Abstract
Across a variety of species, the amygdala appears to play a key role in the detection and avoidance of potential dangers (e.g., unfamiliar social partners, novel objects or contexts, potential predators, etc.). For many species, seeking out appropriate food sources and avoiding novel, distasteful or potentially tainted food is also a daily concern. Amygdala damage in nonhuman primates has been linked to increased willingness to select unfamiliar or unpalatable foods, as well as inedible items that intact animals typically reject. However, such findings have not always been consistent and have typically been observed in relatively restrictive, laboratory-based testing contexts. We evaluated the food choices of six adult male rhesus monkeys (Macaca mulatta) with bilateral, neurotoxic amygdala lesions and six age- and experienced-matched unoperated control animals. Each animal was able to forage freely in a large enclosure stocked with five preferred and five nonpreferred foods that changed locations each day. While both groups quickly selected palatable foods, monkeys with amygdala lesions consistently selected unpalatable foods that the unoperated control animals generally avoided. Even after repeated presentations of the unpalatable foods, the amygdala-lesioned monkeys failed to change their initial pattern of diminished avoidance. These results are consistent with a general role for the amygdala in danger detection and prevention of harm in the presence of novel or noxious stimuli, regardless of whether such stimuli are conspecifics, predators, objects or foods.
Keywords: amygdala, avoidance, food preference, foraging, nonhuman primate, danger
Introduction
The human and nonhuman primate amygdala has been consistently shown to play a critical role in the avoidance of environmental danger or stressful contexts. For example, when adult macaque monkeys with bilateral neurotoxic amygdala lesions interact either in pairs (Emery et al., 2001) or in small groups (Machado et al., 2008), they are more uninhibited or unrestrained relative to control animals. In addition, when GABAA antagonists are used to disinhibit the nonhuman primate amygdala, animals show decreased social contact, a loss of social play, as well as increased passivity and active withdrawal from social interactions (Málková, Barrow, Lower, & Gale, 2003). Specific deficits in behavioral responses to threatening facial expressions and aggressive gestures have also been demonstrated in monkeys with bilateral neurotoxic amygdala lesions in stable social groups (Machado & Bachevalier, 2006). These abnormalities are not restricted to nonhuman primates, since humans with amygdala damage also have specific deficits in identifying fearful facial expressions (Adolphs et al., 1999), rating the magnitude of fear expressed in the face (Adolphs, Tranel, Damasio, & Damasio, 1995) and assessing the approachability or trustworthiness of unfamiliar individuals (Adolphs, Tranel, & Damasio, 1998). Functional neuroimaging (Hoffman, Gothard, Schmid, & Logothetis, 2007) and electrophysiological recording (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007) studies with nonhuman primates have also demonstrated heightened activity in the amygdala when animals view threatening facial expressions relative to when they view appeasement gestures. Similar results have been present in the human neuroimaging literature for some time (Blair, Morris, Frith, Perrett, & Dolan, 1999; Gur et al., 2002; LaBar, Crupain, Voyvodic, & McCarthy, 2003; Morris, deBonis, & Dolan, 2002; Morris et al., 1996; Phillips et al., 1997; Whalen et al., 2001) and reinforce the similar roles for the human and nonhuman primate amygdala in detecting danger in the social environment.
The amygdala has also been implicated in the avoidance of potentially dangerous objects and animals, as well as the generation of defensive behavior towards these stimuli. For example, rats and mice bred and raised in captivity show increased unconditioned freezing behavior, as well as decreased grooming and feeding when exposed to olfactory cues indicative of natural predators (e.g., cat fur or chemical components of feline or fox urine/feces). Transient inactivation or permanent lesions of the medial or basolateral nuclei of the amygdala diminish such freezing to predator odors (Blanchard, Canteras, Markham, Pentkowski, & Blanchard, 2005; Muller & Fendt, 2006; Takahashi, Hubbard, Lee, Dar, & Sipes, 2007). For nonhuman primates, snakes can trigger a potent, unlearned fear response (Nelson, Shelton, & Kalin, 2003). Neurotoxic lesions of the nonhuman primate amygdala have been repeatedly shown to blunt fear and avoidance of real or artificial snakes (Aggleton & Passingham, 1981; Amaral et al., 2003; Izquierdo & Murray, 2004; Kalin, Shelton, & Davidson, 2004; Kalin, Shelton, Davidson, & Kelley, 2001; Machado, Kazama, & Bachevalier, 2009; Mason, Capitanio, Machado, Mendoza, & Amaral, 2006; Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999; Prather et al., 2001; Stefanacci, Clark, & Zola, 2003; Zola-Morgan, Squire, Alvarez-Royo, & Clower, 1991). Finally, human subjects with anxiety disorders (i.e., post-traumatic stress disorder, obsessive-compulsive disorder and social or object phobias) or personality disorders display heightened amygdala activation (Birbaumer et al., 1998; Breiter et al., 1996; Donegan et al., 2003; Rauch et al., 1996) and startle reflex potentiation (Pissiota et al., 2003) relative to normal subjects when presented with stimuli relevant to their particular anxiety or phobia.
Another potential danger is the ingestion of inappropriate or unfamiliar foods which can pose serious health risks to primates. The consequences of unwise food selection could range from mild gastrointestinal discomfort to severe pain or even death from poisoning or bacterial infection. Despite the wealth of knowledge described above regarding how the amygdala influences social wariness or avoidance of potentially dangerous objects and animals, we know comparatively less about the role of the amygdala in steering individuals away from potentially dangerous or distasteful foods. Early studies with nonhuman primates using nonspecific lesions that damaged the amygdala, surrounding cortex and fibers of passage (Goulet, Dore, & Murray, 1998; Munoz, Mishkin, & Saunders, 2009) demonstrated that this brain region is essential for normal food choices and avoidance of potentially dangerous foods, such as raw meat (Aggleton & Passingham, 1981, 1982; Baylis & Gaffan, 1991; Weiskrantz, 1956). More recently, similar nonhuman primate studies using axon-sparing, neurotoxic amygdala lesions have demonstrated that this structure is mainly involved in avoidance of unpalatable foods and inedible objects that normal animals find of little interest (Machado & Bachevalier, 2007a, 2007b; Murray, Gaffan, & Flint, 1996; Stefanacci et al., 2003). However, it should be noted that the most severely disturbed food preferences following selective amygdala lesions have been observed in forced-choice testing paradigms (Aggleton & Passingham, 1981, 1982; Baylis & Gaffan, 1991; Machado & Bachevalier, 2007a; Murray et al., 1996; Stefanacci et al., 2003; Weiskrantz, 1956).
