at synaptic sites, and regulate their gating.
Proteomic analyses have identified CNIH proteins as additional AMPA receptor auxiliary subunits (Schwenk et al., 2009). These studies also show that CNIH-2 and -3 increase AMPA receptor surface expression and slow channel deactivation and desensitization. Also, CNIH-2/3 are found at postsynaptic densities of CA1 hippocampal neurons and are incorporated into ~70% of neuronal AMPA receptors. Yet, based on biochemical analyses, Schwenk et al. proposed that TARPs and CNIH-2/3 associate predominantly with independent AMPA receptor pools.
Here, we investigated possible modulatory actions of TARP and CNIH proteins at the same AMPA receptor complex. We find that transfection of TARPs (γ-4, γ-7 or γ-8) causes AMPA receptors to resensitize upon continued glutamate application. γ-8-containing hippocampal AMPA receptors, however, do not display resensitization suggesting that an endogenous regulatory mechanism prevents this. We find that co-expression with CNIH-2 – but not CNIH-1 – abolishes γ-8-mediated resensitization. γ-8 and CNIH-2 co-fractionate and co-immunoprecipitate in hippocampal extracts while, also, co-localizing at hippocampal synapses. Furthermore, genetic disruption of γ-8 markedly and selectively reduces CNIH-2 and GluA protein levels, indicative of a tri-partite protein complex. Recapitulating hippocampal AMPA receptor gating and pharmacology in transfected cells requires co-expression of GluA subunits with both γ-8 and CNIH-2. In hippocampal neurons, over-expressing γ-8 promotes resensitization and altering CNIH-2 levels modulates synaptic AMPA receptor gating and extra-synaptic pharmacology. In cerebellar granule neurons from stargazer mice, CNIH-2 transfection alone does not rescue synaptic responses but, when dually expressed, CNIH-2 synergizes with γ-8 to enhance transmission. Together, these findings demonstrate that hippocampal AMPA receptor complexes are controlled by both CNIH-2 and γ-8 subunits.
Results
TARPs γ-4, γ-7 and γ-8 impart resensitization kinetics upon AMPA receptors
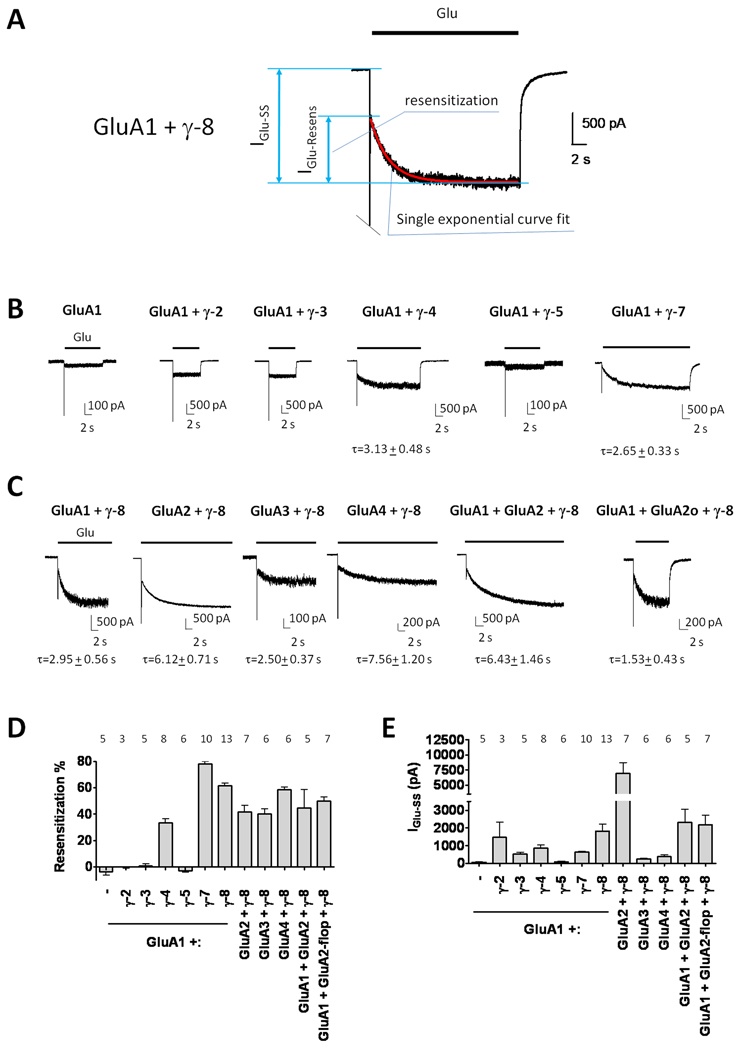
Previous studies in heterologous cells showed that co-transfection of γ-7 with GluA1 or GluA2 creates AMPA receptor complexes that, upon prolonged glutamate application, show unexpected desensitization kinetics that are quite different than kinetics from GluA subunits expressed either alone or with γ-2 (Kato et al., 2008; Kato et al., 2007). Here, we find that γ-8 transfection imparts GluA1 with a similar kinetic signature, characterized by glutamate-induced channel opening, rapid but incomplete desensitization, followed by an accumulation of current which achieves a large steady-state level (Figure 1A). We designate this reversal of desensitization as “resensitization” and quantify this as the fraction of steady-state current that accrues from the trough of the initial desensitization (Figure 1A). For GluA1 co-expressed with γ-8, resensitization accounts for ~60% of the steady-state current and develops with a tau of 2.95 seconds (Figure 1A, C, D). The extent of resensitization is independent of glutamate-evoked current amplitude and extracellular calcium (Figure 1E, S1).
Figure 1.
AMPA receptors co-expressed with γ-4, γ-7 or γ-8 show resensitization. (A) Representative trace of glutamate-evoked current in a HEK293T cell co-transfected with GluA1 and γ-8. Glutamate-evoked current rapidly desensitizes and then gradually resensitizes (IGlu−Resens), until reaching steady-state (IGlu−SS). Resensitization time course fits to a single exponential curve (red line). (B) TARPs γ-4, γ-7 and γ-8, but not γ-2, γ-3 or γ-5 impart resensitization to GluA1. (C) Glutamate-evoked currents with GluA1-4 subunit homomers and GluA1/2 heteromers co-expressed with γ-8 show resensitization. Philanthotoxin was added to isolate the currents mediated by GluA1 + GluA2-flop + γ-8 from those by GluA1 + γ-8. Note that the time constants for resensitization (τ) depend upon GluA subunits and TARPs. (D) γ-4, -7 and -8 exhibit resensitization. (E) Resensitization does not depend on the size of the elicited steady state current. Numbers of repetitions are indicated above the bar graphs. Summary data are mean ± S.E.M. See also Figure S1.
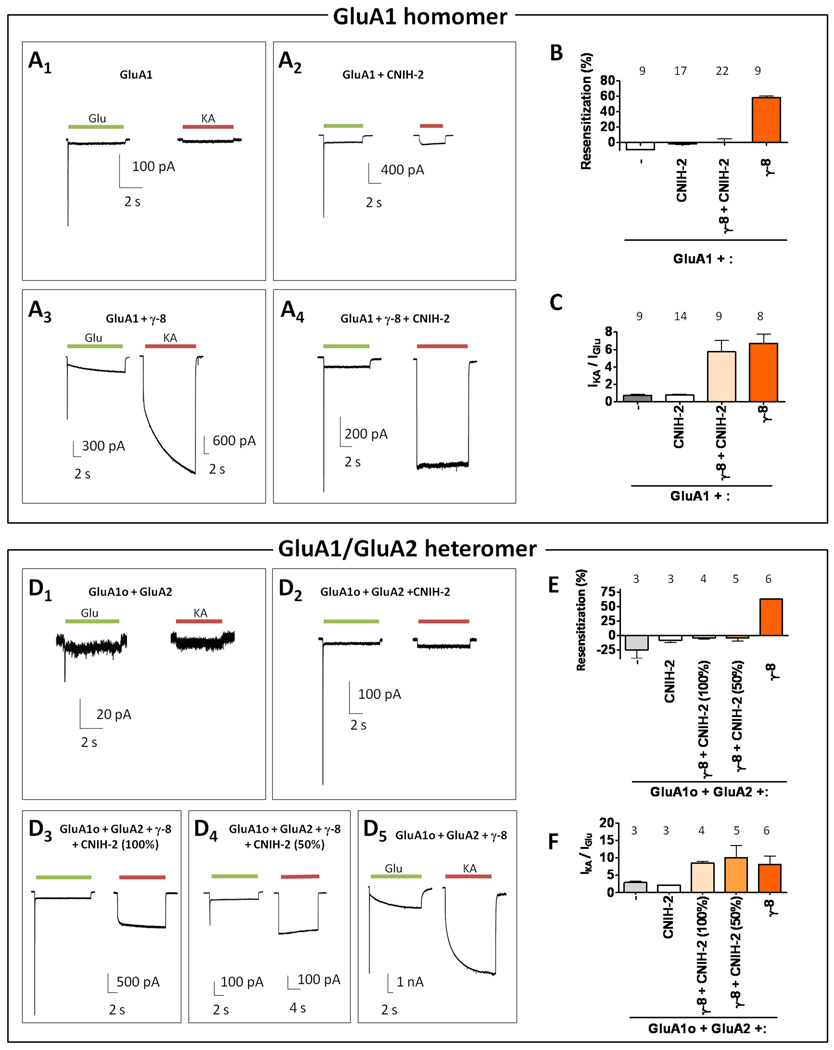
Resensitization shows remarkable TARP-dependent specificity. This phenomenon is not seen in receptors composed of GluA1 alone or GluA1 containing γ-2, γ-3 or γ-5 (Figure 1B, D). By contrast, resensitization is evident when GluA1 is co-expressed with γ-4, γ-7 or γ-8. Resensitization accounts for approximately 35% of the steady-state current for γ-4-containing receptors, and fully 80% for γ-7 containing receptors (Figure 1B, D). Channel resensitization is qualitatively similar when γ-8 is co-expressed with each GluA1-4 subunit and also when γ-8 is co-expressed with heteromeric GluA1/2 receptors (Figure 1C). Comparison of the kinetics of resensitization between subunits shows that GluA2-containing receptors resensitize more slowly than GluA2-lacking receptors. In addition, differences in resensitization kinetics can be observed between AMPA receptors expressing flip (i) or flop (o) splice variants; γ-8-containing GluA1/2o receptors resensitize more rapidly than do γ-8 containing GluA1/2i receptors (Figure 1C). Thus, resensitization is unique to γ-4, -7 and -8 and appears to occur with all GluA subunit combinations.
