Abstract
Oral transmission of human immunodeficiency virus (HIV) in adult populations is rare. However, HIV spread across fetal/neonatal oropharyngeal epithelia could be important in mother-to-child transmission. Analysis of HIV transmission across polarized adult and fetal oral epithelial cells revealed that HIV transmigrates through both adult and fetal cells. However, only virions that passed through the fetal cells – and not those that passed through the adult cells – remained infectious. Analysis of expression of anti-HIV innate proteins beta-defensins 2 and 3, and secretory leukocyte protease inhibitor in adult, fetal, and infant oral epithelia showed that their expression is predominantly in the adult oral epithelium. Retention of HIV infectivity after transmigration correlated inversely with the expression of these innate proteins. Inactivation of innate proteins in adult oral keratinocytes restored HIV infectivity. These data suggest that high-level innate protein expression may contribute to the resistance of the adult oral epithelium to HIV transmission.
Keywords: HIV transepithelial migration, oral epithelium, HIV inactivation
Introduction
The interaction of HIV with the mucosal epithelia of the oropharyngeal, gastrointestinal, and genital (anorectal and cervicovaginal) tracts – which involves the translocation of the virus from the mucosal surface to HIV-susceptible intraepithelial and subepithelial immune cells – is the first step in the establishment of systemic HIV infection (Anderson et al.; Pope and Haase, 2003). However, the ability of HIV to initiate systemic infection through exposure to these various epithelia varies considerably. While transmission through the adult oral cavity is rare (Page-Shafer et al., 2006; Tudor-Williams and Lyall, 1999), mother-to-child transmission (MTCT) through the neonatal oral and/or gastrointestinal epithelia is common (De Cock et al., 2000; Luzuriaga, 2007; Mandelbrot et al., 1999). Transmission is also more frequent through the adult anogenital tract (Anderson et al.; Pope and Haase, 2003) than it is through the adult oral route (Scully and Porter, 2000). Thus, different mucosal epithelia transmit HIV infection to different degrees, but the mechanisms underlying these differences are poorly understood.
Orally transmitted HIV in the fetus/neonate may come from amniotic fluid in utero (Jaspan et al., 2004; Maiques et al., 2003; Mundy et al., 1987), and from amniotic and cervicovaginal fluids at birth and in breast milk postnatally (Nussenblatt et al., 2005; Semba, 2000; Semba and Neville, 1999; Willumsen et al., 2000). While antiretroviral therapy (ART) has been shown to reduce the rates of mother to child transmission (MTCT) of HIV, the rate of MTCT without ART has been estimated to be about 15% in Europe and 25–30% in African and Asian countries (De Cock et al., 2000; Luzuriaga, 2007). By contrast, the rate of oral HIV transmission in adults has been estimated to be only about 0.004% per exposure (del Romero et al., 2002; Page-Shafer et al., 2002; Royce et al., 1997; Vittinghoff et al., 1999), suggesting the mechanisms of HIV transmission via fetal/neonatal and adult oropharyngeal epithelia are different.
Application of HIV to human vaginal, cervical and intestinal tissue explants ex vivo leads to the migration of HIV across these epithelia (Hladik et al., 2007; Maher et al., 2005; Shen et al.; Shen et al., 2009; Shen et al.). These results indicate that the virus can transmigrate across intact mucosal epithelia, allowing it to infect intraepithelial and submucosal HIV-susceptible immune cells and therefore initiate systemic infection. In vitro studies using single-layer, polarized epithelial cells show that virus transmigration is mediated by transepithelial transcytosis without infection of the epithelial cells. HIV transepithelial transcytosis has been well documented in polarized cells of vaginal, endometrial, and intestinal origin (Bobardt et al., 2007; Bomsel, 1997; Hocini et al., 2001; Hocini and Bomsel, 1999; Meng et al., 2002; Saidi et al., 2007). However, HIV transcytosis via adult and infant/fetal oral epithelial cells has not been well investigated, even though differences between adult and infant/fetal oral epithelia may help to account for the higher rate of oral transmission in infants.
To better understand the mechanisms underlying resistance and susceptibility to HIV transmission across fully developed and developing oral epithelia, respectively, we established monostratified polarized oral epithelial cells from fully developed, mature, adult epithelium and the developing, less mature, fetal oral epithelium. Using these polarized epithelial cell models, we show that the HIV virions can traverse both adult and fetal oral epithelial cells by transcytosis. However, during passage through the adult cells – but not through the fetal cells – infectivity of the virions is greatly diminished. High-level expression of the anti-HIV innate proteins beta-defensins (HBD) 2 and 3 and secretory leukocyte protease inhibitor (SLPI) in adult oral epithelial cells are associated with reduction or loss of HIV infectivity.
Results
HIV transcytosis across polarized oral epithelial cells
To study HIV transepithelial transmission across well-developed adult oral and developing fetal oral epithelia, we established monostratified polarized epithelial cells originating from adult tongue and tonsil, as well as fetal tongue and oropharyngeal mucosal epithelia. To compare HIV transcytosis of oral epithelial cells to that of genital epithelial cells, we also used polarized adult endometrial and cervical epithelial cells. Cells were grown on microporous filter inserts, and their polarity was confirmed by immunodetection of tight junction proteins and measurement of paracellular permeability and transepithelial resistance (TER). The tight junction proteins occludin (Figure 1A) and ZO-1 (data not shown) were both found to be localized to the lateral membranes of polarized cells, consistent with the presence of tight junctions. To confirm the functional status of the tight junctions, paracellular permeability and TER were measured in polarized cells that were either untreated or treated with EDTA to dissociate the tight junctions. A substantial increase in [3H] inulin passage from the apical surface to the basolateral compartment and a decrease in TER in EDTA-treated cells in comparison to control, EDTA-untreated cells were observed. These findings indicate the formation of a tight, polarized, epithelial monolayer (Figure 1B).
Figure 1.
