Abstract
Major depressive disorder (MDD) is characterized by hypersensitivity to negative feedback that might involve frontocingulate dysfunction. MDD subjects exhibit enhanced electrophysiological responses to negative internal (errors) and external (feedback) cues. Whether this dysfunction extends to remitted depressed (RD) subjects with a history of MDD is currently unknown. To address this issue, we examined the feedback-related negativity (FRN) in RD and control subjects using a probabilistic punishment learning task. Despite equivalent behavioral performance, RD subjects showed larger FRNs to negative feedback relative to controls; group differences remained after accounting for residual anxiety and depressive symptoms. The present findings suggest that abnormal responses to negative feedback extend to samples at increased risk for depressive episodes in the absence of current symptoms.
Keywords: Depression, Remission, Feedback-related Negativity, Reinforcement Learning, Action Monitoring, Anterior Cingulate Cortex, Executive Function, Punishment, Negative Bias
Introduction
Major Depressive Disorder (MDD) is a common disorder with a chronic course characterized by the recurrence of depressive episodes. Emerging evidence indicates MDD is associated with widespread impairments in cognitive function including excessive sensitivity to negative reinforcement, negative processing biases, and deficits in adjusting behavior after errors or negative feedback [1]. While some studies report recovery of executive functions after remission [2], others note persistent deficits in planning, monitoring, and attention [3].
Dysfunctions within brain areas implicated in reinforcement learning, such as the basal ganglia and the anterior cingulate cortex, might play a role in abnormal responses to negative feedback in MDD [4,5]. The feedback-related negativity (FRN) may be used as an electrophysiological index of responses to feedback [6,7] and has been localized to the anterior cingulate cortex, among other regions [7,8]. Using a speeded task, Tucker et al. [9] demonstrated that, compared with controls, moderately depressed patients showed larger FRNs, particularly following negative feedback (but see [10]).
Although these findings suggest that MDD subjects are characterized by abnormal responses to negative feedback, it is unclear whether this abnormality is a result of current depressed mood. The goal of this study was to examine FRN responses to negative feedback in remitted depressed (RD) individuals, who are not currently experiencing depressive symptoms, and can thus provide critical information about vulnerabilities associated with MDD [11]. Using a probabilistic punishment learning task, we hypothesized that, relative to controls, RD subjects would show increased sensitivity to negative feedback as indexed by (1) stronger response bias against the more frequently punished stimulus; and (2) larger FRNs.
Method
Participants
Twenty-seven participants (12 RD, 15 controls) were recruited from the community through advertisements. All participants completed the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV) [12]. RD subjects were enrolled if they had (1) at least one Major Depressive Episode within the past 10 years; (2) less than two threshold or subthreshold Major Depressive Episode symptoms in the last 8 weeks, neither of which included anhedonia or depressed mood; and (3) a Beck Depression Inventory (BDI-II) [13] score <14. Three RD subjects reported past substance abuse occurring more than 1 year before the study; no participants met criteria for lifetime substance dependence. Controls reported no current or past Axis I disorder. At the time of testing, no participants were taking psychotropic medications or receiving psychotherapy. The RD and control groups did not differ on age (M±SD: 24.83±4.15 vs. 25.20±6.42 years), male/female ratio (1/11 vs. 2/13), and education (16.13±1.51 vs. 16.47±1.36 years) (Ps>.35). For the RD sample, the mean number of prior Major Depressive Episodes was 2.6 (range: 1–10), the mean age of MDD onset was 18.4 years (range: 11–30), and the mean time since recovery was 41.7 months (range: 4–96). Participants received $15/hour for completing the SCID, $10/hour for the EEG session, and an additional $10 in “winnings”. Participants provided written informed consent to a protocol approved by the Committee on the Use of Human Subjects at Harvard University.
Data collection and reduction
Probabilistic punishment task
This task consisted of 240 trials, divided into 3 blocks of 80 trials, and was adapted from Tripp and Alsop [14]. Each trial started with a fixation point presented for 1000 ms that was replaced by the target stimulus presented for 350 ms (Fig. 1). Target stimuli consisted of 10 different patterns of circles and squares containing 17 shapes (either 7 squares and 10 circles or 10 squares and 7 circles). For each trial, subjects pressed a button with either their left or right hand (counterbalanced) to indicate whether the target contained more circles or squares. Within each block, stimuli were presented an equal number of times (n=40) in a pseudo-randomized sequence. A blank screen followed target stimuli for 1500 ms. For each block, 20 correct responses were followed by negative feedback (“You lose 10 cents!”) for 1500 ms. To induce a response bias, an asymmetrical reinforcement ratio was used: correct responses for one stimulus were punished three times more frequently (n=15) than correct responses for the other stimulus (n=5). Participants were given $10 to start the task.
