Remarkable advances in our understanding of innate and adaptive immunity have shed light on why inflammation is centrally involved in the pathogenesis of many diseases traditionally not viewed as primarily inflammatory in nature. One key area of progress has been the discovery and study of innate immune receptors, referred to as pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nod like receptors (NLRs). PRRs recognize molecules commonly produced by microbial pathogens broadly defined as pathogen associated molecular patterns (PAMPs), but also self molecules that are indicators of cell injury or death. These self molecules, called damage associated molecular patterns (DAMPs), include reactive oxygen species, intracellular crystalline deposits, normal molecules in abnormal locations (ATP, heat shock proteins; nuclear proteins), and many others 1, 2. DAMPs may be induced or change location because of cell injury or death by any number of causes (genetic, traumatic, toxic, metabolic), and their recognition by innate immune system receptors will initiate responses that include local and systemic inflammation. This is why diseases with just about any underlying cause may be expected to have an inflammatory component. Importantly, innate immune responses have evolved to amplify and modify responses to best combat diverse infections, but these amplified and specialized responses, if inappropriately targeted, may themselves become intrinsic to the progression of disease processes. One way this can occur is by innate immune stimulation of adaptive immune responses mediated by T lymphocytes. In fact, normal protective T cell-mediated immunity requires a kick-start by the innate immune system, a condition that ensures that we do not readily mount powerful and potentially harmful adaptive immune responses when there is no real damage or danger present.
One of major ways in which innate immunity promotes T cell mediated immunity is through the activation of antigen presenting cells (APCs) such as dendritic cells (DCs). DCs process and present protein antigens, in the form of peptides bound to MHC molecules, for recognition by the antigen receptor on T cells (TCR). DCs express many PRRs that can become activated by PAMPs and DAMPs, leading to enhanced antigen processing capabilities, increased MHC expression, and migration from infected or damaged tissues into lymph nodes where naïve T cells are located. Importantly, PRR signaling in DCs also increases the expression of cell surface costimulatory molecules, which bind to different receptors on T cells. Concomitant signaling by the TCR and the costimulatory receptor are essential for activation of naïve T cells. The result is clonal expansion and differentiation into effector T cells capable of microbial defense and/or tissue injury. Several molecules possess costimulatory activity, but arguably the most important are CD80 (B7-1) and CD86 (B7-2) on activated DCs, which bind to CD28 on T cells 3. Our understanding of the essential role of CD80 and CD86-mediated costimulation for T cell activation has lead to the development of drugs for autoimmune diseases 4. For example, CTLA-4 Ig (abatacept and belatacept) is a fusion protein blocker of CD80/CD86 approved for treatment of rheumatoid arthritis 5. In this issue of Circulation, Vinh and colleagues report that CTLA-4 Ig treatment of mice prevents angiotensin or DOCA/salt induced hypertension, and the drug effect is mimicked by genetic CD80/CD86 deficiency 6. These results support the concept that hypertension belongs in the list of cardiovascular diseases for which immunomodulatory therapy should be considered.
Given that cell injury from many different causes can induce innate responses, and the role of innate immunity in enhancing the ability of APCs to activate T cells, it is not surprising that T cell responses may be found in the setting of many different disorders, including cardiovascular diseases. The participation of T cells in atherosclerosis was first described in the 1980's by Goran. Hansson and colleagues 7, and there is now an extensive literature describing how T cells contribute to atherosclerotic lesion growth and destabilization of advanced lesions. More recently, T cells have been shown to contribute to inflammatory processes within adipose tissues in the setting of metabolic syndrome 8. The possibility that T cells contribute to hypertension was addressed more than 25 years ago in studies of DOCA/salt induced hypertension in thymectomized control mice 9. However, as recently reviewed elsewhere 10, there has only been modest attention paid to T cells in hypertension. Based on work from several laboratories, and in particular studies from David Harrison's laboratory, T cells may now be taking on a larger role as culprits in hypertensive disease.
Setting the stage for Vinh and colleagues current paper on costimulatory blockade, the Harrison group previously showed a requirement for T cells in angiotensin II or desoxycorticosterone acetate (DOCA)-salt induced hypertension in mice 11. In wild type mice, they saw a striking effect of angiotensin II infusion on T cell activation and on infiltration of T cells into periadventitial fat. They used an adoptive transfer approach into lymphocyte deficient (Rag-1-/-) mice to demonstrate that T cells are required for development of full blown hypertension in either angiotensin II or DOCA-salt models. Furthermore, adoptive transfer of T cells lacking the angiotensin type I receptor or a functional NADPH oxidase resulted in blunted angiotensin II-dependent hypertension and decreased aortic superoxide production. In the current study, Vinh and coworkers addressed the potential involvement of B7/CD28 T cell costimulation axis in mouse hypertension. They found that CTLA4-Ig ameliorated angiotensin II -induced hypertension, and reduced vascular superoxide production. CTLA4-Ig treatment blocked the ability of angiotensin II to increase numbers of circulating activated T cells, and the drug also inhibited T cell infiltration into periadventitial fat. Moreover, CTLA-4 Ig also blocked angiotensin-induced increases in T cell TNF-α and IFN-γ production. Cognizant of the fact that costimulator expression on APCs is critical for T cell activation, Vinh and colleagues examined secondary lymphoid tissue DCs and found that CD86 (but curiously not CD80 or class II MHC) was selectively increased in angiotenisn II-treated mice. All the effects of CTLA-4 Ig treatment were also found in CD80/CD86-deficienct mice as well as wild type mice transplanted with CD80/CD86-deficienct bone marrow. Remarkably, transplantation of wild type bone marrow into CD80/CD86-deficient mice completely restored angiotensin II-induced effects on hypertension and T cells. CTLA4-Ig also prevented DOCA-salt induced hypertension and associated T cell activation and aortic infiltration. Finally, CTLA4-Ig could also reverse established angiotensin II and DOCA-salt induced hypertension.