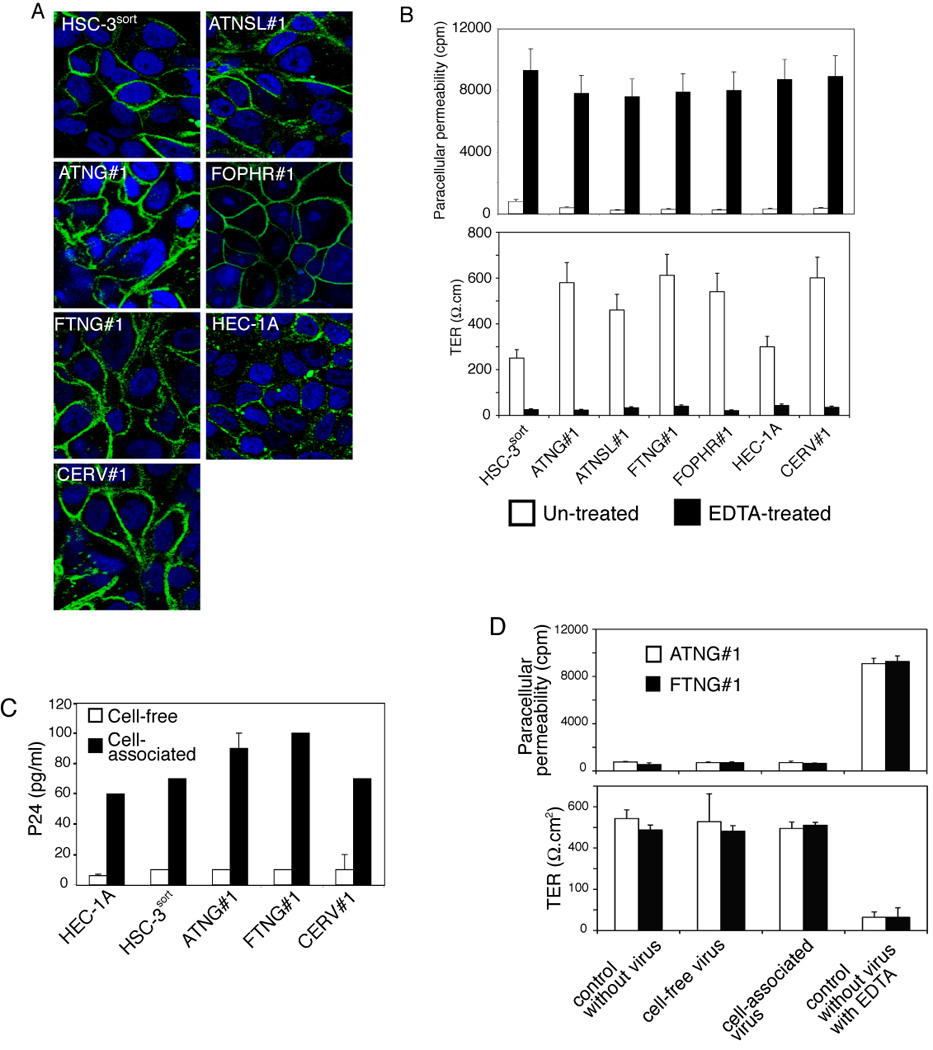
Establishment of polarized adult and fetal oral epithelial cells and transcytosis of HIV. (A) Formation of tight junctions in polarized adult and fetal oropharyngeal cells. Cells were grown under polarizing conditions on Transwell filter inserts for 7–14 days. Expression of occludin, a marker for tight junctions, was examined by immunostaining. Cell nuclei were stained in blue. Cells were analyzed by confocal microscopy and images were obtained approximately 3 µm below the apical surface of polarized cells in the x-y (horizontal) plane. (B) Transepithelial resistance (TER) and paracellular permeability of polarized adult and fetal oral epithelial cells were examined by measuring electrical resistance of monolayers and passage of radioactive 3H-inulin through paracellular spaces. As a positive control, cell junctions were disrupted by incubating cells with 10 mM EDTA before measurement. Error bars show ± standard error of the mean (s.e.m.) (n = 3). (C) Transcytosis of cell-free and cell-associated HIV. Cell-free (20 ng p24/insert) and cell-associated HIV-1SF33 (106 PBMCs) were added to the apical membranes of polarized endometrial (HEC-1A), adult tongue (HSC-3sort, ATNG#1), fetal tongue (FTNG#1), and adult cervical (CERV#1) epithelial cells. After 3 h, culture media from the lower chambers were examined for HIV-1 p24 using an ELISA. (D) Analysis of the integrity of polarized oral epithelial cells after HIV transcytosis. For transcytosis assays, cell-free and cell-associated HIV-1SF33 were added to the apical membranes of polarized adult (ATNG#1) and fetal tongue (FTNG#1) epithelial cells for 3 h. One set of filter inserts was without virus, and the next set of inserts was without virus and treated with EDTA for 30 min prior to measurement. TER and paracellular permeability of polarized cells were examined by Millicell-ERS voltohmmeter and radioactive 3H-inulin passage, respectively.
HIV transcytosis was examined using cell-free and cell-associated dual (R5/X4)-tropic HIV-1SF33 virus, which was added to the apical membranes of polarized cells. For cell-associated virus, we used HIV-infected peripheral blood mononuclear cells (PBMCs). Previous reports have shown that 3 h incubation of either cell-free or cell-associated virus with polarized epithelial cells is sufficient for HIV transcytosis across these cells (Bomsel, 1997; Carreno et al., 2002; Hocini et al., 2001). Therefore, this time period was used for our transcytosis experiments. HIV transcytosis was evaluated by detection of HIV-1 p24 in the lower chamber of the filter inserts using an ELISA p24 detection assay. We compared transcytosis of cell-free and cell-associated HIV-1SF33 virions in polarized endometrial (HEC-1A), adult tongue (HSC-3sort, ATNG#1), fetal tongue (FTNG#1), and adult cervical (CERV#1) epithelial cells (Figure 1C). Transcytosis of cell-free HIV-1SF33 virus in all polarized cells was at a low level, i.e., about 0.005% of viruses (from the original inoculum) transmigrated the polarized monolayer. In contrast, transcytosis of cell-associated HIV-1SF33 virus was about 10-fold higher. No PBMCs were detected in the lower chambers, indicating that PBMCs did not migrate into lower chambers through polarized cells.
To determine whether the interaction of cell-free virus or HIV-infected PBMCs from the apical surface of epithelial cells altered the integrity of polarized epithelial cells, we examined the TER and paracellular permeability of the adult and fetal polarized monolayers during transcytosis. As a control, cell junctions were disrupted by incubation of polarized cells with 10 mM EDTA. We found that TER and paracellular permeability of polarized epithelial cells were not changed by transcytosis of either cell-free or cell-associated HIV-1SF33 virions (Figure 1D).