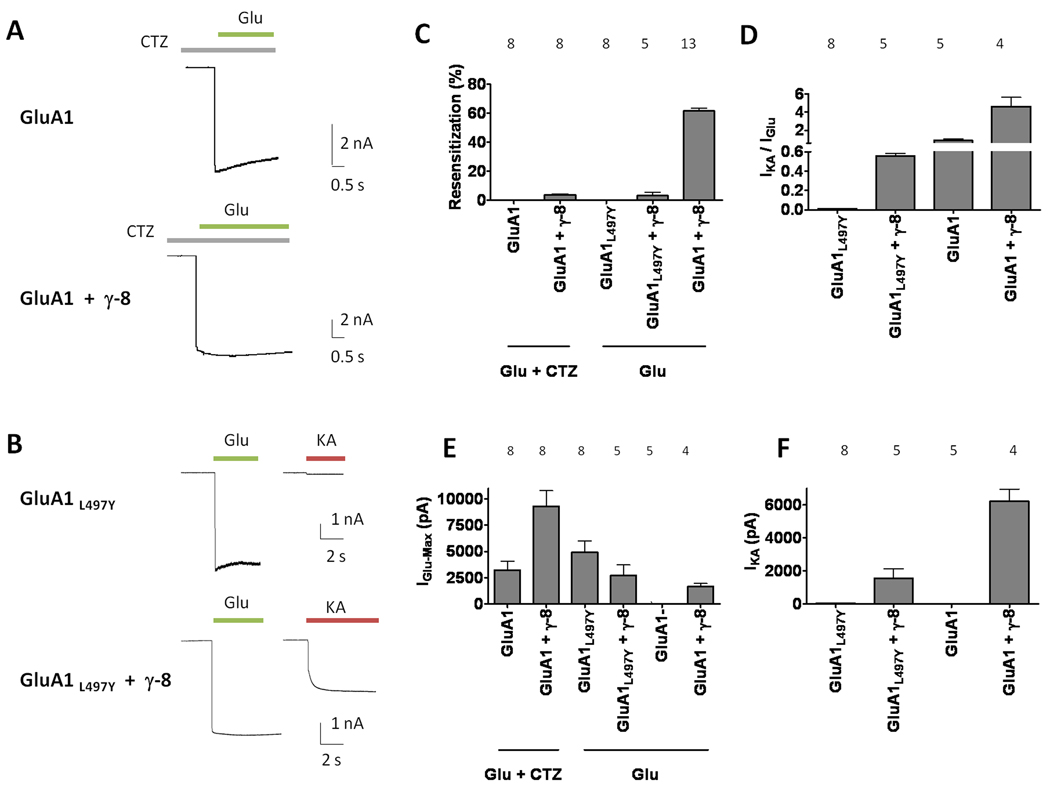
This kinetic phenotype could result from mechanisms unrelated to an apparent “reversal” of desensitization. To evaluate these possibilities, we first performed experiments in the presence of cyclothiazide (CTZ), which blocks desensitization of all GluA-flip isoforms. Results showed that CTZ abolished the delayed current run up in GluA1 receptors conferred by co-expression of γ-8, suggesting that this phenomenon reflects a reversal in desensitization (Figure 2A, C). Further confirmation came from studies examining the effects of γ-8 on the mutant GluA1L497Y receptor, which does not show glutamate-evoked desensitization (Stern-Bach et al., 1998). Consistent with the results found with CTZ, γ-8 expression did not produce the delayed increase in current when co-expressed with GluA1L497Y (Figure 2B, C). As previously published for γ-2 (Tomita et al., 2007b), γ-8 transfection did not significantly enhance glutamate-evoked currents from GluA1L497Y (Figure 2E). On the other hand, γ-8 increased the ratio of kainate / glutamate evoked currents from GluA1L497Y, confirming association of γ-8 with this non-desensitizing receptor mutant (Figure 2D, F). These data show that the γ-8-mediated resensitization reflects reversal of desensitization in AMPA receptors.
Figure 2.
Blocking desensitization occludes γ-8-mediated resensitization. (A) Representative traces of glutamate-evoked currents from GluA1 and GluA1 + γ-8 in the presence of CTZ, which blocks desensitization. (B) Typical traces of glutamate-evoked currents from the non-desensitizing mutant GluA1L497Y with or without γ-8. Resensitization percentage is IGlu−Resens/IGlu−max. (C) CTZ or the L497Y mutation abolishes resensitization even in the presence of γ-8. (D) The ratio of kainate / glutamate-evoked currents confirms γ-8 incorporation. (E, F) Steady state current levels elicited by glutamate (E) and kainate (F). Summary data are mean ± S.E.M. See also Figure S2.
TARPs have a four transmembrane domain core and a cytoplasmic C-terminal tail, and alignment of the six TARP isoforms does not show unique homologies amongst γ-4, γ-7 and γ-8. To investigate which domains mediate resensitization, we generated three pairs of reciprocal chimeras that replaced in γ-2 and γ-8 the partner’s N-terminus through second transmembrane domain (NT-TM2), the third through fourth TM domain (TM3-TM4) and C-terminal domain, respectively. When co-transfected with GluA1, these six chimeras interacted with and produced functional AMPA receptors with large kainate-evoked currents, indicating co-expression of functional TARP proteins (Figure S2). Exchange of the C-terminal domains did not influence resensitization for γ-8 or γ-2 (Figure S2, V–VI), whereas both the NT-TM2 and TM3–TM4 chimeras showed no resensitization for either the γ-8 or γ-2 host protein (Figure S2, I–II and III–IV respectively). Thus, these results indicate that resensitization requires non-continuous regions within the body of γ-8.
Hippocampal AMPA receptors do not exhibit resensitization
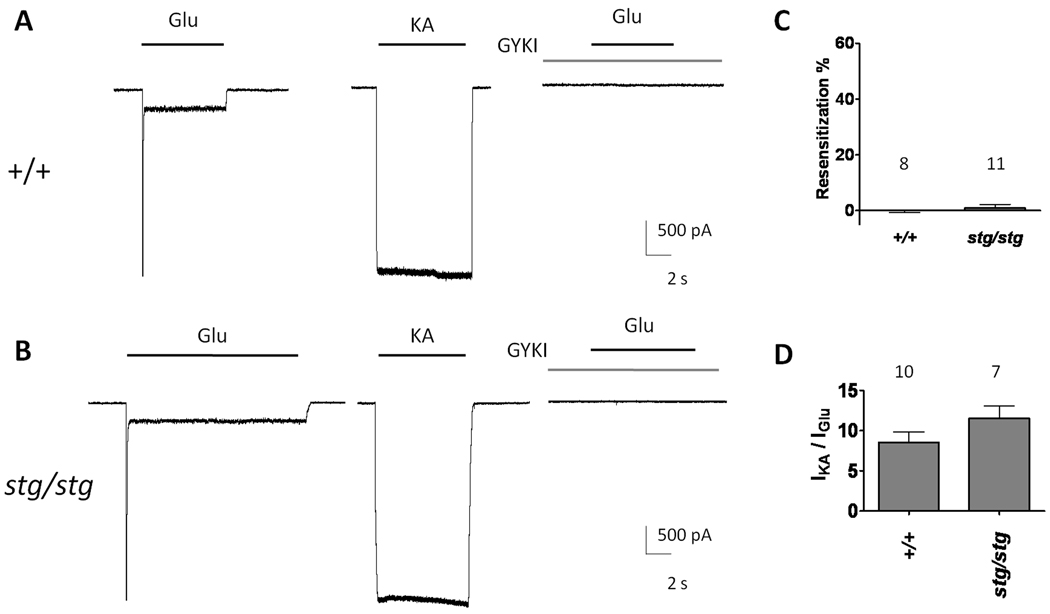
Genetic studies have established that most AMPA receptor complexes in hippocampal neurons contain γ-8 (Fukaya et al., 2006; Rouach et al., 2005). Consistent with previous studies, GYKI 53784-sensitive, hippocampal AMPA receptors showed no evidence of resensitization in response to glutamate (Figure 3A, C). Because AMPA receptors in γ-8 knockout mice have been shown to associate with γ-2 (Menuz et al., 2008; Rouach et al., 2005), the possibility exists that γ-2 containing AMPA receptors, which do not display resensitization, might mask resensitization of hippocampal receptors. To test this hypothesis, we recorded glutamate-evoked currents from acutely isolated pyramidal neurons isolated from stargazer mice, which are deficient in the γ-2 subunit. We observed that glutamate-evoked currents from hippocampal AMPA receptors from stargazer mice also did not display resensitization and kainate / glutamate current ratios, similar to wild-type hippocampal neurons (Figure 3B–D). These results indicate that γ-2 expression is not responsible for the absence of resensitization in γ-8 containing AMPA receptors.
Figure 3.
AMPA receptor-mediated responses from hippocampal pyramidal neurons show no resensitization. (A) Typical traces recorded from acutely isolated hippocampal neurons from wild-type (+/+) and (B) stargazer mice (stg/stg). Responses are blocked by a selective AMPA receptor antagonist, GYKI 53784 (20 µM). (C) Resensitization percentages from wild type and stargazer acutely isolated hippocampal neurons. (D) Kainate- to glutamate-evoked current ratios in wild type and stargazer are similar. Summary data are mean ± S.E.M.