The impact of brain lesions on other forms of goal directed behavior (i.e., social behavior) can be highly dependent on context (Bachevalier & Málková, 2006; Emery & Amaral, 2000; Kling & Brothers, 1992). Therefore, it is important to compliment previous controlled studies of food choices with analogous experiments in semi-naturalistic contexts. In the only previous assessment of food choices conducted in a semi-naturalistic environment, monkeys with selective amygdala lesions showed elevated preferences for familiar unpalatable foods relative to control animals (Machado & Bachevalier, 2007b), which was consistent with similar, more-controlled studies (Baylis & Gaffan, 1991; Stefanacci et al., 2003). However, the operated animals in the study by Machado and Bachevalier were tested with the same preferred and unpalatable foods both prior to and following surgery. It is possible that those animals gained knowledge about the low reinforcement value of the novel and inappropriate foods included in that study (such as lemon wedges and garlic cloves) before receiving amygdala lesions. Therefore, that study could not make conclusions about the role of the amygdala in food neophobia (avoidance of, and reluctance to taste, unfamiliar foods), which has been highlighted in previous rodent studies (Burns, Annett, Kelley, Everitt, & Robbins, 1996; Burns, Everitt, & Robbins, 1994; Rollins, Stines, McGuire, & King, 2001).
In the course of a multidisciplinary analysis of amygdala function in the nonhuman primate, we conducted the current experiment to compare the foraging behavior of monkeys with neurotoxic amygdala lesions with unoperated control animals. In addition to including foods that rhesus monkeys readily eat (e.g., apples, grapes, peanuts, etc.), we also included unfamiliar, unpalatable foods to specifically examine the role of the nonhuman primate amygdala in food neophobia.
Materials and methods
All experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and developed through consultation with the veterinary staff at the California National Primate Research Center (CNPRC). All protocols were also approved by the University of California, Davis, Institutional Animal Care and Use Committee.
Subjects & housing
Twelve adult male, rhesus monkeys (Macaca mulatta) were randomly assigned to receive either bilateral ibotenic acid lesions of the amygdala (A-IBO; n = 6) or to act as unoperated controls (CON; n = 6). For complete rearing, animal selection and husbandry details, refer to Emery and colleagues (2001). Briefly, each was born and reared with its mother at the CNPRC in one of twelve half-acre enclosures containing approximately seventy animals each. Animals were relocated to indoor housing at the CNPRC when they were between 5 and 8 years old. The current study did not begin until the animals ranged in age between 7 and 10 years and weighed 10 – 15 kg. During the present study, animals were housed in individual cages (66 cm width × 61 cm length × 81 cm height) and allowed visual access to other male macaques participating in a separate study. The housing room was maintained on a twelve-hour light/dark cycle. All animals were maintained on a diet of fresh fruit, vegetables and monkey chow (Lab Diet #5047, PMI Nutrition International Inc., Brentwood, MO), with water available ad libitum. The animals were fed at approximately 07:00 and 15:30 daily and were not food-deprived in any way during this experiment. Prior to the current experiment, these animals also participated in studies of emotional reactivity to potentially dangerous objects (Mason et al., 2006), social behavior (Emery et al., 2001; Machado et al., 2008), resting hormonal stress response (Emery et al., 1998), as well as emotional reactivity to video presentations of social stimuli.
Neuroimaging, surgeries and histological lesion assessment
Neuroimaging, surgical and histological lesion assessment procedures have been reported in detail previously (Emery et al., 2001). Briefly, each animal assigned to the A-IBO group received a magnetic resonance imaging (MRI) scan before surgery to determine individualized stereotaxic coordinates for ibotenic acid injections into the amygdala (Saunders, Aigner, & Frank, 1990). For these scans, A-IBO animals were anaesthetized and placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). A Phillips 1.5T Gyroscan magnet and a T1-weighted Inversion Recovery pulse sequence were used to acquire the images. These images were overlaid with a matrix of 1 mm × 1 mm squares and the stereotaxic locations of intended ibotenic acid injections throughout the amygdala were determined.
Five A-IBO animals underwent a two-stage lesion of the amygdala, with each stage separated by one week. One animal (case 26085) underwent a single-stage bilateral lesion surgery, and all animals recovered similarly. A midline incision and craniotomies were made over the amygdala. A 10 µl Hamilton syringe (26 gauge needle) was used to inject 0.5 – 1.5 µl of ibotenic acid (Biosearch Technologies Inc., Novato, CA) at each injection site. The single-stage bilateral lesion was carried out using two identical Hamilton syringes to simultaneously inject ibotenic acid into each amygdala. After all injections were completed, the wound was then closed in layers. Animals assigned to the CON group did not receive any invasive surgical procedures, nor did they receive MRI scans. These animals were, however, sedated, brought to the surgical suite, had their head shaved, were intubated and brought to a surgical level of anesthesia. These animals remained sedated for the average length of the neurotoxic lesion surgeries experienced by A-IBO animals.
At the completion of all behavioral testing, the A-IBO animals were deeply anesthetized and perfused intracardially with 4% paraformaldehyde in sodium phosphate buffer. The brain was then blocked stereotaxically, removed from the skull, cryoprotected and stored at −70°C until sectioned. Frozen sections were cut on a sliding microtome in the coronal plane at a thickness of 30 µm. Every eighth section was mounted onto glass slides and stained for Nissl substance with 0.25% thionin solution to visualize and measure lesion extent. For five normal rhesus monkey brains (kindly provided by Dr Peter Rapp, National Institute on Aging) and the six A-IBO animals’ brains, the amygdala as a whole, as well as four subnuclei (lateral, basal, accessory basal and central) were drawn on a template rhesus monkey atlas using a Leica stereomicroscope and camera lucida (Wetzlar, Germany). The volume of the amygdaloid tissue remaining in the A-IBO cases was then compared to the volume of the amygdala and subnuclei in the normal animals to calculate the percentage of the total volume damaged in the left and right hemispheres, as well as the arithmetic mean across hemispheres. Similar percent damage measurements were made for the adjacent rostral hippocampus (defined as the portion of the hippocampus extending from the rostral tip to a level at the posterior extent of the uncus) as well as the entorhinal cortex.
Apparatus
Food preferences were measured while animals explored a large, familiar, indoor enclosure (5.56 m long × 1.91 m wide × 2.13 m tall), constructed from galvanized steel pipe and chain link fencing (Emery et al., 2001). Ten clear polycarbonate boxes (8 cm long × 8 cm wide × 5 cm deep) were attached around the inside perimeter of the cage at regular intervals, approximately 1 m apart and 60 cm above the floor.
All animals had been previously trained to reliably enter and exit the testing enclosure in less than 15 sec. Because the polycarbonate boxes were a new addition to the enclosure, each animal was initially given one familiarization session prior to any testing without food present. Animals were released into the enclosure and rewarded for exploring by the experimenter scattering raisins onto the floor. After 10 min of free ad libitum reward delivery, the animal was temporarily placed into one of the two lateral release cages and all food boxes were baited with raisins. The animal was re-released into the enclosure, allowed to freely explore and recover raisins from the boxes. Once the animal recovered a raisin from each of the 10 boxes, the training session ended. All animals recovered food rewards from the boxes during this single habituation session.