Expression of HIV coreceptors in adult and fetal oral epithelial cells
HIV co-receptors CXCR4, CCR5, galactosyl ceramide (GalCer), and heparan sulfate proteoglycans (HSPG) may play a critical role in HIV transcytosis (Alfsen and Bomsel, 2002; Alfsen et al., 2005; Bobardt et al., 2007; Bomsel, 1997; Yahi et al., 1994). It has been well documented that polarized endometrial and vaginal epithelial cells do not express CD4, but may express one or more of these HIV co-receptors (Bobardt et al., 2007; Bomsel, 1997; Hocini et al., 2001; Hocini and Bomsel, 1999; Saidi et al., 2007). We used FACS and immunofluorescence assays to determine whether CD4, CXCR4, CCR5, GalCer, and HSPG are expressed in polarized oral epithelial cells. Analysis of CD4 expression in adult and fetal epithelial and cervical and endometrial cells showed that CD4 was not detectable in any of these cell lines (data not shown). Detection of the HIV co-receptors GalCer, HSPG, CXCR4, and CCR5 in polarized oral epithelial cells by FACS revealed that primary adult and fetal oral epithelial cells, as well as cervical epithelial cells expressed all of these receptors (Figure 2A). Expression of CXCR4 and CCR5 in immortalized adult HSC-3sort tongue cells was not detectable, and only low-level expression of these receptors was found in immortalized HEC-1A endometrial cells. Confocal microscopy showed that these receptors were present on the apical surfaces (Figure 2B).
Figure 2.
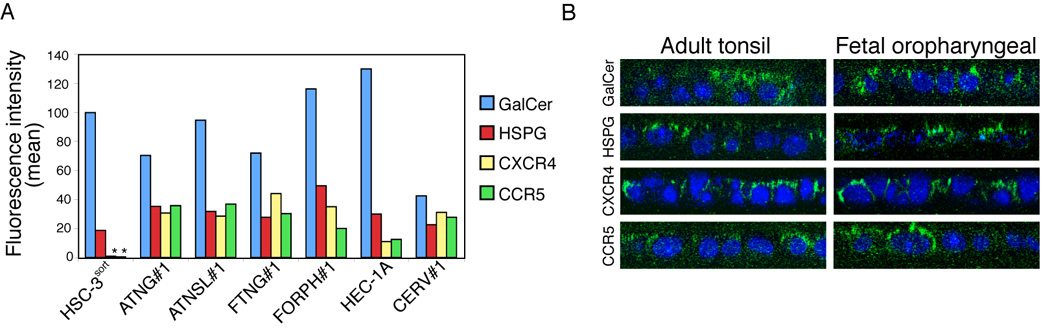
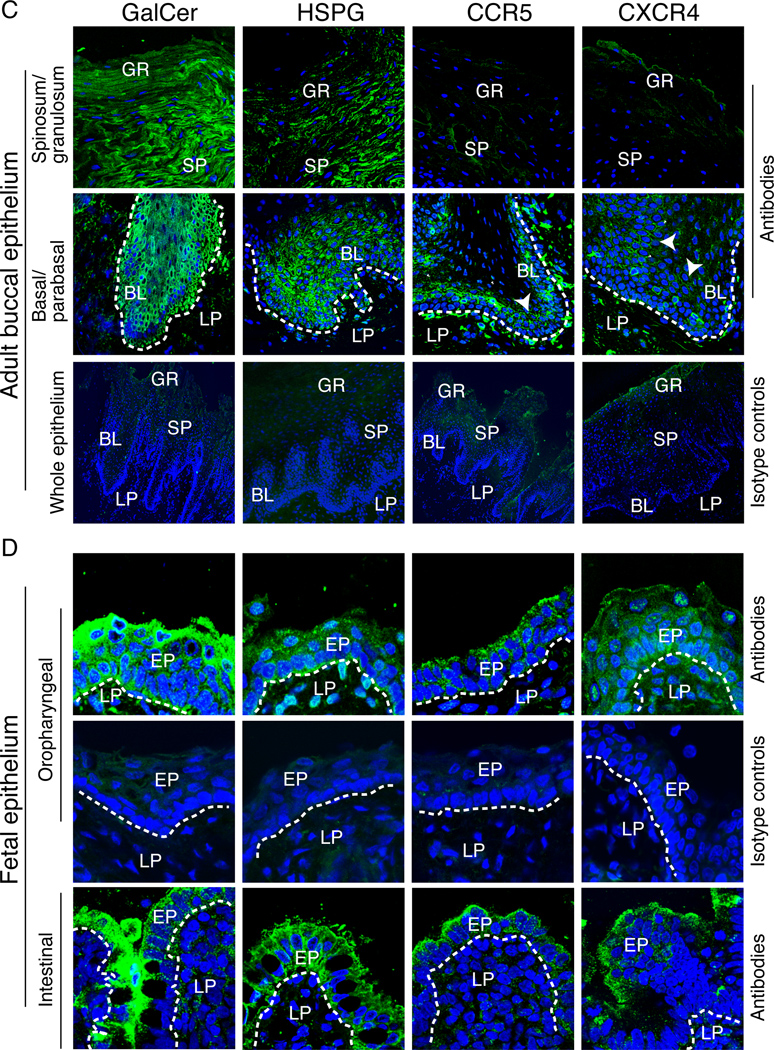
Expression of HIV-1 co-receptors in adult and fetal oral and fetal intestinal epithelia. (A and B) Expression of HIV-1 co-receptors in polarized epithelial cells. (A) Expression of CXCR4, CCR5, GalCer, and HSPG in adult (HSC-3sort and ATNG#1 tongue, and ATSL#1 tonsil) and fetal (FTNG#1 tongue and FORPH#1 oropharyngeal) oral epithelial cells, as well as in adult endometrial (HEC-1A) and cervical (CERV#1) epithelial cells, was examined by flow cytometry. The mean fluorescence intensity is shown. *, not detected. (B) Localization of HIV co-receptors on the apical surfaces of polarized oral epithelial cells. Polarized adult and fetal oral epithelial cells were fixed with 4% paraformaldehyde, and non-permeabilized cells were immunostained for HIV co-receptors (green). Nuclei were counterstained in blue. Cells were analyzed by confocal microscopy and images are shown in the x-z (vertical) plane. Original magnification is ×600. (C and D) Expression and localization of HIV-1 co-receptors in adult (C), fetal oral (D, upper panels), and fetal intestinal (D, lower panels) epithelia. Tissue sections were immunostained with antibodies to GalCer, HSPG, CCR5 or CXCR4 (all green) or their isotype controls. Cell nuclei were stained in blue. Only merged panels are shown. GR, granulosum; SP, spinosum; BL, basal; LP, lamina propria; EP, epithelium. White lines indicate the border between the lamina propria and the mucosal epithelium. Original magnification is ×600 (A, upper and middle panes, B, all panels). Original magnification is ×400 (A, lower panels).