Fig. 1.
Schematic representation of the probabilistic punishment learning task.
Participants were administered a second version of the task, in which correct responses of one stimulus were disproportionally reward. Unlike prior studies [15], this reward version failed to elicit a response bias toward the more frequently rewarded stimulus in controls. In light of these unsatisfactory psychometric properties, data in the reward condition were not further analyzed (data are available upon request).
Behavioral analyses
Performance was decomposed into response bias and discriminability. Unlike our prior work using a reward task [15], here response bias assesses the systematic preference for the response paired with less frequent punishment, and was calculated as:
where S1 and S2 is the stimulus associated with less vs. more frequent punishment, respectively.
Discriminability assesses the subject’s ability to distinguish between the two stimuli and was used as an indicator of general task performance (see [15] for formula, which was modified by adding 0.5 to the matrix cells). After the task, participants completed the BDI-II and Mood and Anxiety Symptom Questionnaire (MASQ) [16] to assess current level of depressive symptoms, general distress, anhedonia, and anxious arousal. In addition, the BIS subscale of the Behavioral Activation and Inhibition Scale (BIS/BAS) [17] was used to assess sensitivity to punishment, negative affect, and inhibition associated with negative outcomes.
Scalp event-related potentials
EEG was recorded using a 128-channel Electrical Geodesics system (EGI Inc., Eugene, OR) at 250 Hz with 0.1–100 Hz analog filtering referenced to the vertex. Data was processed using Brain Vision Analyzer (Brain Products GmbH, Germany). Offline data were segmented and re-referenced to an average reference. Each trial was visually inspected for movement artifacts and automatically removed with a ±75 μV criterion. Eye-movement artifacts were corrected by Independent Component Analysis. Artifact-free EEG epochs were extracted beginning 200 ms before and ending 600 ms after feedback presentation. ERPs were averaged for blocks 2 and 3 to allow adequate exposure to the differential reinforcement schedule. ERPs were filtered at 1–30 Hz and a 200 ms pre-stimulus baseline correction was used. The FRN was scored as the most negative peak 250–450 ms after feedback presentation relative to the immediately preceding positivity at midline sites (Fz, FCz, Cz).
Source localization analyses
LORETA [18] was used to estimate intracerebral current density underlying the FRN following published procedures [19]. Using all 128 channels, current density was computed within a 280–400 ms post-feedback time window, which captured the mean peak latency of the FRN (327 ms) across the midline sites.
Statistical analyses
For response bias and discriminability, separate mixed ANOVAs with Group (RD, Control) and Block (1,2,3) were performed. Mixed ANOVAs were used to analyze the FRN with Group and Site (Fz, FCz, Cz) as factors. Follow-up independent t-tests were performed to decompose significant effects. The Greenhouse-Geisser correction was used when appropriate. For the LORETA data, the groups were contrasted at each voxel (N=2,394) using unpaired t-tests; a combination of P value (0.01) and cluster extent (5 voxels) threshold was used to identify significant findings. Two-tailed Pearson correlations were performed over all participants between the FRN and self-report measures. Hierarchical regressions were performed to examine whether group differences remained after partialing out the variance associated with self-report measures (performed separately for the MASQ, BIS and BDI-II). Group was entered in the second step whereas the self-report measure was entered on the first step.
Results
Self-report and behavioral data
Compared to controls, RD subjects reported higher BDI-II (2.33±2.10 vs. 0.53±1.06; t(25)=2.89, P<0.017), MASQ general distress/depression (18.17±5.17 vs. 14.80±2.36; t(25)=2.25, P=0.055), and anhedonic depression (52.83±9.14 vs. 44.80±10.33; t(25)=2.11, P<0.045) scores. No groups differences were found for the BIS (21.08±3.31 vs. 19.93±2.89; t(25)=0.96, P>0.30) or the other MASQ subscales (Ps>0.18).
A significant main effect for Block emerged for response bias, F(2,50)=4.24, P<0.035, ε=0.74. Post-hoc tests indicated that response bias toward the less frequently punished stimulus was significantly higher in block 3 (0.23±0.44) than block 1 (0.05±0.19) and block 2 (0.13±0.35), ts(26)>2.27, Ps<0.035. Contrary to our hypotheses, no effects involving Group emerged (Ps>0.71). An analysis for discriminability revealed no differences across blocks or between groups (Ps>0.12), indicating that groups did not differ with respect to task difficulty.