Food preference pilot testing
The 10 foods used in the formal experiment were chosen based on a food preference pilot study using two normal adult rhesus macaque males otherwise uninvolved in the study. The two males were presented with a random series of 66 paired combinations of 12 foods in a Wisconsin General Testing Apparatus (WGTA). Each food was presented with each other food once per day for 7 days. The twelve foods were: 1) red apple, 2) Thompson seedless grapes, 3) carrots, 4) shelled, unsalted peanuts, 5) lemon slice with peel, 6) fruit-flavored breakfast cereal (Froot Loops™; Kellogg’s, Battle Creek, MI), 7) chocolate chips, 8) pitted, green olives, 9) raw red onion, 10) meat-flavored dog treats (Snasages™; Del Monte Foods, San Francisco, CA), 11) hot dog (Vienna Sausage™; ConAgra Foods, Omaha, NE), 12) canned, cooked baby clams (World Finer Foods, Bloomfield, NJ). Each animal was allowed to select only one of the two foods during each trial. A hierarchy of food preference was determined. Foods 1 – 5 listed above were the most frequently selected during pilot testing and assigned to the “Preferred Food” category for the formal study described below. Apples, grapes and carrots were a common component of each animal’s standard diet at the CNPRC and were therefore familiar. Peanuts and lemons were unfamiliar to all animals at the start of this experiment. Foods 8 – 12 above were the least preferred foods during pilot testing and were assigned to the “Nonpreferred Foods” category in the formal study. Each of these foods was novel to all experimental animals at the start of this study. Foods 6 and 7 were not consistently preferred or rejected, and were therefore not used in the formal study.
Foraging food preference testing
Food preference testing occurred over 10 days. On each day, three A-IBO animals and three controls were tested between 10:00 and 12:00, and the remaining animals were tested between 13:00 and 15:00. Animals alternated each day between the morning and afternoon testing sessions. The ten foods were presented within the clear polycarbonate boxes mounted around the testing enclosure. The spatial location of each food rotated daily to control for any predisposed exploratory patterns. Six spatial arrangements were generated in which no food was presented at the same location twice, or presented adjacent to the same food twice. The animals experienced these six arrangements in the same order over the course of the 10 test days.
All foods were cut to be approximately the same size (i.e., approximately 3 grams or approximately the size of a grape). Prior to each testing session and with the animals out of sight, the experimenter entered the enclosure and placed one piece of each food into the appropriate foraging box. Animals were introduced individually into the testing arena from one of two release cages at either end of the enclosure. The specific release cage alternated every day to minimize any bias in exploratory pattern. The observer sat adjacent to the cage and started a stopwatch once the animal was released into the enclosure to record the latency to remove each food from its box. The observer also rated the animal’s reaction to each food (from positive to negative) according to the following scale: 1 = place food in mouth immediately after removal from box; 2 = remove from box, discard to floor, then place food in mouth from floor later; 3 = remove from box and discard to floor; 4 = leave in box. Since rhesus monkeys commonly store foods in their cheek pouches, there was no way for the observer to quantify if the animal actually ingested any of the foods. The trial ended after 10 min and the animal was removed from the testing enclosure. If a food was not recovered from a box, a latency of 600 sec was recorded on the datasheet. Each box was cleaned with water between animals to prevent odor contamination.
Data analysis
Food selection score and latency to select a food (relative to entering the enclosure) were the primary dependent variables analyzed in this study. For both measures, one animal (A-IBO case 25627) rarely retrieved any food from the foraging boxes. Z-scores were calculated for the mean latency to select Preferred and Nonpreferred Foods across the 10 test days to determine how far this animal deviated from both the CON and A-IBO group means. This animal differed substantially from both CON (Preferred Foods: Z = 3.04; Nonpreferred Foods: Z = 0.72) and A-IBO means (Preferred Foods: Z = 14.36; Nonpreferred Foods: Z = 3.14). This animal was therefore eliminated from all analyses.
Data for both behavioral measures were divided into 5 two-test-day blocks to capture changes in the groups’ food preferences over time. Within each block, data were averaged. Neither measure was normally distributed for either group, as determined with the Shapiro-Wilk test and by inspecting the skewness and kurtosis ratios. All data were therefore log10(x+1) transformed prior to statistical analyses (Sokal & Rohlf, 1995), but non-transformed values were used for illustration purposes. All data were analyzed using General Linear Model ANOVAs with Group (2) as a between subjects factor and Block (5) as a within subjects factor with repeated measures using the SPSS 17.0 statistical analyses package. A Huynh-Feldt correction was used to adjust the degrees of freedom if group variances did not remain equal across the five testing blocks. Alpha was set at p < .05 in all tests. Because main effects of Block do not provide any specific information regarding amygdala function, these effects were omitted from the Results section below.
Finally, for A-IBO animals, Pearson product-moment correlation matrices were generated to determine if the extent of intended damage to the amygdala (as a whole or separate subnuclei) or unintended damage to the rostral hippocampus or entorhinal cortex significantly influenced any behavioral parameters measured.
Results
Lesion extents
A detailed description of each A-IBO animal’s lesion has been provided before (Emery et al., 2001). However, a brief description of this group as a whole is provided here and a summary of the histological lesion assessment for each animal is displayed in Table 1. Ibotenic acid injections were intended to damage the entire amygdaloid complex but primarily targeted the lateral, basal and accessory basal nuclei, since these nuclei have the most substantial direct connections with the neocortex. For the most part, the lesions across the A-IBO group were quite successful in achieving their goal, with average bilateral damage to the amygdala as a whole ranging from 66.5% to 84.0%. Damage to the lateral and basal nuclei was even more complete, ranging from 84.2% to 99.3% in the lateral nucleus bilaterally and from 82.2 % to 98.3% in the basal nucleus bilaterally. Average bilateral damage was slightly less in the accessory basal nucleus, ranging from 55.7% – 93.8%. The central nucleus was damaged extensively but less than the deep nuclei (range: 55.8% – 83.4%). The more superficial areas of the amygdala, such as the medial nucleus and the periamygdaloid cortex, were typically spared. Nevertheless, it is likely that these superficial nuclei were heavily denervated since a majority of their input arises from the lateral, basal and accessory basal nuclei (Pitkanen & Amaral, 1998).
Table 1.