To determine whether the expression of HIV co-receptors in established, polarized cells in vitro correlates with their expression in oral mucosal epithelia in vivo, we immunostained adult and fetal biopsy tissue sections for the HIV receptors CD4, CXCR4, CCR5, GalCer, and HSPG. Expression of CD4 was not detected in either adult or fetal oral epithelia. CXCR4 and CCR5 were detected mostly within basal and parabasal cells (Figure 2C) in 6 of 7 (85.7%) buccal tissue samples; the more superficial layers of the adult buccal epithelia were negative. GalCer and HSPG were both detected within all layers of the adult buccal epithelia (Figure 2C). In fetal oral and intestinal tissues, CXCR4 and CCR5 were detected in 5 of 10 (50%) buccal, 7 of 9 (55.5%) oropharyngeal, and 4 of 8 (50%) intestinal tissue samples (Figure 2D). All were positive for GalCer and HSPG, primarily in the more superficial cell layers.
HIV transmission across adult oral epithelial cells leads to viral inactivation, whereas HIV transcytosed across fetal epithelial cells retains infectious activity
In the above experiments, we showed that the amount of HIV that transmigrated across fetal and adult oral epithelia was about similar (Figure 1C). We next sought to determine if the levels of infectious virus were similar. To determine the infectivity of transmigrated cell-free virions, we performed transcytosis assays with cell-free HIV-1SF33 in polarized adult tongue and tonsil, fetal tongue and oropharyngeal, and adult endometrial and cervical epithelial cells. The infectious activities of transmigrated virions were examined in target PBMCs using a reverse transcriptase (RT) assay after 7 days. In this analysis, no RT activity was detected in target PBMCs infected with cell-free virions transcytosed through any of these cells (data not shown). Similar data were obtained from 4 independent experiments. This observation may have reflected the low levels of cell-free HIV transcytosis via polarized epithelial cells (Figure 1C).
To examine the infectious activity of transmigrated, cell-associated virions, HIV-1SF33 - infected PBMCs were added to the apical surfaces of polarized adult and fetal tongue epithelial cells, and adult endometrial cells. After 3 h one third of the culture medium from the lower chamber was collected for detection of HIV-1 p24 by ELISA. The remaining media, along with the target PBMCs, were cultured for the next 7 days, and the PBMCs were used for RT assays. Detection of HIV-1 p24 using an ELISA assay showed that the rates of HIV transcytosis through adult and fetal tongue epithelial cells and adult endometrial cells were comparable. These findings indicate that about the same numbers of virions transmigrated through each cell type. However, virions that transmigrated across adult tongue epithelial cells showed no RT activity in target PBMCs (Figure 3A). In contrast, virions that passed through fetal tongue epithelial cells and adult endometrial cells did show RT activity in target PBMCs. These data indicate that the cell-associated virions that had transmigrated across adult oral epithelial cells were not infectious in the target PBMCs, but that those that had passed through fetal oral epithelial and adult endometrial cells retained infectious activity.
Figure 3.

Analysis of infectious activity of HIV transcytosed through adult and fetal oral epithelial cells. (A) Transcytosis of cell-associated HIV-1SF33 virus across adult (ATNG#1) and fetal tongue (FTNG#1) epithelial cells and adult endometrial cells was examined. After 3 h one third of culture media with PBMCs from the lower chambers was collected and used for an ELISA p24 assay. The rest of the media were maintained with target PBMCs for the next 7 days, and the infectious activity of the virus was determined using an RT assay. Data shown are mean ± sem from 3 filter inserts. Similar results were obtained in 3 independent experiments. (B) Transcytosis of cell-associated HIV-1SF33 through adult endometrial HEC-1A, cervical (CERV#1), tongue (HSC-3sort, ATNG#1, ATNG#2), and tonsil (ATSL#1, ATSL#2) epithelial cells, as well as fetal tongue (FTNG#1, FTNG#2, FTNG#3) and oropharyngeal (FORPH#1, FORPH#1) epithelial cells. Infectious activity of transcytosed virions was examined using an RT assay after 7 days of culturing PBMCs from the lower chambers. Each data symbol (circle) indicates one experiment, and each experiment used three separate filter inserts. n, number of filter inserts. Horizontal lines represent median values. (C) Cell-associated R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 were used for transcytosis through endomethrial (HEC-1A), adult tongue (HSC-3sort, ATNG#1), tonsil (ATSL#1), and fetal oropharyngeal (FORPH#1, FORPH#2, FORPH#3) epithelial cells. After 1, 2, and 3 weeks of transcytosis, the infectious activity of virions in PBMCs was examined using an RT assay. Results shown are from one representative experiment out of two experiments. Error bars show ± s.e.m. (n = 3).
Analysis of the RT activity of PBMCs infected with transmigrated, cell-associated HIV-1SF33 virions in multiple experiments using several different adult and fetal oral epithelial cell lines (Figure 3B) showed that virions that had transmigrated across adult oral epithelial cells had a strong tendency to lose their infectious activity for target PBMCs. In contrast, the infectious activity of virions that had traversed fetal oral epithelial cells was well preserved. HIV-1SF33 virions that transmigrated through adult endothelial and cervical epithelial cells were also infectious (Figure 3B).
Primary HIV isolates lose their infectious activity after transmigration in adult oral epithelial cells but not in fetal oral epithelial cells
To determine the infectious activity of clinical HIV-1 isolates after their transmigration across adult and fetal oral epithelial cells, we performed transcytosis assays with cell-associated X4-tropic HIV-192UG029 and R5-tropic HIV-1SF170 primary isolates in adult endometrial, tongue and tonsil, and fetal tongue and oropharyngeal polarized epithelial cells (Figure 3C). The infectious activity of transmigrated virions was examined after 1, 2, and 3 weeks in target PBMCs. These data showed that virions that traversed adult oral epithelial cells were not infectious, but those transmitted through adult endometrial and fetal oral epithelial cells were infectious in PBMCs and that their RT values increased with time. Transmigration of infectious virions was also detected through polarized primary cervical epithelial cells (data not shown). Thus, transmission of HIV-1 clinical isolates through adult oral epithelial cells caused their complete inactivation, whereas viral transmigration through fetal and cervical epithelial cells did not affect their infectious activity.