The findings from this study by Vinh A et al. and the previous study form the Harrison's laboratory discussed above 11 clearly establish that T cells contribute to hypertensive disease and vascular pathology in mouse models. Many questions are raised by this work, which will require further investigation, in order to fully understand how T cells contribute to murine disease models and to determine if they contribute to human disease. The adaptive immune system has evolved to rely on exquisitely specific clonally distributed antigen receptors for lymphocyte antigen recognition and activation. Therefore, when T cells are implicated in a disease process, one of the first questions that arise is what antigens are being recognized? The authors emphasize that the requirement for CD80/86-mediated costimulation in the mouse models indicates that antigen recognition by T cells is key, rather than non-specific perivascular accumulation of previously activated/memory T cells of any specificity. A similar argument has been made for proatherogenic T cell responses, which are dependent on CD80/CD86 mediated costimulation 12. Nonetheless, the actual specificity of the pathogenic T cells in hypertension is important to know in order to understand the underlying mechanism of the disease process and also for the possible development of therapies based on antigen-induced tolerance, rather than nonspecific immunosuppression. This may not be an easy question to answer. For example, the identity of the relevant antigens recognized by pro-atherogenic T cells remains uncertain after decades of study, although there are strong candidates, 13. There are multiple mechanisms for maintenance of T cell tolerance to self proteins, which kill off or inactivate self-reactive T cells during thymic development and in peripheral tissues. Therefore, barring the unlikely role of infection in the mouse models, the emergence of T cells that contribute to the development of hypertension is a failure of tolerance to normal self molecules, or a reaction against altered self molecules which accumulate because of the abnormal conditions that underlie disease (e.g. injury to the vascular wall from direct or indirect effects of angiotensin).
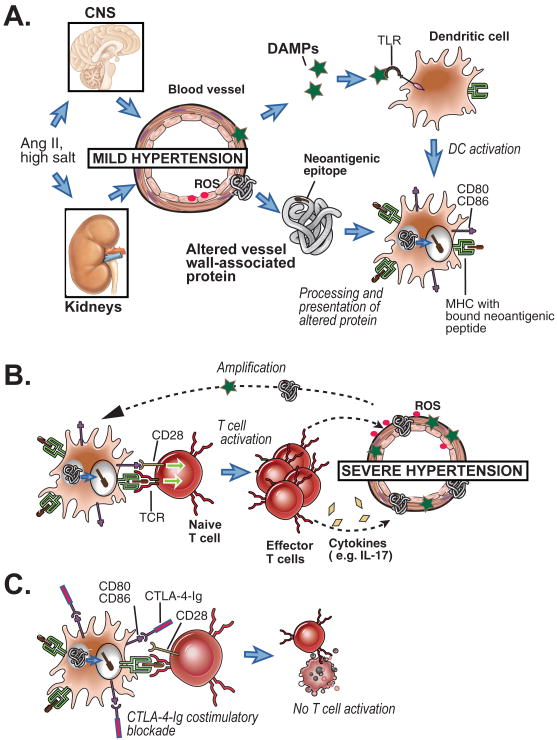
The second major question raised by the work reported here and in previous papers is the mechanism by which T cell activation promotes hypertension. The presence of activated T cells in a perivascular distribution suggests a local effect of cytokines that may diffuse into arterial walls, and alter vascular smooth muscle and/or or endothelial function, but this has not been proven. In support of this idea, Harrison's group has reported that IL-17 is required for sustained hypertension and vascular pathology, and they found increased IL-17 producing T cells in blood and IL-17 in the aortic media in angiotensin IL-treated mice 14. Another group reported that aldosterone, a key mediator in hypertension, promotes differentiation of Th17 cells 15. Thus T cells that produce IL-17 cells may play an important local role in this disease. The Harrison's group has also reported that anteroventral third cerebral ventricle (AV3V) lesions and hydralizine each prevented angiogetensin II induced hypertension, T cell activation, and perivascular inflammation 16. They interpret these findings as evidence that modest hypertension, mediated through CNS dependent mechanisms, occurs first in the angiotensin model, and this leads to T-cell activation, which then induces perivascular inflammation and drives the progression to severe hypertension. This hypothetical sequence of events would be consistent with the concept that some initial vascular damage caused by mild hypertension is necessary to activate the innate immune system and upregulate costimulatory molecules expression, leading to loss of T cell tolerance to blood vessel-associated self antigens or activation of T cell responses to altered-self antigens, possibly generated by oxidative stress. This paradigm (see Figure) is reminiscent of Svendsen's thymectomy study 9 mentioned earlier, which showed an initial thymus independent (i.e. T cell independent) phase of hypertension followed by a later long lasting thymus dependent phase in DOCA/salt treated mice.