FRN data
A significant effect for Site emerged, F(2,50)=20.42, P<0.001, ε=0.55, due to larger FRN at Fz relative to FCz and Cz, ts(26)>−4.49, Ps<0.0002. A main effect of Group was also significant (F(1,25)=7.50, P<0.015), due to an overall larger FRN for RD than control subjects (0.54±1.89 vs. 2.69±2.14 μV) (Fig. 2). [The main effect of Group was confirmed also when the factor Condition order (punishment vs. reward condition run first) was added to the ANOVA on FRN values, F(1,23)=6.76, P<0.017.] Thus, further analyses focused on the FRN averaged across the three sites. Pearson correlations indicated that higher BDI-II total scores were related to larger FRN, r=−0.38, P<0.05. A similar relation was found for BIS scores (r=−0.40, P<0.04) and MASQ general distress/depression subscale (r=−0.37, P=0.057), whereas scores on the MASQ anhedonic depression subscale were not related to the FRN (P>0.39).
Fig. 2.
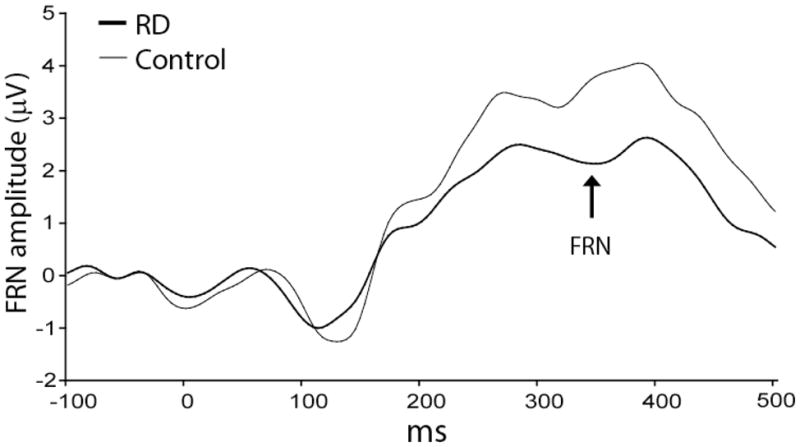
Averaged ERP waveforms from 200 ms before to 500 ms after the presentation of negative feedback during the probabilistic punishment task for individuals with remitted depression (RD; heavy line) and controls (light line) averaged across Fz and FCz and Cz.
Hierarchical regressions revealed that Group was a significant predictor of FRN amplitude after accounting for the MASQ general distress/depression and anhedonic depression scores (R2=0.26; ΔR2=0.17; ΔF(1,23)=5.39, P<0.030) or BIS scores (R2=0.33; ΔR2=0.27; ΔF(1,23)=6.09, P<0.025). A similar (albeit less robust) pattern was observed for BDI-II scores (R2=0.26; ΔR2=0.11, ΔF(1,24)=3.58, P=0.07).
LORETA analyses revealed no differences between RD participants and controls.
Discussion
The goal of the present study was to examine electrophysiological correlates of negative feedback processing in unmedicated individuals with a history of MDD but no current depressive symptoms using a probabilistic punishment learning task. As predicted, individuals with RD showed larger FRNs than controls. The magnitude of the FRN increased with depressive symptoms consistent with Tucker et al. [9], as well as with self-reported sensitivity to punishment (BIS). Unlike Tucker et al., however, the depression scores were well below the clinical threshold (BDI-II<6); thus, correlational findings involving the BDI should be interpreted with caution. Notably, group differences remained after accounting for self-report measures of anxiety/punishment sensitivity (BIS), general distress and anhedonic symptoms (MASQ subscales), suggesting that the enhanced FRN in RD subjects was not due to the presence of residual clinical symptoms (we note, however, that group differences in FRN were slightly reduced when considering BDI scores). Collectively, these findings indicate that dysfunctional neural responses to negative feedback are not only observed in currently depressed subjects [9], but extend to subjects at increased vulnerability to MDD in the absence of clinical symptoms, and support prior reports that RD subjects are characterized by negative cognitive styles [20]. Whether abnormal ERP responses to negative feedback reflects a consequence (“scar”) of a prior MDE or a trait-like risk factor for MDD cannot be evaluated in the present study. Future studies in at-risk samples prior to the first MDE are required to test this critical differentiation.
The ability to learn response-outcome associations (i.e., develop a response bias toward a less frequently punished stimulus) was similar for RD subjects and controls. This result suggests that negative feedback did not have a detrimental effect on behavioral performance in a remitted state [4]. Thus, negative reinforcement learning, at least as probed by the current task, was not exacerbated in the current RD sample. This finding is not necessarily at odds with Pizzagalli et al. [15] who reported that individuals with elevated depressive symptoms, who might also be at risk for future Major Depressive Episodes, were impaired at developing a response bias toward a more frequently rewarded stimulus. Unfortunately, in the present study, we were unable to test whether RD subjects are similarly impaired in positive reinforcement learning. In light of emerging evidence indicating that positive and negative reinforcement learning might rely on different mechanisms [5,21], studies using psychometrically matched reward and punishment learning tasks will be needed to identify putative markers that might be associated with increased vulnerability to depression.