Lesion extents for animals with bilateral ibotenic acid amygdala lesions
| Intended Damage | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | Lateral Nucleus |
Basal Nucleus |
Accessory Basal Nucleus |
Central Nucleus |
|||||||||||||||
| Case | L | R | Avg | L | R | Avg | L | R | Avg | L | R | Avg | L | R | Avg | ||||
| 24349 | 61.2 | 89.7 | 75.4 | 79.1 | 100.0 | 89.5 | 77.9 | 100.0 | 88.9 | 49.6 | 100.0 | 74.8 | 46.1 | 68.0 | 57.1 | ||||
| 25468 | 75.6 | 87.7 | 81.7 | 93.2 | 99.5 | 96.3 | 95.2 | 100.0 | 97.6 | 81.0 | 99.6 | 90.3 | 59.5 | 84.4 | 72.0 | ||||
| 25571 | 95.5 | 70.4 | 82.9 | 100.0 | 89.2 | 94.6 | 100.0 | 90.3 | 95.2 | 100.0 | 70.2 | 85.1 | 90.9 | 75.9 | 83.4 | ||||
| 25627 | 71.9 | 80.3 | 76.1 | 98.5 | 92.5 | 95.5 | 97.2 | 96.8 | 97.0 | 58.3 | 89.1 | 73.7 | 47.9 | 63.7 | 55.8 | ||||
| 25942 | 73.6 | 59.3 | 66.5 | 94.5 | 73.9 | 84.2 | 94.4 | 70.0 | 82.2 | 66.0 | 45.5 | 55.7 | 60.2 | 61.3 | 60.8 | ||||
| 26085 | 83.3 | 84.7 | 84.0 | 98.5 | 100.0 | 99.3 | 97.2 | 99.4 | 98.3 | 93.0 | 94.6 | 93.8 | 59.6 | 65.4 | 62.5 | ||||
| Mean | 76.8 | 78.7 | 77.8 | 94.0 | 925 | 93.2 | 93.6 | 92.7 | 93.2 | 74.6 | 83.2 | 78.9 | 60.7 | 69.8 | 65.2 | ||||
| Unintended Damage | |||||||
|---|---|---|---|---|---|---|---|
| Rostral Hippocampus |
Entorhinal Cortex |
||||||
| Case | L | R | Avg | L | R | Avg | |
| 24349 | 1.3 | 1.9 | 1.6 | 49.3 | 40.7 | 45.0 | |
| 25468 | 0.0 | 0.0 | 0.0 | 83.6 | 83.5 | 83.6 | |
| 25571 | 2.5 | 0.0 | 1.2 | 49.9 | 78.0 | 64.0 | |
| 25627 | 1.1 | 3.8 | 2.5 | 57.3 | 66.6 | 62.0 | |
| 25942 | 1.7 | 0.0 | 0.9 | 60.2 | 77.9 | 69.1 | |
| 26085 | 4.6 | 0.8 | 2.7 | 33.1 | 57.7 | 45.4 | |
| Mean | 1.9 | 1.1 | 1.5 | 55.6 | 67.4 | 61.5 | |
Data presented are the percentage of normal volume damaged within the amygdala as a whole, the lateral, basal, accessory basal and central nuclei, as well as the adjacent rostral hippocampus (from anterior tip to posterior extent of the uncus) and entorhinal cortex. L – percentage of damage to the left hemisphere; R – percentage of damage to the right hemisphere; Avg – average of L and R.
Unintended damage to adjacent areas was mild in most cases. Five of six cases showed minor cell loss in the rostral hippocampus (range: 0.0% – 2.7%), and more caudal portions of this structure were completely undamaged. All animals received minor to extensive cell loss in the piriform cortex and ventral claustrum, and all cases received extensive damage to the rostral field of the entorhinal cortex (range: 45.0% – 83.6%) that is located ventral to the amygdala.
Food selection latencies
To first verify the assignment of foods into “preferred” and “nonpreferred” categories, we focused on CON animals and compared the average selection latency across all 10 test days to the maximum session duration (600 seconds). One-sample t-tests revealed that CON animals selected the apples, grapes, carrots, peanuts and lemons significantly faster than the maximum session duration (range: t(5) = −3.4 – −26.3, all p < .05). By contrast, the latency to select the olives, onions, dog treats, hot dogs and smoked clams was not significantly different from the maximum session duration. Therefore, in all subsequent analyses, the foods were grouped into Preferred Foods (apples, grapes, carrots, peanuts and lemons) and Nonpreferred Foods (olives, onions, dog treats, hot dogs and smoked clams). We also analyzed dog treats and hot dogs separately as a group of Meat-containing Foods since previous studies indicated that amygdala lesions result in unusual ingestion of these foods that are typically rejected by normal monkeys (Aggleton & Passingham, 1981, 1982; Baylis & Gaffan, 1991; Murray et al., 1996; Stefanacci et al., 2003; Ursin, Rosvold, & Vest, 1969; Weiskrantz, 1956).
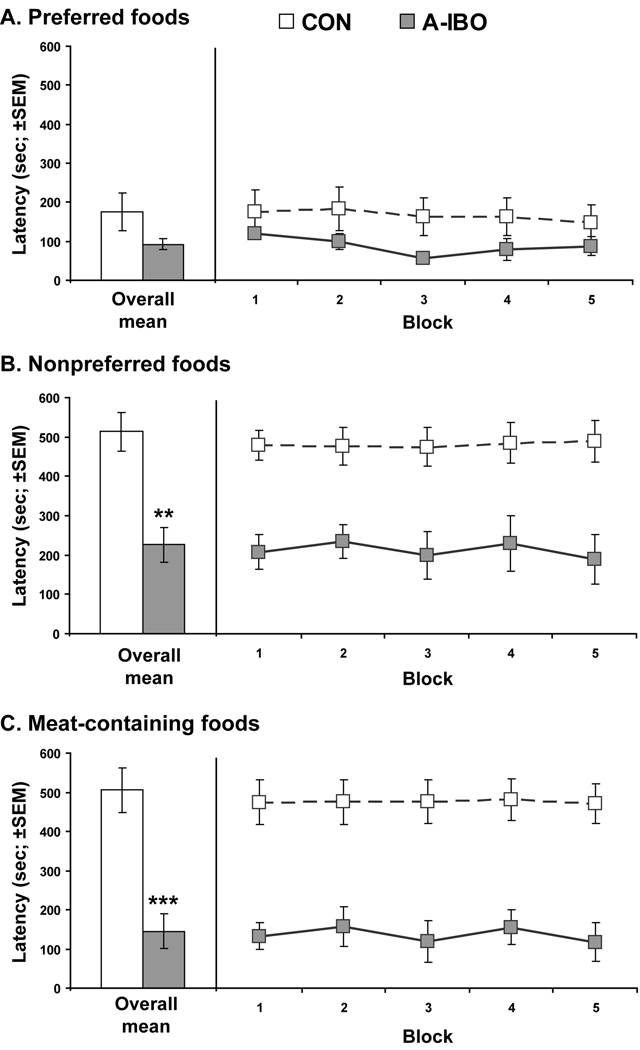
Food selection latencies for the three general categories are shown for each animal in Table 2. Analysis of food selection latencies across the five testing blocks revealed that the groups did not differ in selection latency for Preferred Foods [Group effect: F(1,9) = 1.75, ηp2 = .16, p = .22]. By contrast, A-IBO animals selected Nonpreferred Foods [Group effect: F(1,9) = 12.77, ηp2 = .59, p < .01] and Meat-containing Foods [Group effect: F(1,9) = 21.46, ηp2 = .71, p = .001] significantly faster than CON animals (Figure 1). There were no significant Group × Block interactions for any of the food categories, indicating that heightened preference or diminished avoidance for Nonpreferred Foods and Meat-containing Foods by A-IBO animals was present on their first exposure to these foods and remained constant throughout the 10 testing sessions.
Table 2.