CNIH-2 specifically blocks γ-8 mediated resensitization
Recently, CNIH-2/3 was shown to modulate AMPA receptor pharmacology and kinetics (Schwenk et al., 2009). Because CNIH-2 is enriched in the hippocampus (see Figure 5 below), we investigated the extent to which CNIH-2 could alter γ-8 induced resensitization and AMPA receptor pharmacology. Fitting with previous studies, we found that CNIH-2 increases the magnitude of currents evoked by glutamate (Figure S3A). By generating chimeric constructs composed of CNIH-2 and CNIH-1, a CNIH-2 homologue that does not functionally modulate AMPA receptors, we found that first extracellular domain of CNIH-2 plays a key role to enhance glutamate-evoked currents (Figure S3B). In addition, we found that CNIH-2, like TARPs, converts CNQX from an antagonist to a partial agonist, albeit more weakly (Figure S3D) (Menuz et al., 2007). We observed that transfection of CNIH-2 alone with GluA1 neither promoted resensitization nor increased the ratio of kainate / glutamate-evoked currents. However, co-expression of CNIH-2 with γ-8 completely suppressed γ-8 mediated resensitization, while maintaining a high kainate / glutamate ratio (Figures 4A–C). Evaluation of the CNIH-1 / 2 chimeras revealed that the first extracellular domain of CNIH-2 is necessary for CNIH-2 to block γ-8-mediated resensitization (Figure S3C). We explored further the mechanism for CNIH-2 modulation of γ-8-containing receptors by employing a tandem construct, which links GluA1 to γ-8 (Morimoto-Tomita et al., 2009; Shi et al., 2009). Expression of this GluA1 / γ-8 tandem yielded glutamate-evoked currents that showed resensitization characteristic of γ-8 containing AMPA receptors (Figure S3E). Co-transfecting CNIH-2 with this tandem largely, but not completely, reversed this resensitization and maintained a high kainate / glutamate ratio (Figure S3E). These data demonstrate that γ-8 and CNIH-2 can simultaneously interact with a single AMPA receptor complex.
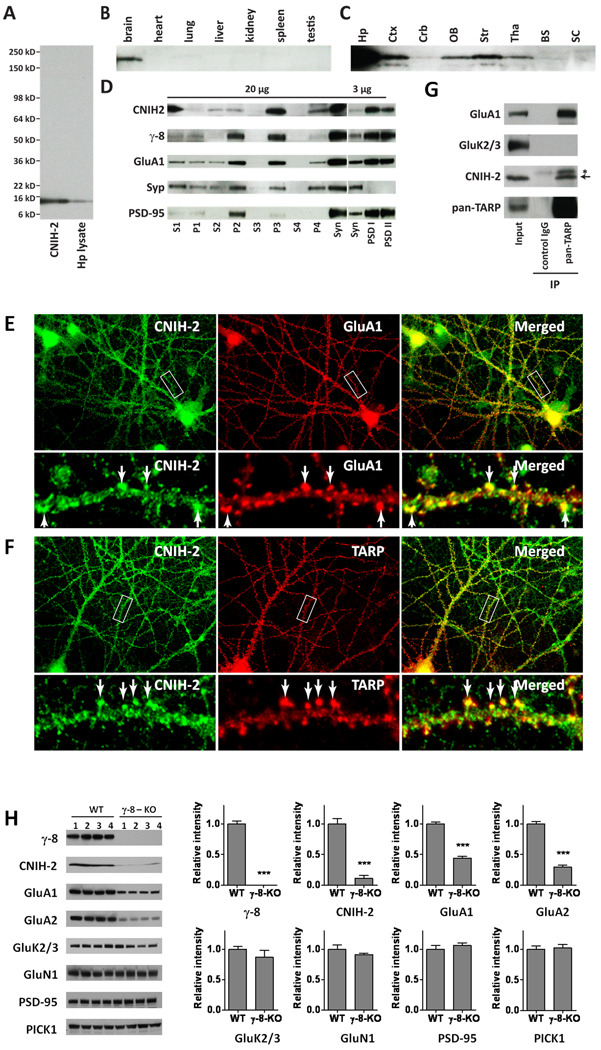
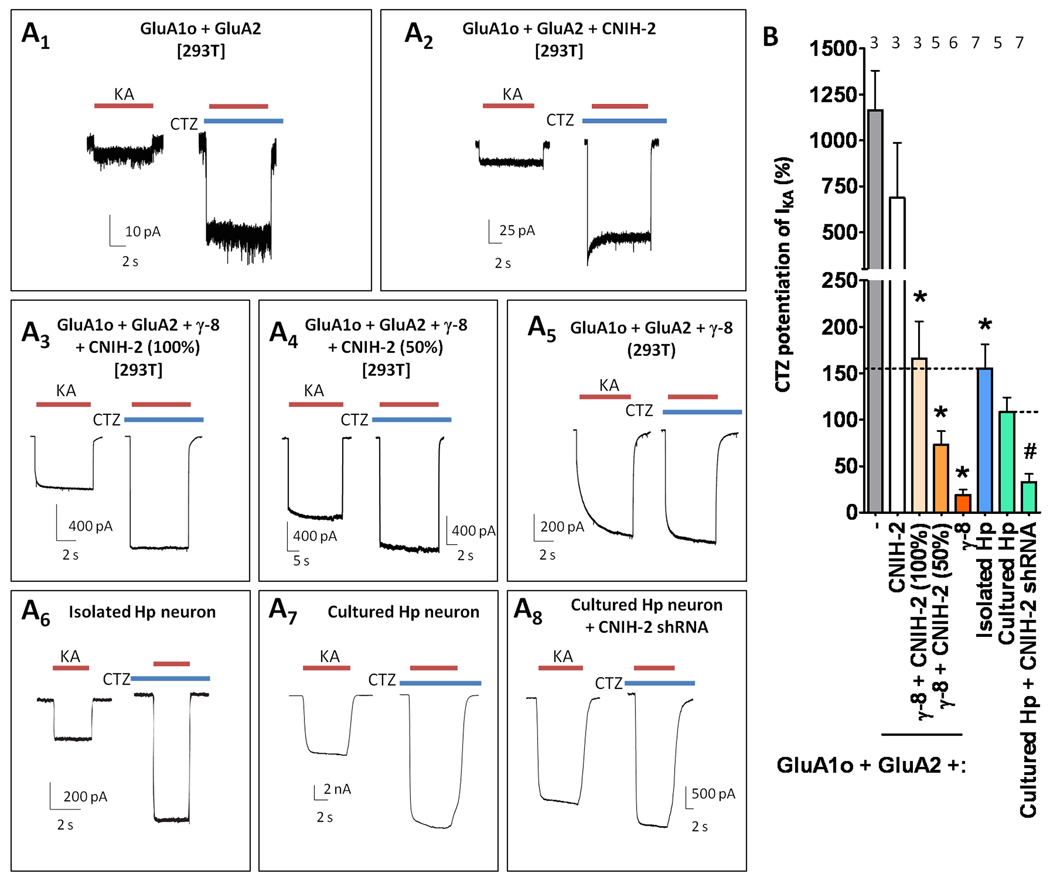
Figure 5.
CNIH-2 and γ-8 interact within a hippocampal AMPA receptor complex. (A) Immunoblot of mouse hippocampal (Hp) lysates reveals that the CNIH-2 antibody detects a single band of ~15 kD that co-migrates with CNIH-2 from transfected HEK cells. (B) Immunblot shows that CNIH-2 is enriched in brain and not detectable in several peripheral tissues. (C) Brain regional immunoblot reveals that CNIH-2 is particularly enriched in the hippocampus (Hp) with intermediate expression levels in the cerebral cortex (Ctx), olfactory bulb (OB), striatum (Str), and thalamus (Tha) and lower levels within the cerebellum (Crb). CNIH-2 was not detectable in brain stem (BS) or spinal cord (SC). (D) Subcellular fractionation shows enrichment of CNIH-2 in the synaptosomal (Syn) and microsomal (P3) fractions with a noticeable concentration in PSD fractions. This distribution generally resembles that of γ-8 and GluA1. PSD-95 and synaptophysin (Syp) serve as controls. (E, F) Immunocytochemistry reveals colocalization of CNIH-2, GluA1 (E) and TARPs (F) in the dendrites and dendritic spines of cultured hippocampal neurons. Boxes denote from where the zoomed images were taken. (G) Immunoprecipitation analysis shows that TARP complexes in hippocampus contain CNIH-2 and GluA1. As a control, GluK2/3 was absent from the TARP complex. (H) Western blots on hippocampal extracts from four wild type and four γ-8 knockout mice reveal a large reduction of CNIH-2 and GluA subunits in the mutant. Knockout of γ-8 did not affect protein levels of kainate receptor subunits GluK2/3, NMDA receptor subunit NR1, PSD-95 or PICK1.
Figure 4.
CNIH-2 blocks γ-8 mediated resensitization. (A1–4) Representative traces of glutamate- and kainate-evoked responses recorded from recombinant cells expressing GluA1 alone or with γ-8 and / or CNIH-2 as indicated. Note that CNIH-2 blocks γ-8 mediated resensitization. (B) Resensitization percentages from GluA1 receptor combinations. (C) Kainate- to glutamate-evoked current ratios (IKA/IGlu) from GluA1 receptor combinations. Note that CNIH-2 has little effect on (IKA/IGlu) with GluA1 or GluA1 + γ-8. (D1–5) Representative traces of glutamate- and kainate-evoked responses recorded from recombinant cells expressing GluA1o/2 heteromeric receptors either alone with either γ-8 and / or CNIH-2. (E) Resensitization percentages from various GluA1o/2 receptor combinations. Note that CNIH-2 blocks γ-8 mediated resensitization in heteromeric GluA1o/2 receptors. (F) CNIH-2 has minimal effects on (IKA/IGlu) from GluA1o / GluA2 receptors. (E, F) Note that a 50% reduction in the amount of CNIH-2 transfected relative to GluA subunit does not affect inhibition of resensitization or kainate / glutamate current ratios. Summary data are mean ± S.E.M. See also Figure S3.