Analysis of anti-HIV innate protein expression and secretion in polarized cells
To understand the mechanisms of HIV inactivation in adult oral epithelial cells and the preservation of infectious activity in fetal oral epithelial cells, we examined the expression of anti-HIV innate proteins beta-defensins (HBD) 1, 2 and 3 and secretory leukocyte protease inhibitor (SLPI) (Dale et al., 2001; Dunsche et al., 2002; Jana, Gray, and Shugars, 2005; Moutsopoulos et al., 2007; Quinones-Mateu et al., 2003; Sun et al., 2005) in both cell types. In an immunofluorescence assay, we found that about 70% of adult and fetal oral epithelial cells and about 80% of cervical epithelial cells were positive for HBD1 (data not shown). We also found that 60–80% of adult oral epithelial cells were positive for HBD2, HBD3, and SLPI (Figure 4A). However, the fetal oral epithelial cells had an approximately 5- to 10-fold lower level of expression of these molecules than did the adult cells. Furthermore, the expression of HBD2, HBD3, and SLPI in adult cervical epithelial cells was 2- to 5-fold lower than that in adult oral epithelial cells. None of these proteins was detected in HEC-1A endometrial cells (data not shown).
Figure 4.
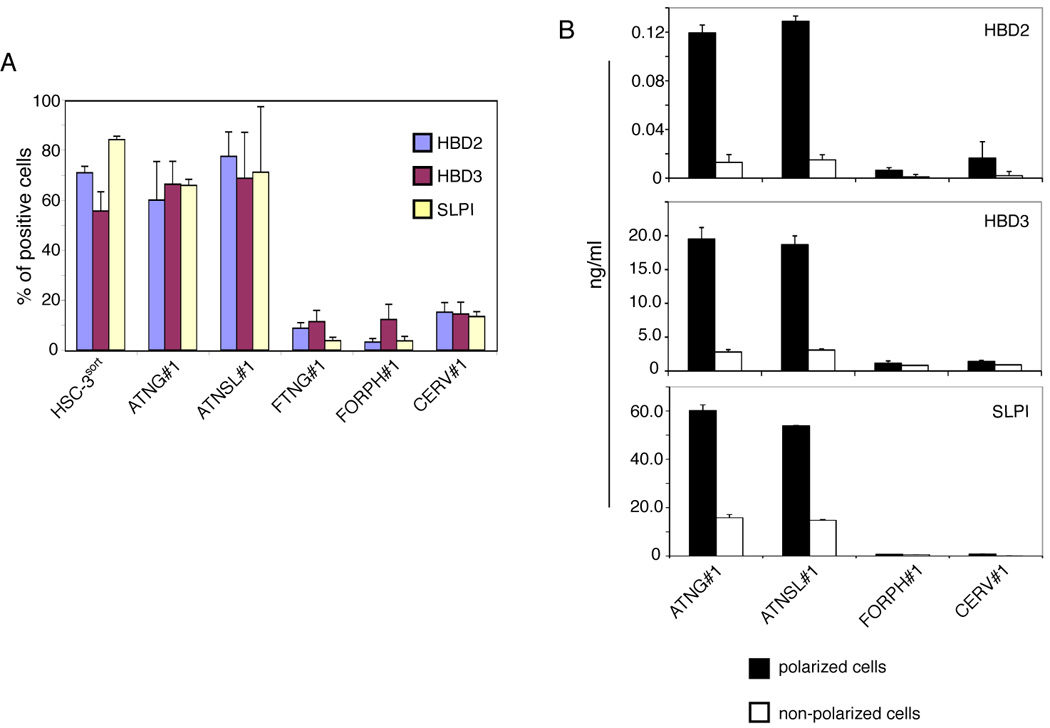
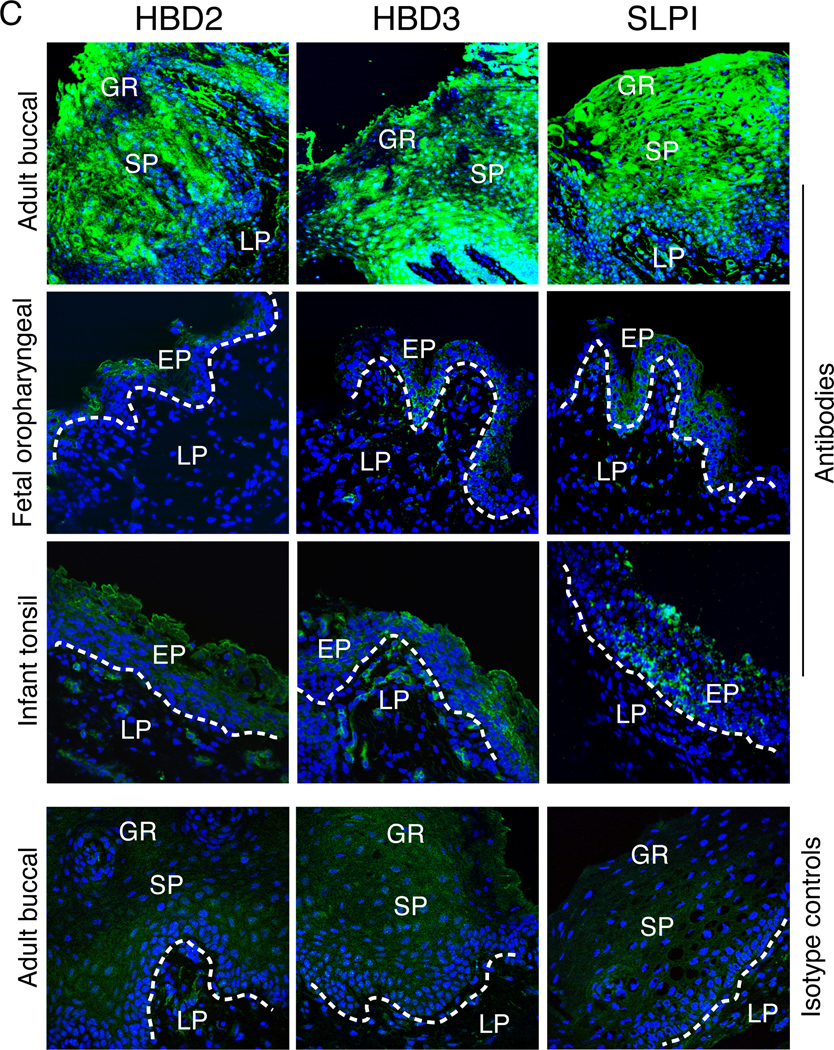
Expression of anti-HIV innate proteins in adult, fetal, and infant oral epithelial cells. (A) Expression of anti-HIV innate proteins in polarized epithelial cells. Adult and fetal epithelial cells were grown under polarizing conditions and immunostained for HBD2, HBD3 and SLPI. Cells were analyzed by confocal microscopy and positive cells were counted. Error bars show ± s.e.m. (n = 3). (B) Secretion of HBD2, HBD3, and SLPI from polarized and non-polarized adult tongue and tonsil, fetal oropharyngeal, and adult cervical epithelial cells. Culture media were collected separately from apical and basolateral membranes of polarized cells. In parallel experiments, culture media were collected from non-polarized cells. Secretion of HBD2, HBD3, and SLPI was measured using an ELISA detection kit for each protein. Error bars show ± s.e.m. (n = 3). (C) Expression of anti-HIV proteins in adult buccal, fetal oropharyngeal, and infant tonsil epithelia. Tissue sections were immunostained with antibodies to HBD2, HBD3, or SLPI (all green) or their isotype controls. Cell nuclei are counterstained in blue. Cells were analyzed by confocal microscopy. Original magnification is ×600. GR, granulosum; LP, lamina propria; EP, epithelium.