Food selection latencies
| Preferred Foods |
Nonpreferred Foods |
Meat-containing Foods |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | ||
| 25042 | 81.2 | 32.2 | 28.1 | 39.1 | 23.8 | 400.0 | 316.5 | 271.9 | 258.2 | 252.1 | 317.0 | 308.3 | 327.5 | 317.5 | 311.8 | ||
| 25516 | 171.4 | 297.6 | 324.1 | 211.0 | 363.0 | 466.1 | 559.1 | 570.7 | 550.8 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | ||
| 25630 | 447.1 | 414.7 | 318.2 | 391.7 | 185.0 | 600.0 | 600.0 | 600.0 | 600.0 | 574.4 | 600.0 | 600.0 | 600.0 | 600.0 | 536.0 | ||
| 25648 | 67.8 | 172.1 | 142.0 | 83.5 | 106.5 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | ||
| 26225 | 237.5 | 183.2 | 146.8 | 148.3 | 146.9 | 600.0 | 600.0 | 558.3 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | 600.0 | ||
| 26268 | 106.3 | 57.40 | 64.20 | 143.9 | 109.8 | 402.1 | 375.9 | 441.5 | 497.8 | 501.1 | 309.3 | 322.5 | 312.5 | 344.5 | 352.8 | ||
| Mean | 185.2 ± 143.1 | 192.9 ± 144.8 | 170.6 ± 125.2 | 169.6 ± 123.8 | 155.8 ± 114.7 | 511.4 ± 100.0 | 508.6 ± 128.2 | 507.1 ± 129.3 | 517.8 ± 133.5 | 521.3 ± 137.3 | 504.4 ± 148.2 | 505.1 ± 147.0 | 506.7 ± 144.7 | 510.3 ± 139.2 | 500.1 ± 133.0 | ||
| A-IBO | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | ||
| 24349 | 146.2 | 93.8 | 31.4 | 31.3 | 71.9 | 193.0 | 195.1 | 179.0 | 292.4 | 225.0 | 39.0 | 60.0 | 39.3 | 208.0 | 52.3 | ||
| 25468 | 86.2 | 79.6 | 85.0 | 142.0 | 133.5 | 362.6 | 410.3 | 443.0 | 489.1 | 429.9 | 216.75 | 324.0 | 317.0 | 322.8 | 319.8 | ||
| 25571 | 99.6 | 180.2 | 89.9 | 163.2 | 166.6 | 91.6 | 257.4 | 190.5 | 263.2 | 111.6 | 70.0 | 109.8 | 47.5 | 86.3 | 63.8 | ||
| 25942 | 180.7 | 106.7 | 49.4 | 36.1 | 44.8 | 284.2 | 142.2 | 41.9 | 52.1 | 33.3 | 183.0 | 74.5 | 39.8 | 56.3 | 36.3 | ||
| 26085 | 117.7 | 56.8 | 35.5 | 35.1 | 42.5 | 173.9 | 247.9 | 210.9 | 125.3 | 212.9 | 195.0 | 267.8 | 188.5 | 153.3 | 146.0 | ||
| Mean | 126.1 ± 37.9 | 103.4 ± 46.7 | 58.24 ± 27.5 | 81.5 ± 65.3 | 91.86 ± 55.6 | 221.1 ± 104.6 | 250.6 ± 100.5 | 213.1 ± 144.8 | 244.4 ± 168.6 | 202.5 ± 149.3 | 140.8 ± 80.4 | 167.2 ± 120.5 | 126.4 ± 124.0 | 163.3 ± 105.9 | 124.8 ± 116.7 | ||
Data presented are the food selection latencies for each animal in Group CON or A-IBO. Within each food category, B1 – B5 indicate two-day testing blocks used for analysis. Group means (± standard deviation) are also shown. Note that data for animal 25627 are not show in this table since this animal’s data were not included in final analyses.
Figure 1.
Selection latencies for Preferred Foods (A), Nonpreferred Foods (B) and Meat-containing Foods (C) for the two experimental groups. White bars and symbols indicate control animals (CON), whereas gray bars and symbols indicate amygdala-lesioned animals (AMYG). The mean across the 10 test days is given on the left side of each graph, and the mean for each two-day block is given on the right side of each graph to show the trends over time. ** p < .01, *** p < .001
We also performed follow-up analyses (one-way ANOVAs) of the mean selection latency for individual foods in the Nonpreferred and Meat-containing Foods categories to determine if select foods were driving the overall group differences more than others. This analysis revealed that A-IBO animals selected onions [F(1,9) = 7.11, p < .05], dog treats [F(1,9) = 8.33, p < .05], hot dogs [F(1,9) = 12.87, p < .01] and smoked clams [F(1,9) = 15.16, p < .01] significantly faster than CON animals. The groups did not differ in their selection latency for olives [F(1,9) = 1.75, p = .22].
Despite these group differences, the food selection behavior of the A-IBO animals was not completely indiscriminate. A-IBO animals selected Preferred Foods and Meat-containing Food significantly faster than Nonpreferred Foods across the 10 testing sessions [Preferred Foods vs. Nonpreferred Foods: t(4) = −3.53, p < .05; Meat-containing Foods vs. Nonpreferred Foods: t(4) = 2.87, p < .05]. The latency to select Preferred Foods and Meat-containing Foods did not differ for A-IBO animals [t(4) = −.95, p = .39]. This pattern was, however, different from CON which selected Preferred Foods significantly faster than Nonpreferred Foods and Meat-containing foods across the 10 testing session [Preferred Foods vs. Nonpreferred Foods: t(5) = −5.51, p < .01; Preferred Foods vs. Meat-containing Foods: t(5) = −5.50, p < .01]. These latter two categories did not differ for CON animals [t(5) = .461, p = .66].
Food selection rating scores
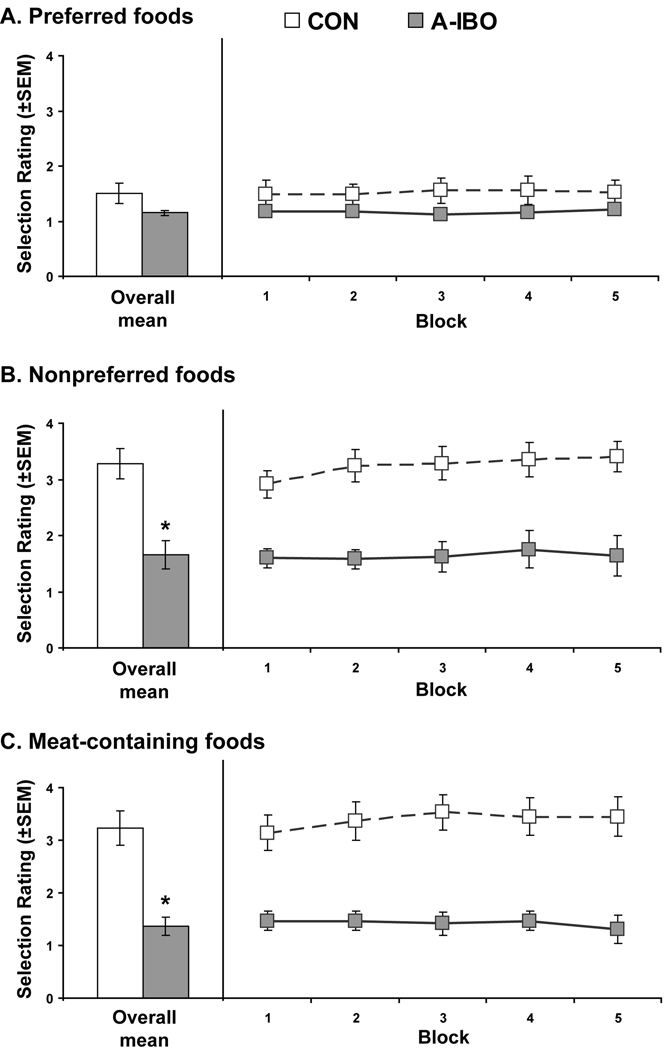
A very similar pattern of results emerged from analysis of the food selection rating scores. Food selection rating scores for the three general categories are shown for each animal in Table 3. The groups again did not differ in rating scores for Preferred Foods [Group effect: F(1,9) = .02, ηp2 = .002, p = .89]. However, A-IBO animals received significantly lower food retrieval ratings (i.e., fewer rejections) than CON animals for Nonpreferred Foods [Group effect: F(1,9) = 7.48, ηp2 = .43, p < .05] and Meat-containing Foods [Group effect: F(1,9) = 8.32, ηp2 = .45, p < .05] (Figure 2). There were no significant Group × Block interactions for any of the three food categories. More focused analyses of group differences in selection rating for specific foods in the Nonpreferred and Meat-containing categories revealed that A-IBO animals received consistently lower ratings than CON animals only for smoked clams [F(1,9) = 10.33, p < .01] and hot dogs [F(1,9) = 10.83, p < .01].