We also evaluated the effects of CNIH-2 on γ-8 containing GluA1o/2 receptors, which predominate in hippocampal neurons (Geiger et al., 1995). CNIH-2 alone did not induce resensitization or alter the kainate / glutamate ratio of GluA1o/2 heteromers. Similar to GluA1 homomers, CNIH-2 co-expression abolished γ-8 mediated resensitization while maintaining TARP-dependent, hippocampal neuronal-like increased kainate / glutamate current ratios (Figures 4D–F, 3D). Furthermore, reducing the amount of CNIH-2 co-transfection by 50% also inhibited γ-8 mediated resensitization and did not alter kainate / glutamate current ratios (Figure 4E, F).
We next evaluated the specificity of CNIH-2 suppression for γ-8-mediated resensitization. Previous studies showed that LY404187 induces tri-phasic kinetics on AMPA receptors that qualitatively resemble TARP-mediated resensitization (Quirk et al., 2004). Indeed, we found that LY404187 conferred ~60% resensitization on GluA1o/2 expressing cells. Importantly, LY404187-induced resensitization was not affected by co-transfection with CNIH-2, indicating that the effects of CNIH-2 on AMPA receptor resensitization are γ-8 dependent (Figure S3F).
γ-8 and CNIH-2 co-localize and co-fractionate in hippocampus
To determine whether CNIH-2 and TARPs interact in hippocampal neurons, we generated antibodies to CNIH-2. By immunoblotting, our CNIH-2 antibody is specific and selectively interacts with a ~15 kD band in hippocampal extracts that co-migrates on SDS-PAGE with CNIH-2 expressed in heterologous cells (Figure 5A). This protein band is present in brain but not in our survey of peripheral tissues (Figure 5B). CNIH-2 protein is expressed at highest levels in the hippocampus, intermediate levels in the cerebral cortex, striatum olfactory bulb and thalamus and lower levels in the cerebellum consistent with its mRNA distribution (Figure 5C) (Lein et al., 2007). Subcellular fractionation of brain extracts revealed enrichment of CNIH-2 in microsomal and synaptosomal fractions, particularly within the PSD. This distribution resembled that of γ-8 and GluA1. PSD-95 also was enriched in PSD fractions, and synaptophysin was absent from the PSD (Figure 5D). Incubation of hippocampal slices with a membrane-impermeant biotinylation reagent detects CNIH-2 and GluA1 on cell surface (Figure S4). Immunofluorescent staining of hippocampal cultures showed punctate labeling for CNIH-2 along dendrites and dendritic spines, where CNIH-2 co-localized with both TARPs and GluA1 (Figure 5E, F). CNIH-2 also localized to dendritic puncta not containing GluA1 or TARPs.
We evaluated in vivo association of CNIH-2 and TARPs by co-immunoprecipitation. Solubilized extracts of hippocampus were incubated with pan-TARP antibodies and adherent complexes were captured on protein A-coupled beads. Immunoblotting showed that CNIH-2 co-precipitated with TARPs and GluA1. As controls, we found that kainate receptor isoforms GluK2/3 were not present in this complex and that this protein complex did not co-immunoprecipitate with pre-immune IgG (Figure 5G). Subunits of a protein complex are often destabilized when other components are genetically deleted, so we analyzed CNIH-2 in γ-8 knockout mice. As previously published (Rouach et al., 2005), GluA1 and GluA2 levels are decreased by 60–70% in hippocampal of γ-8 knockout mice (Figure 5H). Strikingly, we found that CNIH-2 levels were reduced by >80% in hippocampus from γ-8 knockouts. Of note, we did not observe any changes in the protein levels of kainate or NMDA receptor subunits nor in postsynaptic proteins, PICK-1 and PSD-95 (Figure 5H). Together, these data imply that CNIH-2 is a component of γ-8 containing hippocampal AMPA receptors.
γ-8 expression can induce resensitization in hippocampal neurons
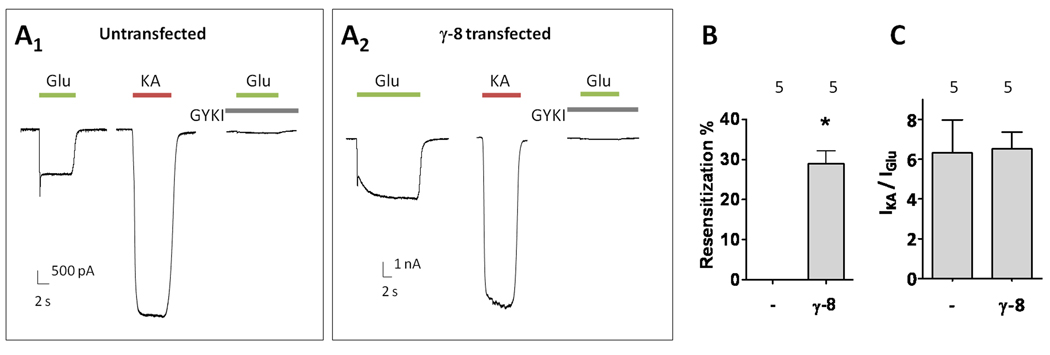
The absence of resensitization in hippocampal AMPA receptors suggests that CNIH-2 may modulate γ-8 containing receptors or that γ-8 induced resensitization is somehow not possible in neurons. To distinguish between these possibilities, we transfected primary hippocampal cultures with γ-8. Untransfected neurons did not display glutamate-evoked resensitization. However, resensitization was clearly evident in γ-8 transfected neurons (Figure 6A, B). The kainate / glutamate ratios in γ-8 transfected neurons were similar to the values detected in non-neuronal cells containing GluA1o/2 and γ-8 subunits (Figure 6C, 4F). As in recombinant systems, CNIH-2 transfection in γ-8-transfected hippocampal neurons blocked resensitization (Figure S5). These data indicate that resensitization can occur in neurons and suggests a balance exists between γ-8 and CNIH-2 in hippocampal neuronal AMPA receptors to modulate channel function.
Figure 6.
Over-expression of γ-8 induces resensitization in hippocampal pyramidal neurons. (A1–2) Representative traces of glutamate- and kainate-evoked responses from untransfected and γ-8 transfected cultured hippocampal neurons. AMPA receptor mediated currents were recorded with 10 µM CPP, 10 µM bicuculline, 1 µM TTX and 300 nM kynurenic acid. (B) Quantification of resensitization. (C) Kainate / glutamate ratios from untransfected and γ-8 transfected cultured hippocampal neurons. Summary data are mean ± S.E.M. * denotes p<0.05. See also Figure S5.
Both CNIH-2 and γ-8 modulate synaptic AMPA receptor gating
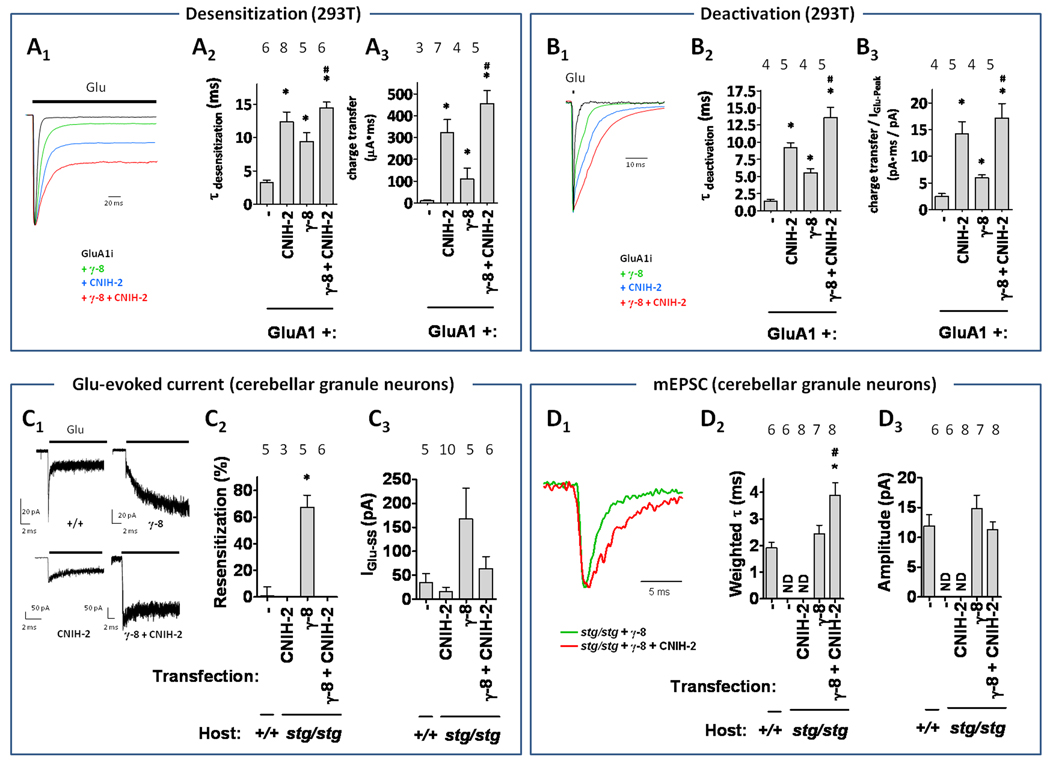
We used fast perfusion electrophysiology (τrise < 1 ms) to evaluate if γ-8 and CNIH-2 synergistically modulate AMPA receptor kinetics. Similar to previous reports, GluA1 subunit expressed alone exhibits fast kinetics (Figure 7A, B), and co-expression of γ-8 slowed deactivation and desensitization rates (Cho et al., 2007; Milstein et al., 2007). CNIH-2 expression slowed deactivation / desensitization rates to a greater degree than γ-8, which is analogous to a previous study comparing γ-2 and CNIH-2/3 (Schwenk et al., 2009). Of note, co-expression of CNIH-2 with γ-8 further slowed deactivation / desensitization rates (Figure 7A, B). Furthermore, analyses of currents resulting from 1 ms and 200 ms glutamate applications revealed that co-expression of γ-8 and CNIH-2 produces more charge transfer than expression of either CNIH-2 or γ-8 alone (Figure 7A, B). To assess the role for endogenous CNIH-2 in hippocampal synaptic function, we sought to knockdown its expression using shRNA and, then, measure pharmacologically isolated, AMPA receptor-mediated miniature excitatory post-synaptic responses (mEPSCs). This shRNA approach reduced, but did not eliminate, CNIH-2 protein expression in transfected HEK 293T cells and cultured hippocampal neurons (Figure S6A–C). Furthermore, CNIH-2 knockdown significantly reduced hippocampal mEPSC charge transfer (Figure S6D) with no effect on rise time (untransfected: 1.0 ± 0.2 vs. CNIH-2 shRNA: 1.0 ±0.3 ms) or frequency (untransfected: 4.4 ± 0.6 vs. CNIH-2 shRNA: 3.1 ±0.5 Hz).