Figure.
Hypothetical scheme for induction of T cell responses in hypertension. This scheme is based on a scheme proposed in Harrison et al.10 with a refocus costimulation of T cells. A. Mild hypertension is initially induced by angiotensin II (Ang II) or high salt, leading to limited vascular wall damage, release of DAMPs and altered self proteins. DAMPs activate dendritic cells (DCs) via TLR signaling, inducing costimulator expression (CD80, CD86). The activated DCs process the altered self proteins and displays peptides from those proteins bound to MHC molecules. B. The DC presents the neoantigen peptide/MHC complexes and costimualtors to naive T cells specific for that antigen, and the T cells are activated, proliferate and differentiation into pathogenic effector T cells specific for altered vessel wall proteins. These T cells infiltrate around blood vessels, become reactivated in that location (not shown), and secrete inflammatory cytokines that further promote vascular dysfunction leading to severe hypertension. More neoantigens and DAMPs will be released from the vessels in severe hypertension, amplifying the T cell response. C. CTLA-4-Ig blocks the interaction of CD80 and CD86 with T cell CD28, thereby preventing naïve T cell activation, and theoretically preventing the pro-hypertensive T cell response shown in B.
Are innate and T cell-mediated immune responses relevant to human hypertensive disease? Circumstantial evidence is consistent with the possibility. Recent studies have shown that plasma levels of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and IL-17 positively correlate with blood pressure in humans 14, 17. In a small study of eight psoriasis patients, mycophenolate mofetil treatment, which mainly targets B and T cells, was shown to reduce blood pressure in essential hypertensive patients 18. Hypertension is a frequent co-morbidity of rheumatoid arthritis 19, 20. Nonetheless, there are no published studies that specifically investigate the effect of abatacept on hypertension in RA patients. In light of the compelling evidence from the work of Vinh et al. on CTLA-4-Ig in mice, it is obviously worthwhile to further study hypertension in patients treated with CTLA-4-Ig. The pathogenesis of essential hypertension may be different in the setting of autoimmune disease, and therefore the experience of costimulatory blockade in RA patients may not predict efficacy in patients with essential hypertension but without autoimmune disease. Given the risks of infection that come with any immunosuppressive therapy, and the efficacy of current anti-hypertensive therapies, it is not yet clear which patients would be appropriate candidates for trials of costimulatory blockade. A reasonable starting place, as suggested by Vinh et al, may be to determine if CTLA-4 Ig is effective as an adjuvant for short term therapy of malignant hypertension. Certainly the evidence from animal models in support of a role for T cells in hypertension is currently very strong, warranting serious consideration of clinical trials of drugs targeting T cell activation or effector functions for high blood pressure.
Acknowledgments
Funding: A.H.L. is supported by N.I.H. Grant HL087282 (AHL)
Footnotes
Disclosures: The authors have declared that no conflict of interest exists.
References
- 1.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 2.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20:530–537. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linsley PS, Nadler SG. The clinical utility of inhibiting cd28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 5.Schiff M. Abatacept treatment for rheumatoid arthritis. Rheumatology. doi: 10.1093/rheumatology/keq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy J, Weyand C, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the b7/cd28 t cell costimulation axis prevents experimental hypertension. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–847. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 9.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of doca and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/b7-2 costimulation regulates plaque antigen-specific t-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 13.Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrada AA, Contreras FJ, Marini NP, Amador CA, Gonzalez PA, Cortes CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF, Fardella CE, Kalergis AM. Aldosterone promotes autoimmune damage by enhancing th17-mediated immunity. J Immunol. 2010;184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 16.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (c-reactive protein, interleukin-6, and tnf-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 18.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 19.Cohen AD, Weitzman D, Dreiher J. Psoriasis and hypertension: A case-control study. Acta Derm Venereol. 2010;90:23–26. doi: 10.2340/00015555-0741. [DOI] [PubMed] [Google Scholar]
- 20.Tobin AM, Veale DJ, Fitzgerald O, Rogers S, Collins P, O'Shea D, Kirby B. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. The Journal of Rheumatology. 2010;37:1386–1394. doi: 10.3899/jrheum.090822. [DOI] [PubMed] [Google Scholar]