Table 3.
Food selection rating scores
| Preferred Foods |
Nonpreferred Foods |
Meat-containing Foods |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | ||
| 25042 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 2.2 | 2.1 | 2.0 | 2.0 | 2.2 | 2.0 | 2.0 | 2.3 | 2.0 | 2.5 | ||
| 25516 | 1.3 | 1.8 | 2.4 | 1.7 | 2.5 | 2.9 | 3.8 | 3.8 | 3.6 | 3.9 | 3.8 | 4.0 | 4.0 | 3.8 | 4.0 | ||
| 25630 | 2.6 | 2.1 | 1.8 | 2.6 | 1.4 | 3.5 | 3.7 | 3.7 | 4.0 | 3.5 | 3.5 | 3.8 | 3.8 | 4.0 | 3.3 | ||
| 25648 | 1.1 | 1.4 | 1.3 | 1.0 | 1.1 | 3.4 | 3.6 | 4.0 | 4.0 | 4.0 | 3.3 | 3.5 | 4.0 | 4.0 | 4.0 | ||
| 26225 | 1.7 | 1.5 | 1.7 | 1.7 | 1.6 | 3.4 | 3.8 | 3.5 | 3.6 | 3.9 | 3.5 | 3.8 | 3.8 | 3.5 | 4.0 | ||
| 26268 | 1.1 | 1.0 | 1.0 | 1.2 | 1.4 | 2.3 | 2.7 | 3.0 | 3.2 | 3.2 | 2.0 | 2.3 | 2.5 | 2.5 | 2.0 | ||
| Mean | 1.5 ± 0.6 | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.5 ± 0.6 | 1.5 ± 0.5 | 3.0 ± 0.6 | 3.3 ± 0.7 | 3.3 ± 0.7 | 3.4 ± 0.8 | 3.5 ± 0.7 | 3.0 ± 0.8 | 3.2 ± 0.9 | 3.4 ± 0.8 | 3.3 ± 0.8 | 3.3 ± 0.9 | ||
| A-IBO | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | B1 | B2 | B3 | B4 | B5 | ||
| 24349 | 1.2 | 1.1 | 1.0 | 1.0 | 1.1 | 1.5 | 1.4 | 1.5 | 2.0 | 1.8 | 1.0 | 1.3 | 1.0 | 1.5 | 1.0 | ||
| 25468 | 1.0 | 1.2 | 1.4 | 1.4 | 1.4 | 2.1 | 2.3 | 2.7 | 3.0 | 3.0 | 1.8 | 2.0 | 2.0 | 2.0 | 2.3 | ||
| 25571 | 1.1 | 1.4 | 1.0 | 1.2 | 1.4 | 1.1 | 1.5 | 1.3 | 1.6 | 1.3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| 25942 | 1.5 | 1.1 | 1.1 | 1.1 | 1.0 | 1.9 | 1.3 | 1.1 | 1.2 | 1.0 | 1.8 | 1.3 | 1.0 | 1.3 | 1.0 | ||
| 26085 | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | 1.5 | 1.5 | 1.6 | 1.1 | 1.2 | 1.5 | 1.5 | 1.8 | 1.3 | 1.0 | ||
| Mean | 1.2 ± 0.2 | 1.2. ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.6 | 1.8 ± 0.8 | 1.7 ± 0.8 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.5 | 1.4 ± 0.4 | 1.3 ± 0.6 | ||
Data presented are the food selection rating scores for each animal in Group CON or A-IBO. All other conventions are identical to Table 2.
Figure 2.
Selection rating scores for Preferred Foods (A), Nonpreferred Foods (B) and Meat-containing Foods (C) for the two experimental groups. For this measure, lower scores indicate that the animals more readily took the food and placed it in their mouth, either immediately or at some point within the session. All conventions are the same as Figure 1. * p < .05
As indicated above, A-IBO animals discriminated between the food categories in terms of latency to select, but their pattern was different from CON animals. This same trend was found for the food selection scores, but the differences between categories fell just short of significance. Specifically, A-IBO animals registered lower food selection scores (indicating that the food was placed in their mouth) for Preferred Foods and Meat-containing Food relative to Nonpreferred Foods across the 10 testing sessions [Preferred Foods vs. Nonpreferred Foods: t(4) = −2.67, p = .056; Meat-containing Foods vs. Nonpreferred Foods: t(4) = −2.56, p = .062]. Average food selection scores for Preferred Foods and Meat-containing Foods did not differ for A-IBO animals [t(4) = −1.29, p = .27]. This pattern was again different from CON which showed lower food selection scores for Preferred Foods relative to Nonpreferred Foods and Meat-containing foods across the 10 testing sessions [Preferred Foods vs. Nonpreferred Foods: t(5) = −9.32, p < .001; Preferred Foods vs. Meat-containing Foods: t(5) = −9.17, p < .001]. These latter two categories did not differ for CON animals [t(5) = −.504, p = .64].