Secretion of HBD2, HBD3, and SLPI was measured for the polarized and non-polarized adult and fetal oral and adult cervical cells using an ELISA assay. These data revealed that all three proteins were secreted at the highest levels only from polarized adult tongue and tonsil epithelial cells (Figure 4B). Their secretion from non-polarized adult tongue and tonsil epithelial cells was about 5–10 fold lower than from polarized adult oral epithelial cells. Secretion of HBD2 and HBD3 from fetal oropharyngeal and adult cervical epithelial cells was about 10–15 fold lower than that of adult oral epithelial cells. High-level SLPI secretion was detected only in adult tongue and tonsil epithelial cells, and SLPI secretion from fetal oropharyngeal and adult cervical epithelial cells was not detectable.
We next examined the expression of anti-HIV proteins in adult and fetal oral biopsy tissues. Immunofluorescence analysis of 5 adult buccal and 6 fetal oropharyngeal (Figure 4C) and 8 buccal (data not shown) tissue explants for HBD2, HBD3, and SLPI showed that HBD2, HBD3, and SLPI were all strongly expressed in the adult oral epithelium (Figure 4C). All three proteins were detected both within cells and in the extracellular environment, consistent with their being secreted. In contrast, expression of these proteins in fetal buccal and oropharyngeal epithelia was mainly negative, and only weak positive signals were observed in only small areas of fetal epithelia (Figure 4C). We also examined HBD2, HBD3, and SLPI expression in 3 infant tonsil epithelia and found that they were all negative or only weakly positive for these proteins.
Functional role of anti-HIV innate proteins in inactivation of HIV during transepithelial transmigration
To determine the functional role of innate proteins in the inactivation of HIV during transcytosis in adult oral epithelial cells, we interfered with innate protein function using two independent approaches: inhibition of innate protein expression and intracellular neutralization. For inhibition of innate protein expression, polarized adult tonsil epithelial cells were treated with siRNA against HBD1, HBD2, HBD3, or SLPI. As a control, we used an unrelated siRNA. Immunostaining of HBD1, HBD2, HBD3, and SLPI after 3 days showed that treatment of cells with specific siRNAs reduced expression of innate proteins by about 60–70% (Figure 5A). A Western blot assay also showed that siRNA-treatment reduced HBD-3 and SLPI expression below detectable levels (Figure 5B). These cells were then used for HIV-1SF33 cell-associated transcytosis assays. The TER of these epithelia was similar to the TER of untreated polarized cells (about 650 Ω/cm2 in both treated and untreated cells), indicating that the siRNA treatment did not disrupt tight junctions. Measurement of the RT activity of target PBMCs after 10 days showed that virions that had traversed polarized cells treated with siRNA against HBD2, HBD3, and SLPI were infectious (Figure 5C). In contrast, virions that had traversed cells treated with either anti-HBD1 siRNA or control siRNA did not cause infection in target PBMCs, i.e., no RT activity was detected in PBMCs after 10 days.
Figure 5.
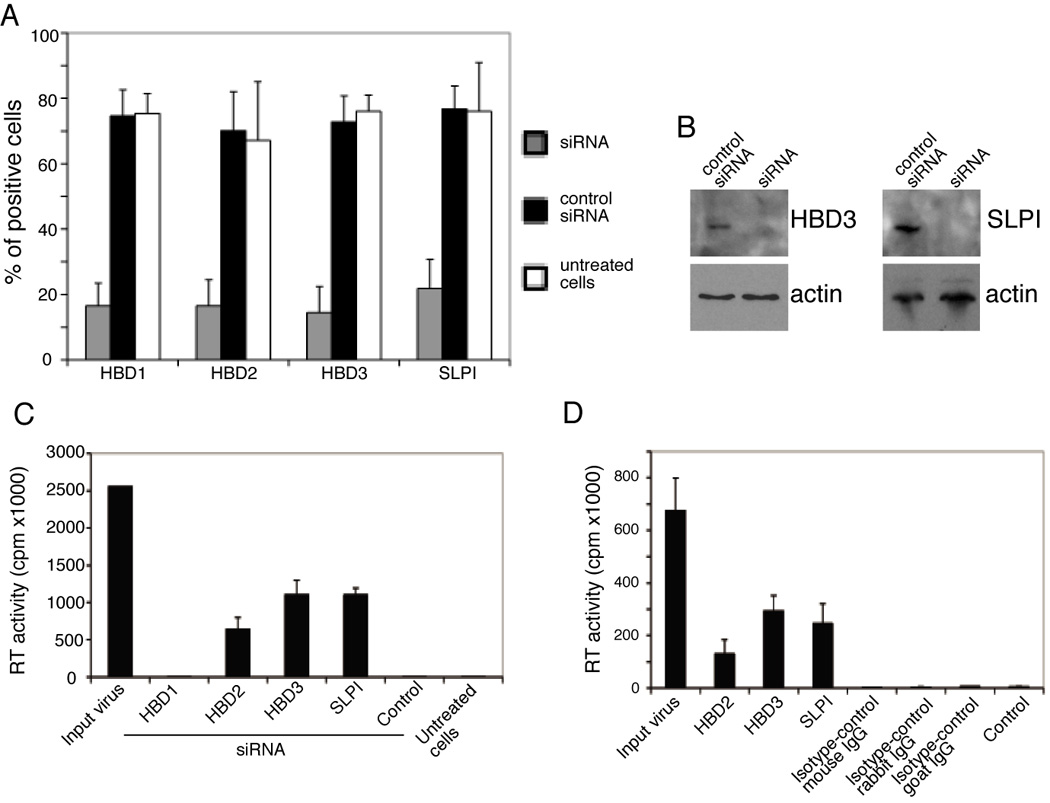
The role of anti-HIV innate proteins in infectious activity of transcytosed HIV-1 through adult oral epithelial cells. (A) Polarized adult tonsil (ATNSL#1) epithelial cells were transfected with siRNA against HBD1, HBD2, HBD3 and SLPI. Control cells were transfected with irrelevant siRNAs or were untreated. After 3 days cells were fixed and immunostained for HBD1, HBD2, HBD3 and SLPI. Expression of innate proteins in cells treated with siRNA and control siRNA, and in untreated cells, was analyzed by confocal microscopy and positive cells were counted in 10 randomly chosen fields. Error bars show ± s.e.m. (B) Polarized adult tonsil (ATNSL#1) epithelial cells were grown in 6-well Transwell inserts and transfected with anti-HBD-3 and SLPI siRNAs and control siRNA. After 3 days cells were extracted and HBD3 and SLPI were detected using a Western blot assay. (C) Polarized adult tonsil cells transfected with specific siRNA or control siRNA and untreated cells were used for transcytosis of cell-associated HIV-1SF33. After 3 h filter inserts were removed and target PBMCs in the lower