Figure 7.
Synaptic AMPA receptor complexes are modulated by both CNIH-2 and γ-8. Scaled representative traces of 200 ms (desensitization, A1) and 1 ms (deactivation, B1) glutamate-evoked responses onto recombinant cells expressing GluA1 alone or with γ-8, and / or CNIH-2 as indicated. (A2,B2) Calculated weighted tau of desensitization (A) or deactivation (B) fit with a bi-exponential function. (A3, B3) Calculated charge transfer from 200 ms (A) and 1 ms (B) glutamate application onto recombinant cells expressing GluA1 alone or with γ-8, and / or CNIH-2 as indicated. * denotes p<0.05 when compared to GluA1. # denotes p<0.05 when compared to γ-8. Representative traces (C1) and quantified resensitization (C2) and steady-state current (IGlu-SS) following glutamate application to wild type (+/+) or transfected stargazer (stg/stg) cerebellar granule neurons. Currents evoked from γ-8 transfectants show resensitization and this is prevented by co-transfection with CNIH-2. Note that CNIH-2 alone restored glutamate-evoked currents in 3 of 10 transfected neurons. (D) Synaptic AMPA receptor-mediated mEPSCs were recorded from cultured cerebellar granule neurons from wild type or stargazer mice transfected with CNIH-2 and / or γ-8. (D1) CNIH-2 synergizes with γ-8 to slow AMPA-receptor mEPSC decay constant (D2) without affecting mEPSC amplitude (D3) in transfected cerebellar granular neurons. Note the absence of synaptic AMPA receptor responses in stargazer neurons transfected with CNIH-2 alone (ND, not detectable). * denotes p<0.05 when compared to +/+. # denotes p<0.05 when compared to stg/stg + γ-8. Summary data are mean ± S.E.M. See also Figure S6.
To more directly measure CNIH-2 effects on extra-synaptic and synaptic AMPA receptors, we utilized cultured stargazer cerebellar granule neurons, which lack functional AMPA receptors as well as TARP (Chen et al., 2000) and CNIH-2/3 subunits (Schwenk et al., 2009). Similar to our heterologous cell findings (Figure 4), bath application of glutamate to γ-8-transfected stargazer granule cells produced a resensitizing current that was inhibited by co-expression of CNIH-2 (Figure 7C). Transfection of CNIH-2 alone did not rescue synaptic AMPA receptors whereas transfection with γ-8 produced mEPSCs that decayed with a tau of ~2.5 ms (Figure 7D). Importantly, co-expression of CNIH-2 with γ-8 slowed mEPSCs (tau ~4 ms) and did not have significant effects on amplitude relative to wild type or γ-8-transfected stargazer granule cells (Figure 7D). Taken together, these results show that CNIH-2 can modulate decay kinetics of synaptic AMPA receptors through synergic actions with γ-8-containing receptors.
Both γ-8 and CNIH-2 regulate extra-synaptic hippocampal AMPA receptor function
We next evaluated for CNIH-2 modulation of cyclothiazide (CTZ) actions on kainate-evoked currents (IKA) from AMPA receptors, for which the hippocampal neuronal phenotype has yet to be recapitulated with co-expression of GluA and TARP subunits. Previous studies found that CTZ potentiates kainate-evoked currents ~2 fold in hippocampal neurons (Patneau et al., 1993), whereas in oocytes injected with GluA1 + γ-8, CTZ augments kainate-evoked currents by only ~40% (Tomita et al., 2007a). In the present studies, CTZ minimally potentiated kainate-evoked currents from GluA1o/2 + γ-8 (Figure 8A5, B). By contrast, CTZ potentiation of kainate-evoked currents for GluA1o/2 alone was ~12 fold (Figure 8A1, B), which was not significantly different from CTZ-potentiated kainate-evoked currents from GluA1o/2 + CNIH-2 (~7 fold). Importantly, co-expression of CNIH-2 with γ-8 modulated GluA1o/2 receptors to yield CTZ potentiation of kainate currents of ~2 fold, which was quantitatively similar to that observed in acutely isolated hippocampal neurons (Figure 8A3, 8A6, B). CNIH-2’s effect on CTZ-mediated potentiation of kainate-evoked currents was sensitive to a 50% reduction in the amount of CNIH-2 transfected, which minimized the potentiation of kainate currents to near γ-8 alone levels (Figure 8A4). These data suggest that CNIH-2 stoichiometry in AMPA receptors may modulate CTZ pharmacology (Figure 8B). Furthermore, this requirement for both γ-8 and CNIH-2 to produce hippocampal AMPA receptor-like kainate / CTZ pharmacology was also observed for transfections with GluA1i / GluA2 heteromeric receptors (Figure S7).
Figure 8.
Recapitulation of hippocampal AMPA receptors pharmacology requires both CNIH-2 and γ-8. (A) Representative traces of kainate-evoked responses in the presence or absence of CTZ from (A1–5) recombinant cells, (A6) acutely isolated hippocampal (Hp) neurons, or (A7–8) cultured hippocampal neurons. (B) Quantification of CTZ potentiation of kainate-evoked responses. * denotes p<0.05 when compared to GluA1o/2 and # denotes p<0.05 when compared to untransfected cultured hippocampal neurons. The dotted line represents the mean CTZ-induced IKA potentiation observed in acutely isolated or cultured hippocampal neurons. Note that a 50% reduction in the amount of CNIH-2 transfected relative to GluA subunit reduces CTZ potentiation of kainate-evoked currents, which is qualitatively similar to the effect of CNIH-2 shRNA transfection in cultured hippocampal neurons. Summary data are mean ± S.E.M. See also Figure S7.
Cultured hippocampal neurons transfected with CNIH-2 shRNA exhibited reduced CTZ potentiation of IKA (Figure 8B). CNIH-2 knockdown also produced resensitization in only one out of nine hippocampal neurons (data not shown), supporting the hypothesis that complete elimination of CNIH-2 expression is necessary to reveal γ-8-mediated resensitization, whereas a graded stoichiometric mechanism likely explains CNIH-2’s effect on kainate / CTZ pharmacology. Collectively, these results indicate that γ-8 and CNIH-2 are required to recapitulate native hippocampal AMPA receptor complexes.
Discussion
The present studies demonstrate that TARP isoforms γ-4, γ-7, γ-8 can impart a unique resensitization signature upon AMPA receptors. This resensitization is characterized by a delayed accumulation of current flux upon continued application of glutamate. The absence of resensitization in CA1 hippocampal neurons, whose AMPA receptor complexes predominantly contain γ-8, indicates that additional proteins regulate hippocampal AMPA receptors. Indeed, we find that CNIH-2 specifically blocks resensitization of γ-8-containing AMPA receptors. Also, reconstitution of hippocampal kainate / CTZ pharmacology requires interaction between γ-8 and CNIH-2. Whereas CNIH-2 alone cannot traffic AMPA receptors to synapses of stargazer granule neurons, CNIH-2 synergizes with γ-8 to control synaptic gating and charge transfer. Hippocampal CNIH-2 protein occurs as postsynaptic densities, associates with γ-8-containing AMPA receptors and relies on γ-8 complexes for stability. Taken together, these data suggests that both γ-8 and CNIH-2 associate within a native hippocampal AMPA receptor complex to control transmission.
AMPA receptor resensitization is a novel property of specific TARP isoforms
The prototypical TARP, stargazin, was initially suggested to serve primarily as a chaperone for AMPA receptor trafficking to the cell surface and synapse (Chen et al., 2000). Subsequent biophysical studies showed that TARPs also have profound effects on AMPA receptor pharmacology and channel gating. TARPs generally increase AMPA receptor affinity for glutamate (Kott et al., 2007; Priel et al., 2005; Tomita et al., 2005) and non-competitive antagonists (Cokic and Stein, 2008), increase the efficacy of kainate (Tomita et al., 2005; Tomita et al., 2007a; Turetsky et al., 2005), and alter the pharmacology of competitive antagonists (Kott et al., 2009; Menuz et al., 2007) and CTZ-like potentiators (Tomita et al., 2006). The effects of TARPs on AMPA receptor gating include slowing of AMPA receptor deactivation and desensitization and augmentation of glutamate-evoked steady-state currents (Bedoukian et al., 2006; Priel et al., 2005; Turetsky et al., 2005). At the single channel level, TARPs can increase open channel probability and burst duration (Tomita et al., 2005). Through these effects, TARPs typically augment charge transfer during synaptic transmission.
Our studies identify AMPA receptor resensitization as a new gating characteristic conferred by specific TARP isoforms. Resensitization occurs only in AMPA receptors assembled with γ-4, γ-7, and γ-8. Whereas resensitization is qualitatively similar with these three TARPs, the magnitude of resensitization is greatest with γ-7. The present studies demonstrate that γ-8 can bestow resensitization on homomeric receptors of all GluA subunits, as well as on heteromeric receptors. The magnitude of resensitization is similar for homomeric receptors of each GluA subunit, but develops more slowly with GluA2-containing receptors and more rapidly with a receptor having a flop alternatively-spliced GluA subunit.