Correlations between behavior and lesion extent
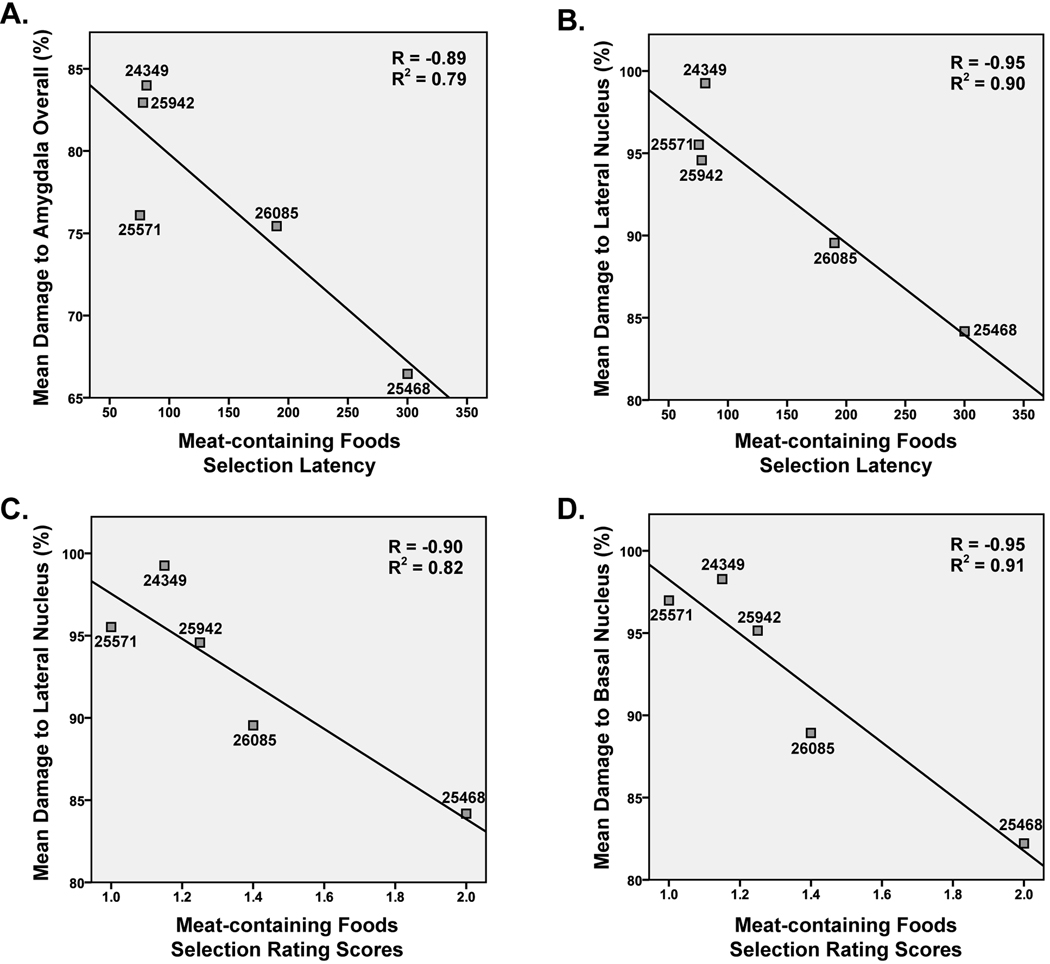
Several significant correlations between intended amygdala damage and mean selection latency or mean selection rating score were found, but only for Meat-containing Foods (Figure 3). There was a significant negative correlation between the latency to select Meat-containing Foods and the amount of amygdala damage overall [r = −.89, p < .05], as well as a negative correlation with damage specifically targeting the lateral nucleus [r = −.95, p < .05] and basal nucleus [r = −.98, p < .01]. Similarly, there was a significant negative correlation between food selection rating score for Meat-containing Foods and damage to the lateral nucleus [r = −.90, p < .05] and basal nucleus [r = −.95, p < .05]. There were no significant correlations between unintended damage to the rostral hippocampus or entorhinal cortex and either behavioral measure.
Figure 3.
Correlations between latency to select meat-containing foods and mean amygdala damage (A) or mean damage to the lateral nucleus. Correlations between food selection rating score and mean damage to the lateral nucleus (C) or basal nucleus (D) are also shown. Lines indicate the best fit to the data in each scatter plot. Individual animal numbers are given for each data point to facilitate comparisons to data shown in Tables 1 – 3. Note that data for animal 25627 are not shown in these plots since this animal’s data were not included in final analyses.
Discussion
The results of this study demonstrate a critical role for the amygdala in avoiding stimuli that normal rhesus monkeys bypass. In particular, when given the choice between five preferred foods and five nonpreferred foods, the amygdala appears to be required for rhesus monkeys to appropriately avoid foods refused by normal monkeys (i.e., meat-flavored foods or cooked baby clams) even on the first exposure. Across all 10 test days, the unoperated control animals either did not select nonpreferred foods at all, or did so very late in the testing session. If nonpreferred foods were selected by control animals, their usual response was to discard them to the floor and not retrieve them during the remainder of the testing session. Amygdala-lesioned monkeys, by contrast, selected nonpreferred foods much earlier in a given testing session. Their most typical response to these foods was to place them in their mouth, either immediately or later in the testing session. This result was substantiated by a significant negative correlation between the extent of amygdala damage and the latency to select Meat-containing Foods. There was also a significant negative correlation between the extent of amygdala damage and food selection rating scores for Meat-containing Foods (lower scores indicate animals put the food in their mouth immediately or dropped it and put it in their mouth later). These two correlations indicate that the more complete the amygdala lesions (especially the lateral and basal nuclei), the faster the selection latencies and the higher the propensity for putting meat-containing foods in the mouth. It is also important to note that the behavioral impairments demonstrated by the amygdala-lesioned animals were not correlated with unintended damage to the rostral hippocampus, which has been associated with reduced anxiety and food neophobia in rats (Bannerman et al., 2002; McHugh, Deacon, Rawlins, & Bannerman, 2004). Our results are consistent with previous assessments of food preference in amygdala-lesioned monkeys in controlled, forced-choice contexts with unpalatable foods (Aggleton & Passingham, 1981, 1982; Baylis & Gaffan, 1991; Murray et al., 1996; Stefanacci et al., 2003).
Since all unpalatable foods used in this study were unfamiliar to the animals, the results presented here provide valuable, new insight into to the role of the nonhuman primate amygdala in both food neophobia and learning to avoid unsavory foods after tasting them. By placing the unpalatable foods in their mouth at some point during their first exposure (exemplified by food rating scores between 1 and 2), the amygdala-lesioned animals clearly demonstrated a lack of typical food neophobia. This finding is consistent with previous amygdala lesion and inactivation studies with rodents (Burns et al., 1996; Burns et al., 1994; Rollins et al., 2001). Moreover, amygdala-lesioned monkeys consistently placed the unpalatable foods in their mouth across the entire 10 days of testing (see Figures 1B, 1C, 2B and 2C, right panels). In other words, increasing familiarity with the taste of these inappropriate foods did not modulate their behavior. This finding is similar to rodent studies where damage to the rodent basolateral amygdala has been shown to diminish conditioned taste aversion (Sakai & Yamamoto, 1999; Simbayi, Boakes, & Burton, 1986; Yamamoto, Fujimoto, Shimura, & Sakai, 1995). However, we cannot be certain whether or not our animals would have continued accepting the novel foods if illness had followed ingestion as typically occurs in rodent studies. Nevertheless, this body of evidence across species indicates an evolutionarily conserved role for the amygdala in avoiding potentially dangerous foods, both on the first exposure and on subsequent ones.