The FRN has been compared to the response-locked error-related negativity (ERN), which occurs after error commission. The ERN may reflect error and conflict detection [22], and unlike the FRN, is internally generated. Interestingly, enhanced ERN amplitude has been reported in MDD subjects (e.g., [23]), suggesting that depression is characterized by both a hypersensitivity to internal (errors) and external (feedback) cues of performance monitoring. Findings of potentiated responses to negative outcomes are intriguing, particularly when considering the role of cognitive diatheses in the etiology of depression. According to Beck’s cognitive theory of depression, for example, increased vulnerability to depression results from the activation of negative schemas about worthlessness, loss, and expected failure when facing stressors [24]. Abnormal responses to negative outcomes might thus be a manifestation of such depressogenic cognitive schemata.
The limitations of the present study should be noted. First, although our task successfully elicited a response bias away from the more frequently punished stimulus, groups did not differ in their behavioral indices of punishment sensitivity. It is unclear whether this null finding is a peculiarity of the present task, or whether the FRN provided a more sensitive probe of punishment sensitivity as abnormal brain activation may emerge in MDD despite spared behavioral performance (see e.g., [25]). Second, no group differences emerged in the LORETA analyses. We note that in two previous studies using a similar reinforcement learning task, differences underlying the neural sources of the FRN were, in part, driven by group differences in the ability to develop a response bias for the more frequently rewarded stimulus (Santesso et al., unpublished data). Thus, task differences and the lack of group differences in behavioral performance might explain the LORETA null findings.
Conclusion
Subjects with a history of MDD were characterized by increased electrophysiological responses to negative feedback, indicating that hypersensitivity to negative reinforcement persists during remission in the absence of current symptoms. Based on these findings, we suggest that the FRN provides a sensitive tool for examining subtle impairments in negative reinforcement learning in RD and might be useful to predict future depressive episodes.
Acknowledgments
Source of Support
This work was supported by grants from NIMH (R01 MH68376, DAP; F31 MH7424601, CMD), Harvard College Research Program (KTS, TMM), and a McMasters Fund Research Grant (CMD). Dr. Pizzagalli has received research support from GlaxoSmithKline and Merck & Co., Inc. for research unrelated to this project.
The authors would like to thank Elena Goetz, Jeffrey Birk, Kyle Ratner and Nancy Brooks for their assistance.
Footnotes
Disclosures
All authors report no competing interests.
References
- 1.Pizzagalli DA, Dillon DG, Bogdan R, Holmes A. Reward and punishment processing in the human brain: Clues from affective neuroscience and implications for depression research. In: Vartanian O, Mandel D, editors. Neuroscience of decision making. Psychology Press; in press. [Google Scholar]
- 2.Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, et al. Executive function improvement upon remission of recurrent unipolar depression. Eur Arch Psychiatry Clin Neurosci. 2005;255:373–380. doi: 10.1007/s00406-005-0577-7. [DOI] [PubMed] [Google Scholar]
- 3.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Elliot R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal responses to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28 :559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- 5.Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- 6.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 7.Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 8.Van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: Implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 2004;56:267–276. doi: 10.1016/j.bandc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol. 2003;112:667–678. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- 10.Ruchsow M, Hernberger B, Beschoner P, Gron G, Spitzer M, Kiefer M. Error processing in major depressive disorder: Evidence from event-related potentials. J Psychiatric Res. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Scher CD, Ingram RE, Segal ZE. Cognitive reactivity and vulnerability: Empirical evidence of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders – Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- 14.Tripp G, Alsop B. Age-related changes in sensitivity to relative reward frequency. NZ J Psychol. 1999;28:30–36. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- 15.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and divergent validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Carver CL, White TL. Behavioral inhibition, behavioral activation, and affective responses to impeding reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 18.Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, et al. Low resolution brain electromagnetic tomography LORETA functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res: Neuroimaging. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 19.Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ. Affective judgments of faces modulate early activity (~160 ms) within the fusiform gyri. NeuroImage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- 20.Haeffel GJ, Abramson LY, Voelz ZR, Metalsky GI, Halberstadt L, Dykman BM, et al. Negative cognitive styles, dysfunctional attitudes, and the remitted depression paradigm: a search for the elusive cognitive vulnerability to depression factor among remitted depressives. Emotion. 2005;5:343–348. doi: 10.1037/1528-3542.5.3.343. [DOI] [PubMed] [Google Scholar]
- 21.Frank MJ, Seeberger L, O’Reilly RC. By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neurosci Biobehav Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- 25.Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29:203–215. doi: 10.1016/j.neuroimage.2005.07.002. [DOI] [PubMed] [Google Scholar]