chambers were cultured for the next 10 days and RT activity was determined. Input virus, the RT value of HIV-infected PBMCs before transcytosis. Control, cells transfected with irrelevant siRNA. Untreated cells, cells were not treated with specific or control siRNA. Error bars show ± s.e.m. (n = 3). Data shown are from one representative experiment out of two experiments. (D) Antibodies to HDB2, HBD3, and SLPI were internalized into adult tongue epithelial cells (ATNG#1) using antibody delivery reagents. As a control, cells were treated with matched isotype antibodies. After 16 h, cell-associated HIV-1SF33 transcytosis was performed, and after 7 days the RT activity of traversed virions was determined in PBMCs from the lower chamber. Input virus, the RT value of HIV-infected PBMCs before transcytosis. Control, transcytosis in the absence of antibodies. Error bars show ± s.e.m. (n = 3). Similar results were obtained in 4 independent experiments.
In the next set of experiments, we neutralized intracellular innate proteins in adult tongue epithelial cells using specific antibodies. Antibody-mediated intracellular neutralization of viruses and proteins has been shown (Bomsel et al., 1998; Burns et al., 1996; Devito et al., 2000; Edelson and Unanue, 2001; Krautz-Peterson et al., 2008; Wright et al., 2006). Antibodies against HBD2, HBD3, or SLPI and isotype control antibodies were added to polarized adult tongue epithelial cells with the lipid-based antibody-delivery reagent Ab-DeliverIN™ (OZ Biosciences) to promote their internalization. The TER in these epithelia was similar to the TER of untreated polarized cells (about 600 Ω/cm2 in both treated and untreated cells), indicating that the antibody-delivery reagent did not disrupt tight junctions. Measurement of SLPI secretion from cells treated with anti-SLPI antibodies showed that internalization of antibodies completely blocked the secretion of SLPI, indicating that antibodies in the cytoplasm may inhibit secretion of innate proteins. Antibody-internalized cells were then used for HIV-1SF33 cell-associated transcytosis. Measurement of the RT activity of target PBMCs after 7 days showed that virions that had traversed polarized cells treated with antibodies against HBD2, HBD3, and SLPI were infectious (Figure 5D). The RT values of these target PBMCs further increased about 3–5 fold by the 14-day time point (data not shown). In contrast, virions that had traversed cells treated with isotype control antibodies did not cause infection in target PBMCs, i.e., no RT activity was detected in PBMCs after 7 and 14 days. Thus, reduction of innate protein expression by siRNA and their intracellular neutralization by specific antibodies in adult tonsil and tongue epithelial cells allowed transcytosed virions to remain infectious. These findings strongly suggest that the innate proteins HBD2, HBD3, and SLPI are responsible for the loss of virion infectivity during transcytosis.
Co-localization of HIV with HBD2 and -3 in the vesicular compartment
HBD2 and HBD3 inactivate HIV by direct interaction with the virions (Cole et al., 2002; Wang et al., 2003; Wang et al., 2004; Weinberg, Quinones-Mateu, and Lederman, 2006), which may occur during viral transcytosis. To determine whether HBD2 and HBD3 co-localize with HIV in the vesicular compartment during HIV transcytosis, we performed HIV transcytosis assays in polarized adult tonsil epithelial cells. R5-tropic HIV-1 81-A virus labeled with GFP (HIV-GFP) was added to the apical surfaces of polarized cells and, after 2 h, cells were fixed and immunostained for early endosomal vesicles using antibodies against early endosomal antigen 1 (EEA1) (Figure 6, red). The same cells were also co-stained for HBD2 or HBD3. Confocal microscopy revealed that the HIV-GFP signal (Figure 6, green) colocalized with EEA1, indicating the presence of virions in the early endosomal compartment. Notably, HBD2 and HBD3 (Figure 6, blue) also colocalized with EEA1, indicating that HBD2 and HBD3 were present in the vesicular compartment. Furthermore, HBD2 and HBD3 were also found to colocalize with both the GFP-virions and EEA1 (Figure 6, merged panel, white), suggesting that HBD2 and HBD3 may interact with virions in the vesicular compartment, leading to inactivation of intracellular virus.
Figure 6.