Could the apparent willingness to eat nonpreferred and meat-containing foods by amygdala-lesioned animals be attributed solely to compulsive oral exploration (hyperorality) or indiscriminate reaction to visual stimuli (hypermetamorphosis)? These are consistent behavioral abnormalities observed following large medial temporal lobe lesions in nonhuman primates (Aggleton & Passingham, 1981, 1982; Baylis & Gaffan, 1991; Kluver & Bucy, 1939; Weiskrantz, 1956). Hyperorality of inedible objects has also been shown after selective, neurotoxic amygdala lesions (Machado & Bachevalier, 2007a; Murray et al., 1996; Stefanacci et al., 2003). In fact, the animals studied here also demonstrated elevated oral exploration of the testing environment, but only during initial social behavior assessments (Emery et al., 2001, Experiments 1 and 2). With subsequent testing, these animals no longer showed such hyperorality (Emery et al., 2001, Experiment 3; Machado et al., 2008; Mason et al., 2006). Even within this study, the foraging or exploratory behavior of the amygdala-lesioned animals cannot be classified as indiscriminate. This group’s average latency to select preferred and meat-containing foods was significantly faster (92.2 and 144.9 seconds, respectively) than for nonpreferred foods (226.3 seconds). In addition, the amygdala-lesioned animals’ average food selection scores for preferred and meat-containing foods were consistent with placing these items directly into their mouths (1.2 and 1.4, respectively). However, it was more common for these same animals to discard the nonpreferred foods onto the floor initially and then reacquire them later (average food selection score = 1.7). This pattern of behavior does not closely resemble the classic finding of indiscriminate or compulsive hyperorality or hypermetamorphosis following large temporal lobe lesions.
It is also important to note that amygdala damage did not diminish the normally high-preference that rhesus monkeys show for palatable foods such as apples, grapes, peanuts and carrots, relative to nonpreferred foods. For palatable foods, neither the latency to select the food or how the animals behaved with the foods were different between the experimental groups. This result could indicate that the amygdala does not play a critical role in linking familiar stimuli with previously-learned positive hedonic values. Such appraisals of familiar tastes, textures and odors of foods and the appropriate behavioral responses by amygdala-lesioned animals may have been accomplished by intact circuitry including the basal ganglia, insula, ventromedial prefrontal cortex and orbitofrontal cortex (O'Doherty, Deichmann, Critchley, & Dolan, 2002; Waltz et al., 2009). Activity in these regions of the human brain increases as self-ratings of anhedonia (an inability to experience pleasurable emotions) decrease in severity for both non-clinical individuals and patients with schizophrenia (Harvey, Armony, Malla, & Lepage, 2010). In addition, the current finding that amygdala lesions do not alter strong preferences for palatable foods are consistent with many previous nonhuman primate studies of food preference, both in controlled (Aggleton & Passingham, 1981, 1982; Machado & Bachevalier, 2007a; Murray et al., 1996) and semi-naturalistic settings (Machado & Bachevalier, 2007b).
In summary, this study provides additional information regarding amygdala function in nonhuman primates, and presumably humans as well. In addition to restraining inappropriate social interactions and inhibiting approach behavior in the presence of potentially dangerous predator-like animals or novel objects, the amygdala is also critical for steering primates away from unpalatable and potentially dangerous food sources. Because the studies in this area have examined several types of animals (rodents and nonhuman primates in particular), included several different instrumental behaviors and have now been conducted in both controlled and semi-naturalistic testing contexts, it is also reasonable to conclude that this role for the amygdala likely appeared early in mammalian evolution.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R37 MH57502 and R01 MH41479) and was conducted at the California National Primate Research Center (RR00169). We thank Pamela Tennant, Jeff Bennett, Caroline Chen, and John Ruys for assistance with histology, and Noah Merin for assistance with data collection. We would also like to extend thanks to the technical, animal care and veterinary staff at the California National Primate Research Center for supporting this research. Finally, we thank Dr. Peter Rapp for providing the control brain sections used for histological lesion analysis, as well as Dr. Eliza Bliss-Moreau and two anonymous reviewers for comments on earlier drafts of this manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/BNE
References
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative & Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Passingham RE. An assessment of the reinforcing properties of foods after amygdaloid lesions in rhesus monkeys. Journal of Comparative & Physiological Psychology. 1982;96:71–77. doi: 10.1037/h0077861. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Málková L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behavioral Neuroscience. 2006;120:989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral Neuroscience. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Experimental Brain Research. 1991;86(3):617–622. doi: 10.1007/BF00230535. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neuroscience & Biobehavioral Review. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behavioral Neuroscience. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Burns LH, Everitt BJ, Robbins TW. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 impairs acquisition but not performance of discriminated approach to an appetitive CS. Behavioral and Neural Biology. 1994;61:242–250. doi: 10.1016/s0163-1047(05)80007-x. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Amaral DG. The role of the amygdala in primate social cognition. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford: Oxford University Press; 2000. pp. 156–191. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Emery NJ, Machado CJ, Mendoza SP, Capitanio JP, Mason WA, Amaral DG. Role of the amygdala in dyadic social interactions & the stress response in monkeys. 28th Annual Meeting, Society for Neuroscience; Los Angeles, CA. 1998. p. 780. [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Murray EA. Aspiration lesions of the amygdala disrupt the rhinal corticothalamic projection system in rhesus monkeys. Experimental Brain Research. 1998;119:131–140. doi: 10.1007/s002210050326. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Armony J, Malla A, Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. Journal of Psychiatric Research. 2010;44:707–716. doi: 10.1016/j.jpsychires.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-Expression and Gaze-Selective Responses in the Monkey Amygdala. Current Biology. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A, Brothers L. The amygdala and social behavior. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion. New York: Wiley-Liss; 1992. pp. 353–377. [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007a;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: The effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007b;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): Consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;122:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Barrow KV, Lower LL, Gale K. Decreased social interactions in monkeys after unilateral blockade of GABA-A receptors in the basolateral amygdala. Annals of the New York Academy of Sciences. 2003;985:540–541. [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys: Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17:214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behavioral Brain Research. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Munoz M, Mishkin M, Saunders RC. Resection of the medial temporal lobe disconnects the rostral superior temporal gyrus from some of its projection targets in the frontal lobe and thalamus. Cerebral Cortex. 2009;19:2114–2130. doi: 10.1093/cercor/bhn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Gaffan EA, Flint RW., Jr Anterior rhinal cortex and amygdala: dissociation of their contributions to memory and food preference in rhesus monkeys. Behavioral Neuroscience. 1996;110:30–42. [PubMed] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH. Individual differences in the responses of naive rhesus monkeys to snakes. Emotion. 2003;3:3–11. doi: 10.1037/1528-3542.3.1.3. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. European Journal of Neuroscience. 2003;18:1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. Journal of Comparative Neurology. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rollins BL, Stines SG, McGuire HB, King BM. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiology & Behavior. 2001;72:735–742. doi: 10.1016/s0031-9384(01)00433-4. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neuroscience Research. 1999;35:53–61. doi: 10.1016/s0168-0102(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Experimental Brain Research. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Simbayi LC, Boakes RA, Burton MJ. Effects of basolateral amygdala lesions on taste aversions produced by lactose and lithium chloride in the rat. Behavioral Neuroscience. 1986;100:455–465. doi: 10.1037//0735-7044.100.4.455. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research. 3 ed. New York: Freman; 1995. [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behavioral Neuroscience. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Ursin H, Rosvold HE, Vest B. Food preference in brain lesioned monkeys. Physiology & Behavior. 1969;4:609–612. [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparitive and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neuroscience Research. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]