The TARP-associated resensitization resembles the kinetics of several positive allosteric modulators of AMPA receptors including PEPA (Sekiguchi et al., 2002) and LY404187 (Quirk et al., 2004). For LY404187, time-dependent enhancement in modulation (resensitization) is evident in flip splice variants of homomeric GluA1-4 receptors and depends on a single residue (Ser754), in the flip/flop domain at the interface of adjacent GluA subunits (Quirk et al., 2004; Sun et al., 2002). Structural studies of the ligand-binding core of GluA receptors indicate that desensitization involves weakening of the intermolecular interface between dimeric GluA subunits (Sun et al., 2002). Interestingly, exchange of Asp754 for Ser dramatically increases the rate and extent of desensitization of GluA receptors (Partin et al., 1996) and markedly destabilizes dimerization of the ligand-binding core (Sun et al., 2002). Conversely, pharmacological manipulations that attenuate GluA receptor desensitization, stabilize dimerization of the glutamate ligand-binding modules at least in part through interactions with Ser754 (Sun et al., 2002). Our data suggest a model whereby γ-4, γ-7 and γ-8 promote GluA subunit ligand-binding domain dimerization and thereby partially reverse desensitization. Recent structural analysis of intact GluA2 indicates that juxta-membrane regions also may mediate interactions with auxiliary subunits (Sobolevsky et al., 2009). Future structural studies of GluA with auxiliary subunits are needed to define the molecular mechanism for receptor assembly.
It remains unclear why resensitization is induced specifically by γ-4, γ-7 and γ-8. Although the first extracellular domain of TARPs mediates effects on receptor pharmacology and gating (Bedoukian et al., 2006; Tomita et al., 2005), this region is not specifically conserved between γ-4, -7, and -8 and we find that substituting this region from γ-8 into γ-2 does not induce resensitization. In fact, none of our chimeras that replaced either pairs of transmembrane domains or the C-terminal region between γ-2 and γ-8 interchanged resensitization. Apparently, resensitization requires interactions with discontinuous segments within the 3-dimensional structures of γ-8.
CNIH-2 modulates γ-8 containing AMPA receptors
Previous studies in heterologous cells showed that CNIH-2/3 – like type I TARPs – augment glutamate-evoked currents and also slow receptor desensitization and deactivation (Schwenk et al., 2009), which we confirmed. We also found that CNIH-2 more weakly mimics the effect of TARPs to convert CNQX from an antagonist to a partial agonist. However, unlike type I TARPs, we found that CNIH-2 did not increase the kainate / glutamate ratio from these GluA receptors. These results indicate that TARPs and CNIH-2 modulate AMPA receptors through distinct mechanisms.
To assess for functional interactions, we transfected γ-8 and CNIH-2 together with various GluA constructs and found striking results, which included blockade of γ-8 mediated resensitization. That CNIH-2 suppressed resensitization of a GluA1/γ-8 tandem construct decisively shows that these two classes of associated proteins can both interact with a common AMPA receptor complex, and likely have distinct interaction sites. Importantly, we found that CNIH-2 abolishes γ-8-induced resensitization but left intact the TARP-mediated augmentation of the kainate / glutamate ratio. This suppression of γ-8-mediated resensitization is specific, because we found that CNIH-2 did not blunt pharmacological resensitization induced by LY404187.
We found no effect on resensitization or the magnitude of glutamate-evoked currents with CNIH-1, a homologous protein expressed in peripheral tissues. Taking advantage of this isoform specificity, we constructed a series of chimeras that interchanged regions in CNIH-2 and CNIH-1. This analysis identified the proposed first extracellular loop of CNIH-2 as necessary for modulation of AMPA receptor gating and blunting γ-8-mediated resensitization. This result is consistent with interaction of the CNIH-2 extracellular domain with GluA ligand binding core.
CNIH-2 and γ-8 interact with a common AMPA receptor complex
The biophysical properties of hippocampal AMPA receptors appear to reflect an interaction between γ-8 and CNIH-2 within an AMPA receptor complex. Although most extra-synaptic hippocampal AMPA receptors contain γ-8 (Fukaya et al., 2006; Rouach et al., 2005), we did not detect resensitization in CA1 pyramidal cells. Resensitization also was not observed in hippocampal AMPA receptors from stargazer mice, which depend upon γ-8 but not other TARPs for activity (Menuz et al., 2008; Rouach et al., 2005). Conversely, resensitization was evident in cells transfected with GluA1o/2 + γ-8. Co-expression with CNIH-2 eliminated the resensitization of GluA1o/2 + γ-8 containing cells suggesting that CNIH-2 functionally interacts with γ-8-containing hippocampal AMPA receptors. This interaction hypothesis is further supported by robust co-immunoprecipitation of CNIH-2 TARP-containing AMPA receptors in hippocampus. Also, CNIH-2 co-fractionates and co-localizes with GluA and γ-8 subunits in postsynaptic densities. Importantly, CNIH-2 protein levels are dramatically reduced in hippocampus of γ-8 knockout mice. Together, these data strongly suggest that CNIH-2 protein occurs within native γ-8-containing AMPA receptor complexes.
Further evidence for an interaction between γ-8 and CNIH-2 derives from pharmacological analyses. While CTZ is known to potentiate kainate-induced currents ~2-fold in hippocampal neurons (Patneau et al., 1993), negligible potentiation was observed when γ-8 alone was transfected with GluA1o/2 heteromeric receptors. By contrast, CTZ potentiates kainate-evoked responses by ~2-fold in GluA1o/2 heteromeric receptors co-transfected with γ-8 and CNIH-2. Partial knockdown of CNIH-2 in shRNA-transfected hippocampal neurons recapitulated the reduced CTZ potentiation efficacy observed with γ-8 transfection alone. Interestingly, resensitization was detected in only one out of nine CNIH-2 shRNA-transfected hippocampal neurons. These findings may suggest that more than one CNIH-2 subunit associates with an AMPA receptor-TARP complex and that CNIH-2 regulates neuronal KA / CTZ pharmacology in a graded fashion. Previous studies have shown the number of TARPs per AMPA receptor complex could be variable (Kim et al., 2010; Shi et al., 2009). Future studies are needed to define the stoichiometry of both TARPs and CNIH-2 within native AMPA receptor complexes.
Functional implications of TARP and CNIH-2 co-regulation of hippocampal AMPA receptors
These studies provide important new insights regarding AMPA receptor function. Whereas previous biochemical studies suggested that TARPs and CNIH-2/3 interact predominantly with independent pools of AMPA receptors, our results reveal crucial cooperative interactions. CNIH-2 can promote surface expression of GluA subunits in transfected cells (Schwenk et al., 2009), but this has not been definitively demonstrated in hippocampal neurons. The dramatic loss of extrasynaptic AMPA receptors in γ-8 knockout mice (Fukaya et al., 2006; Rouach et al., 2005) suggests that CNIH-2 cannot efficiently traffic AMPA receptors in these neurons. Of note, CNIH proteins lack a synaptic-targeting PDZ binding site and, in this study, we found that CNIH-2 could not rescue synaptic AMPA receptors in stargazer granule cells. While this work was under final review, Shi et al. also found that CNIH-2 can partially restore extrasynaptic but not synaptic AMPA receptor function in cerebellar granule cells from homozygous or heterozygous stargazer mice (Shi et al., 2010). On the other hand, we find that CNIH-2 can synergize with γ-8 to augment synaptic AMPA receptor function in homozygous stargazer cerebellar granule neurons. Thus, multiple classes of auxiliary subunits acting on a common GluA tetramer provide a combinatorial layer of complexity for regulation of AMPA receptors in diverse cell types and physiological conditions.
Previous studies showed that CNIH protein from both vertebrates and invertebrates mediate endoplasmic reticulum (ER) export of specific growth factors (Hoshino et al., 2007; Roth et al., 1995). It is therefore possible that CNIH-2 transiently interacts with γ-8-containing AMPA receptor complex solely within the ER to modulate function. Indeed, Shi et al. found that over-expressed CNIH-2 accumulates in the Golgi apparatus and does not occur on the neuronal surface (Shi et al., 2010). However, our subcellular fractionation studies indicate that endogenous CNIH-2 is enriched in synaptosomes and is particularly concentrated together with TARPs and AMPA receptors in postsynaptic densities. In addition, electron microscopic data reveal CNIH-2/3 immunoreactivity at postsynaptic sites in hippocampal CA1 neurons (Schwenk et al., 2009). Furthermore, our characterization of neuronal AMPA receptor resensitization and kainate / CTZ pharmacology, together with our analysis of synaptic AMPA receptor gating in hippocampal and stargazer cerebellar granule neurons, suggests that CNIH-2 associates with synaptic and extra-synaptic γ-8-containing AMPA receptors. The dramatic (>80%) loss of hippocampal CNIH-2 protein in γ-8 knockout mice implies a fundamental connection between CNIH-2 and γ-8-containing AMPA receptor protein complexes.
Multiple classes of transmembrane subunits interacting within a native glutamate receptor complex appears to be an evolutionarily-conserved regulatory mechanism. Glutamate receptors in C. elegans are controlled by interactions amongst two classes of auxiliary subunits: suppressor of Lurcher (SOL)-1 and TARPs (Wang et al., 2008). SOL-1 is a transmembrane CUB domain protein, unrelated to CNIH (Zheng et al., 2004). However, another CUB domain protein, Neto2 regulates mammalian kainate receptor trafficking and gating (Zhang et al., 2009). In addition, studies have found recently that another AMPA receptor auxiliary subunit, CKAMP44, associates with AMPA receptors and reduces currents (von Engelhardt et al., 2010). Multiple auxiliary subunits regulate trafficking and gating of voltage-gated calcium channels, and the α2δ subunit also controls the pharmacology of certain calcium channel compounds (Gee et al., 1996). As AMPA receptor modulators show therapeutic potential in numerous neuropsychiatric disorders (Kato and Bredt, 2007), TARP and CNIH proteins provide intriguing pharmacological targets.
Experimental Procedures
Materials
All salts, pre-cast gels and buffers were from Sigma Aldrich (St. Louis, MO), Invitrogen (Carlsbad, CA), Fisher Scientific (Pittsburgh, PA) or Bio-rad Laboratories (Hercules, CA). Antagonist and agonists were from Tocris Bioscience (Ellisville, MO). Polyclonal antibodies against GluK2/3 (04–921), pan-Type I TARP (07–577) and GluA1 (AB1504) and monoclonal antibody against GluR2 (MAB3397) were purchased from Millipore (Billerica, MA). Mouse monoclonal PSD-95 antibody (MA1-046) and polyclonal antibody against PICK-1 (PAI-073) were purchased from Affinity Bioreagents (Rockford, IL). Mouse monoclonal synaptophysin antibody (S5768) was purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibody against NR1 (556308) was purchased from BD Pharmingen (San Jose, CA). Affinity-purified polyclonal antibodies for CNIH-2 were generated by immunizing guinea pigs with the following peptide sequence from human CNIH-2 protein, DELRTDFKNPIDQGNPARARERLKNIERIC. HRP-conjugated anti-guinea pig secondary antibody (706-035-148) and HRP-conjugated native secondary antibody for mouse- and rabbit-derived primary antibodies (21230) were from Jackson Laboratories (West Grove, PA) and Fisher Scientific, respectively.
cDNA cloning
All GluA cDNAs are flip splice variants unless indicated. All GluA and TARP cDNAs were derived from human except for GluA2, which was cloned from rat. shRNA producing plasmids and lentiviral particles were purchased from Sigma-Aldrich. (#1: TRCN0000109842, #2: TRCN0000109844).
Recombinant cell culture and transfection
HEK 293T cells were maintained at 37°C in 5% CO2 high glucose DMEM medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin and split bi- or tri-weekly. HEK 293T cells were plated in 35 mm dishes and were transiently transfected using FuGENE 6 according to manufacturer’s protocols (11814443001: Roche Applied Sciences, Indianapolis, IN). GluA, TARP and CNIH cDNAs were co-tranfected with a GFP-expressing reporter plasmid for identification in electrophysiology experiments. 100% CNIH-2 transfection indicates equal amounts of CNIH-2 and GluA subunit cDNAs and 50% CNIH-2 reduces this ratio by one half. The cells were trypsinized 1 d after transfection and plated on glass cover slips at low density (~5,000/cm2). Experiments were performed 48–72 h post transfection.
Primary cerebellar granule and hippocampal culture and transfection / infection
Stargazer mice were obtained from Jackson Laboratory and maintained at the Yale animal facility under the guidelines of the Institutional Animal Care and Use Committee. Heterozygous male and female mice were mated to obtain homozygous stargazer mice. Cerebellar granule cell cultures were prepared from postnatal day 7–8 (P7-8) homozygous stargazer mice and were transfected at DIV5 as described (Cho et al., 2007). Primary cultures of rat hippocampal neurons were prepared essentially as described (Kato et al., 2008). Briefly, hippocampi dissected from E19 Wistar rat embryos were incubated at 37°C for 10 min in a papain solution (in mM): 5 L-cysteine, 1 EDTA, 10 HEPES-NaOH (pH 7.4), 100 µg/ml bovine serum albumin, 10 unit/ml papain (Worthington) and 0.02% DNase (Sigma). The reaction was stopped by addition of an equal volume of fetal bovine serum. The cells were triturated and washed with Neurobasal (Invitrogen) supplemented with B-27, 100 µg/ml penicillin, 85 µg/ml streptomycin, 0.5 mM glutamine. The cells were plated on 12 mm coverslips coated with poly-D-lysine in 24-well plates at 100,000 cells/well density. cDNA (γ-8, CNIH-2, or γ-8 and CNIH-2)- or CNIH-2 shRNA-Lipofectamine 2000 (Invitrogen) complexes were prepared in Neurobasal medium according to manufacturer’s specifications. Primary neurons (>14 DIV) were incubated with these Lipofectamine complexes in Neurobasal medium (- supplements) for at least 2 h and then returned to the original conditioned medium. Electrophysiological recordings from primary neurons were performed at least 48 h post-transfection. Lentiviral particles for shRNAs were infected at m.o.i = 2.
Acutely isolated neurons
Hippocampal pyramidal neurons from 5 ± 3 month old mice were isolated as previously described (Kato et al., 2008). Briefly, a rapidly dissected brain was immersed in ice cold NaHCO3-bufferd saline solution (in mM): 120 NaCl, 2.5 KCl, 1 MgCl2, 1.25 Na2PO4, 2 CaCl2, 26 NaHCO3 and 10 glucose (pH 7.2), osmolarity 300 ± 2 mOsm/l. Coronal hippocampal slices (400 µm thick) were prepared by a Vibroslice (Campden Instruments) in ice cold NaHCO3-bufferd saline solution and then were recovered at room temperature in continuously oxygenated (95% O2, 5% CO2), NaHCO3-bufferd saline solution for 0.5 – 5 h. The slices were transferred to a Petri dish containing low-Ca2+ HEPES buffered saline (Low-Ca2+ HBS) (in mM): 140 sodium isothionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 15 HEPES (pH7.2), osmolarity 300 ± 2 mOsm/l. Dissected hippocampal CA1–CA3 regions were placed into a holding chamber containing protease type XIV (1 mg/ml, Sigma-Aldrich) dissolved in oxygenated HEPES-buffered Hank’s balanced salt solution (HBSS 6136: Sigma-Aldrich) and maintained at 37°C, pH 7.4, osmolarity 300 ± 5 mOsm/l. After 30 min incubation in the enzyme solution, the tissue was rinsed three times with the Low-Ca2+ HBS and triturated using fire-polished Pasteur pipettes. The cell suspension was placed into a 50 mm plastic Petri dish for electrophysiological recordings. Hippocampal pyramidal neurons were selected on the basis of their characteristic morphology.
Electrophysiology
Agonist-evoked currents were recorded from transfected HEK293T cells, acutely isolated neurons and primary hippocampal cultures as described (Kato et al., 2008). Recordings were made using thick-walled boroscillicate glass electrodes pulled and fire-polished to a resistance of 2–5 MΩ. All cells were voltage-clamped at −80 mV and data were collected and digitized using Axoclamp 200 and Axopatch software and hardware (Molecular Devices, Sunnyvale, CA). For whole cell recordings, the transfected HEK 293T cells were bathed in external solution containing the following (in mM): 117 TEA, 13 NaCl, 5 BaCl2, 1 MgCl2, 20 CsCl, 5 glucose and 10 Na-HEPES pH 7.4 + 0.03. For acutely isolated and culured primary neurons, 10 µM CPP, 10 µM bicuculline, 1 µM TTX and 300 nM 7-chlorokynurenic acid were added in the external solution and the extracellular concentration of NaCl was increased to 130 mM and TEA was omitted. 7-chlorokynurenic acid (7-CK) was omitted for acutely isolated neurons. The intracellular electrode solution contained the following (in mM): 160 N-methyl-D glucamine, 4 MgCl2, 40.0 Na-HEPES pH 7.4, 12 phosphocreatine, 2.0 Na2-ATP pH7.2 ± 0.02 adjusted by H2SO4. For neuronal recordings, 1 mM QX314 were added to the internal solution. For outside-out patches and whole cell recordings using fast perfusion, the internal solution contained (in mM): 130 CsCl, 10 CsF, 10 Cs-HEPES pH 7.3, 10 EGTA, 1 MgCl2 and 0.5 CaCl2 and was adjusted to ~290 mOsm.
The transfected HEK293T cell or the acutely isolated neuron was lifted and perfused with ligand-containing solutions from a sixteen-barrel glass capillary pipette array positioned 100–200 µm from the cells (VitroCom). Each gravity-driven perfusion barrel is connected to a syringe ~30 cm above the recording chamber. The solutions were switched by sliding the pipette array with an exchange rate of less than 20 ms. For fast application experiments with a junction potential rise time of less than 300 µs, rapid solution exchange (1 and 200 ms application for deactivation and desensitization, respectively) from a theta tube containing external solution (in mM: 140 NaCl, 3 KCl, 10 glucose, 10 HEPES pH 7.3, 2 CaCl2, 1 MgCl2) in one barrel and external solution containing glutamate or kainate in the other barrel was driven by a piezoactuator. Glutamate and kainate (1 mM), CNQX (20 µM) and LY404187 (3 µM) were applied where indicated and cyclothiazide (CTZ; 100 or 200 µM) was added to the external for potentiation experiments. The recording from primary cultured neurons was performed on the cover slips where the neurons had grown with the sixteen-barrel pipette array positioned 200–500 µm away from the recorded neurons. Unless otherwise indicated (Figure 2), resensitization percentage was calculated as:
where IGlu−Resens is the current that accrues from the trough of desensitization (Figure 1A). Kainate / glutamate ratios were calculated as:
where IKA−ss and IGlu−ss are the steady state responses evoked by kainate and glutamate application, respectively. CTZ potentiation of kainate-evoked responses was calculated as:
where IKA + CTZ is the steady state current amplitude recorded during kainate + CTZ application and IKA is the steady state current amplitude recorded during kainate application.
Spontaneous AMPA receptor-mediated miniature excitatory post-synaptic currents (mEPSC) from transfected and untransfected cultured primary hippocampal neurons (>14 DIV) were recorded in the presence of 10 µM bicuculline, 50 µM picotoxin, 10 µM CPP, 300 nM 7-CK and 3 µM TTX using an internal solution containing (in mM): 95 CsF, 25 CsCl, 10 Cs-HEPES pH 7.4, 10 EGTA, 2 NaCl, 1 MgCl2, 10 QX-314 and 5 TEA-Cl adjusted to ~290 mOsm with Mg-ATP. mEPSCs used for analysis were collected from a 2 minute period immediately following a 3 minute recording solution equilibrium period, were inspected visually and were selected with a lower limit amplitude cutoff of greater than 15 pA to eliminate any possible contamination from noise and holding current oscillation. Analyses and curve fitting were performed using MiniAnal software (Synaptosoft, Decateur, GA).
Patch clamp recordings from cerebellar granule cells (DIV7-10) were made in external solution containing (in mM): 10 HEPES, 140 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 2.7 MgCl2, and 10 glucose. Patch pipettes were filled with recording solution (pH 7.2, 320 mOsm) that contained (in mM): 130 cesium methanesulfonate, 5 HEPES, 5 Mg-ATP, 0.2 Na-GTP, 20 TEA and 5 EGTA. All recordings were performed at room temperature. To isolate and record AMPA receptor-mediated mEPSCs, tetrodotoxin (0.5 µM), AP-5 (50 µM) and picrotoxin (100 µM) were added to the external solution. mEPSCs were recorded from cerebellar granule cells in whole-cell configuration at a holding potential of −70 mV. The current was analog low-pass filtered at 3 kHz and digitally sampled at 25 kHz. Sampling traces were further filtered with eight-pole low-pass Bessel filter (1KHz, −3dB) for demonstration purposes. Amplitude and frequency of events were analyzed using Minianalysis (Synaptosoft). mEPSCs were fitted with bi-exponential functions to determine decay kinetics (tau).
Subcellular fractionation
Subcelluar fractionations were performed at 4°C essentially as described previously (Kato et al., 2008). From each centrifugation step, the supernatant was reserved and each pellet was resuspended in buffer I and used in the next centrifugation step. Ten rat hippocampi were dissected and homogenized on ice in 10 mL of ice-cold buffer I (0.32 M sucrose, 3 mM HEPES supplemented with 0.1 mg/mL PMSF, pH 7.4). The homogenate was centrifuged at 1000g for 10 min to yield pellet 1 (P1) and supernatant 1 (S1). Each from the following centrifugation steps resulted in the appropriate supernatant and pellets: 12000g for 15 min, 33000g for 20 min, and 260000g for 2 h to yield P2, P3 and P4 pellets, respectively. In a separate fractionation, ten rat hippocampi were separated into synaptosomal fractions via use of a discontinuous sucrose gradient. PSD fractions I and II were obtained by two serial extractions of the synaptosomal fractions with 0.5% TX-100 in 6 mM Tris-HCl (pH 7.5) followed by centrifugations of 100000g for 1 h. For tissue and brain region specific analyses, the P2 fraction was collected from each tissue and brain region and separated via SDS-PAGE for expression comparison.
Co-immunoprecipitation and immunoblotting
Co-immunoprecipitations were carried as described previously (Kato et al., 2008). Briefly, ten rat hippocampi were homogenized in 10 mL of ice-cold buffer I and centrifuged for 20 min at 20000g at 4 °C. The resulting pellet was resuspended in 4 vol (vol/wt) of buffer I and then solubilized at 4 °C with 1.0% TX-100 for 1 h with continuous mixing. After a 1 h centrifugation at 100000g, the supernatant was precleared with protein A-sepharose beads for 1 h and then incubated with 5 µg of affinity purified rabbit anti-pan Type I TARP for 2 h at 4 °C. Then, the antibody / homogenate mixture was incubated with 50 µL of protein A-sepharose resin for 1 h at 4 °C. The antibody / antigen bound resin was then washed 8X with buffer I supplemented with 20 mM NaCl. Bound proteins were eluted with Laemmli buffer containing 5% SDS at 55 °C for 30 min followed by a 10 min incubation at 95 °C. Input protein (0.5%) and 33% of each co-immunoprecipitation were separated via SDS-PAGE and eluted proteins were detected via immunoblotting with appropriate antibodies- GluA1 (1:1000), pan-Type I TARP (1:1000), synaptophysin (1:50), PSD-95 (1:100), γ-8 (1:1000), CNIH-2 (1:1000) and GluK2/3 (1:500). Co-immunoprecipitations of homogenates with 10 µL of pre-immune serum or 5 µg of control IgG served as controls.
Immunocytochemistry
Cultured primary hippocampal neurons (>17 DIV) were washed in Dulbecco’s phosphate buffered saline (D-PBS) and fixed in 4% paraformaldehyde / 4% sucrose for 10 min. Immediately after, neurons were post-fixed in ice cold (−20 degC) methanol for 10 min. Cultures were rinsed and then blocked and permeabilized in D-PBS including 0.1% Triton X-100 and 3% normal goat serum for 1h at room temperature. Cultures were incubated overnight at 4 degC with primary antibody (1:100, GluA1; 2.2 µg/mL, CNIH-2; 1:50, pan-Type I TARP) in D-PBS plus 2% normal goat serum. Cultures were rinsed and incubated with fluorescence-conjugated secondary antibodies (Invitrogen, 1:500) in D-PBS for 1 h at room temperature. After a final rinse, coverslips were mounted and imaged using Leica immunofluorescence microscope systems (Wetzlar, Germany).
Slice biotinylation
400 µm rat hippocampal slices were incubated in slicing buffer (in mM: 124 NaCl, 26 mM NaHCO3, 3 KCl, 10 Glucose, 0.5 CaCl2 and 4 MgCl2) for 1 h. Slices were then placed into biotinylation solution (biotinylation solution = slicing solution except [CaCl2] and [MgCl2] were raised to 2.3 and 1.3 mM, respectively) ~4°C biotinylation solution for 5 min. Surface proteins of the dissected were labeled with sulfo NHS SS biotin (1.5 mg/mL, Pierce) for 30 min on ice and the reaction quenched with glycine (50 mM). Hippocampi were homogenized with Tris buffer (TB: 50 mM Tris, pH 7.4, 2 mM EGTA) then sonicated. Homogenates were centrifuged at 100,000g for 20 min and the pellet was resuspended in TB containing NaCl (TN: TB + 100 mM NaCl). 50 % ULTRA link Neutravidin (Roche) was added and incubated at 4°C for 2 h. Non-bound internal protein solution was removed. Beads were washed with RIPA buffer and and biotinylated surface proteins were eluted by boiling for 5 min in Laemmli buffer containing DTT (7.7 mg/mL). Eluted proteins and internal proteins were separated by SDS-PAGE and detected via western blotting.
Statistics
Data are represented as mean ± SEM and are the result of at least three independent experiments. Analyses involving three or more data sets were performed with a one way ANOVA with a Tukey Kramer post hoc analysis using Graphpad Prism software (Carlsbad, CA). Analyses involving two data sets were performed with an uncorrected student’s t-test or with a student’s t-test with a Welsh correction, only if the variances were statistically different. Significance was set as a p-value of less than 0.05.
Supplementary Material
Acknowledgements
Akihiko S. Kato, Martin B. Gill, Hong Yu, Yuan Tu, Edward R. Siuda, He Wang, Yue-Wei Qian, Eric S. Nisenbaum and David S. Bredt are full-time employee of Eli Lilly and Company. This work was supported in part by grants to Susumu Tomita from the NIMH (R01MH077939) and the NINDS (RC1NS068966).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedoukian MA, Weeks AM, Partin KM. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J Biol Chem. 2006;281:23908–23921. doi: 10.1074/jbc.M600679200. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Cokic B, Stein V. Stargazin modulates AMPA receptor antagonism. Neuropharmacology. 2008;54:1062–1070. doi: 10.1016/j.neuropharm.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs ID, Cull-Candy SG. Transmembrane AMPA receptor regulatory proteins and AMPA receptor function in the cerebellum. Neuroscience. 2009;162:656–665. doi: 10.1016/j.neuroscience.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe M, Akashi K, Natsume R, Kano M, Kamiya H, Watanabe M, et al. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hoshino H, Uchida T, Otsuki T, Kawamoto S, Okubo K, Takeichi M, Chisaka O. Cornichon-like protein facilitates secretion of HB-EGF and regulates proper development of cranial nerves. Mol Biol Cell. 2007;18:1143–1152. doi: 10.1091/mbc.E06-08-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kato AS, Bredt DS. Pharmacological regulation of ion channels by auxiliary subunits. Curr Opin Drug Discov Devel. 2007;10:565–572. [PubMed] [Google Scholar]
- Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, et al. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Yan D, Tomita S. Assembly and stoichiometry of the AMPA receptor and transmembrane AMPA receptor regulatory protein complex. J Neurosci. 2010;30:1064–1072. doi: 10.1523/JNEUROSCI.3909-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott S, Sager C, Tapken D, Werner M, Hollmann M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins gamma2, gamma3, gamma4, and gamma8. Neuroscience. 2009;158:78–88. doi: 10.1016/j.neuroscience.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Kott S, Werner M, Korber C, Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci. 2007;27:3780–3789. doi: 10.1523/JNEUROSCI.4185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Menuz K, Kerchner GA, O'Brien JL, Nicoll RA. Critical role for TARPs in early development despite broad functional redundancy. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Zhang W, Straub C, Cho CH, Kim KS, Howe JR, Tomita S. Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron. 2009;61:101–112. doi: 10.1016/j.neuron.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FJ, Dolphin AC, Clare JJ. Human neuronal stargazin-like proteins, gamma2, gamma3 and gamma4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes. BMC Neurosci. 2003;4:23. doi: 10.1186/1471-2202-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Annals of the New York Academy of Sciences. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Osten P, Stern-Bach Y. Learning from stargazin: the mouse, the phenotype and the unexpected. Curr Opin Neurobiol. 2006;16:275–280. doi: 10.1016/j.conb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Vyklicky L, Jr, Mayer ML. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk JC, Siuda ER, Nisenbaum ES. Molecular determinants responsible for differences in desensitization kinetics of AMPA receptor splice variants. J Neurosci. 2004;24:11416–11420. doi: 10.1523/JNEUROSCI.2464-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Nishikawa K, Aoki S, Wada K. A desensitization-selective potentiator of AMPA-type glutamate receptors. Br J Pharmacol. 2002;136:1033–1041. doi: 10.1038/sj.bjp.0704804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62:633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Suh YH, Milstein AD, Isozaki K, Schmid SM, Roche KW, Nicoll RA. Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16315–16319. doi: 10.1073/pnas.1011706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Tomita S, Byrd RK, Rouach N, Bellone C, Venegas A, O'Brien JL, Kim KS, Olsen O, Nicoll RA, Bredt DS. AMPA receptors and stargazin-like transmembrane AMPA receptor-regulatory proteins mediate hippocampal kainate neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2007a;104:18784–18788. doi: 10.1073/pnas.0708970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of AMPA receptor potentiators. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10064–10067. doi: 10.1073/pnas.0603128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007b;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA receptor trafficking paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.