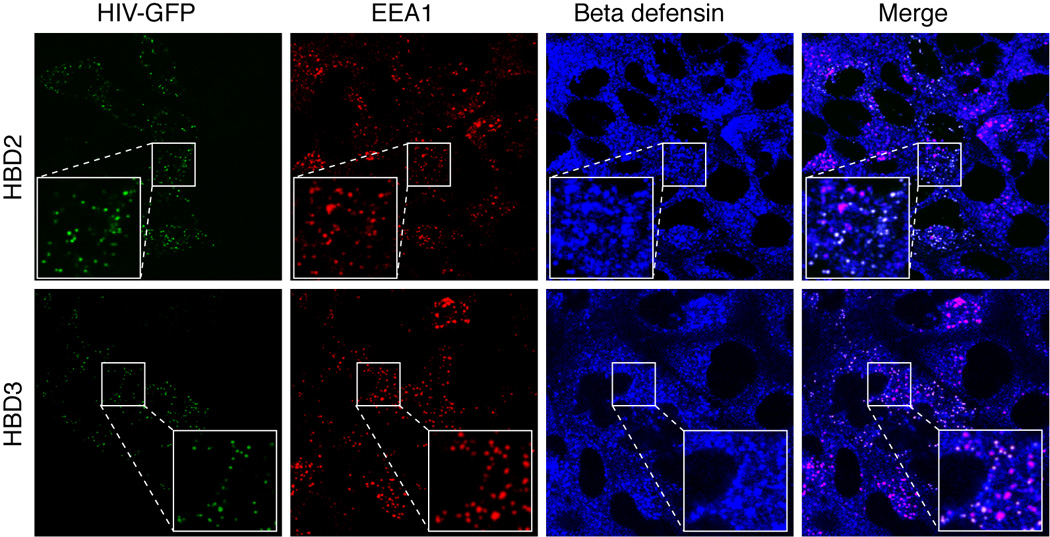
Localization of HIV in the HBD2 and HBD3 containing early endosomal vesicles. GFP-labeled HIV-1 81-A virus was added to the apical surfaces of polarized adult tonsil epithelial cells. Cells were incubated at 37°C for 2 h. Cells were then fixed and co-immunostained for EEA1 (red) and HBD2 or HBD3 (both in blue). Cells were analyzed by confocal microscopy and images were taken about 3 µm below the apical surface. The pink in merged panels indicates co-localization of EEA1 (red) with HBD2 or HBD3 (blue). The white in the merged panels indicates co-localization of HIV (green), HBD2 (blue), and EEA-1 (red), indicating co-localization of HBD2 and virions in the EEA1-positive early endosomes (red).
Discussion
Oral transmission is a potentially important, poorly understood route of HIV infection. Using a monostratified polarized oral epithelial cell model, we showed that HIV can traverse both adult and fetal oral epithelial cells; however, virions transmigrating through adult epithelial cells were rendered noninfectious, whereas those that passed through fetal epithelial cells remained infectious. We further found that HIV inactivation by adult oral epithelial cells was associated with high-level expression of the anti-HIV innate proteins HBD2, HBD3, and SLPI.
Cell-free HIV-1 transcytosis in endometrial HEC-1 cells is substantially lower than that of cell-associated transcytosis (Bomsel, 1997; Hocini and Bomsel, 1999). Our data also showed higher levels of transcytosis for cell-associated than for cell-free HIV across adult and fetal oral epithelial cells (Figure 1C). These data are consistent with a recent report that exposure of the vaginal and cervical mucosa of macaques to cell-associated simian immunodeficiency virus (SIV) leads to rapid transmucosal transmission of SIV (Salle et al.). Efficient transcytosis of cell-associated HIV appears to be mediated by the formation of viral synapses between HIV-infected lymphocytes and the apical surfaces of epithelial cells (Alfsen et al., 2005; Ganor et al.). Interaction of HIV envelope proteins with the heparan sulfate proteoglycan (HSPG) agrin and GalCer, and subsequent cooperation of HSPG and GalCer with beta-1 integrin was critical for the synapse formation between HIV-infected lymphocytes and epithelial cells (Alfsen et al., 2005). Such viral synapses facilitated rapid viral spread via synaptic contact and transcytosis of virions through epithelial cells (Alfsen et al., 2005).
Expression of co-receptors that may play a role in HIV transcytosis, i.e., GalCer, HSPG, CXCR4, and CCR5, in polarized adult and fetal oral epithelial cells and tissues (Figure 2) was consistent with previously reported detection of these receptors in various epithelial tissues, including oral, intestinal, vaginal, and uterine (Bobardt et al., 2007; Dwinell et al., 1999; Kumar et al., 2006; Yeaman et al., 2004; Yeaman et al., 2003). Importantly, we found that, while HIV can transmigrate across intact adult and fetal oral epithelial cells, transmission of infectious virus is inhibited through adult epithelial cells (Figure 3). Higher level expression and secretion of the anti-HIV innate proteins HBD2, HBD3, and SLPI were found in adult oral epithelial cells relative to fetal and infant oral epithelial cells (Figure 4). This difference may at least partially explain the inactivation of HIV transmitted across adult oral epithelial cells. High-level expression of innate proteins, including beta-defensins and SLPI, in the normal adult oral epithelium has been reported previously (Dale et al., 2001; Dunsche et al., 2002; Jana, Gray, and Shugars, 2005; Moutsopoulos et al., 2007; Quinones-Mateu et al., 2003; Sun et al., 2005). However, expression of HBD2, HBD3, and SLPI in the fetal and infant oral mucosal epithelia was barely detectable (Figure 4). This finding suggests that fetuses and infants may have developing mucosal innate immune systems, and therefore transmission of infectious HIV via fetal and neonatal/infant mucosal epithelia could occur more readily. Developmental studies have shown that the level of expression of innate proteins in mammals during their fetal/neonatal period is weaker than during their adult period (Meyerholz et al., 2006; Patil et al., 2005). All these observations are consistent with the finding that there is a higher level of oral transmission of HIV/SIV in neonatal macaques than in (Ruprecht et al., 1998) adult primates (Baba et al., 1996; Ruprecht et al., 1998).
We observed that transcytosis of HIV across polarized endometrial and cervical epithelia did not affect viral infectivity (Figure 3), and this was well correlated with the absence or low-level expression of HBD2, HBD3, and SLPI in these cells (Figure 4). Expression of HBD2, HBD3, and SLPI in the genital mucosa was not stable and depends on the menstrual cycle, i.e., HBD3 and SLPI express during the secretory phase, and HBD2 expression has been detected only during menstruation (King, Critchley, and Kelly, 2003; King et al., 2003; Moriyama et al., 1999).
Restoration of HIV infectious activity in adult oral epithelial cells by siRNA-mediated silencing of HBD2, HBD3, and SLPI gene expression and by antibody-mediated intracellular neutralization of these proteins supports the idea that they play a critical role in reduction of viral infectivity (Figure 5). HBD2 and HBD3 were present in the vesicular compartment of polarized oral epithelial cells and co-localized with HIV virions (Figure 6). This finding suggests the possible intravesicular interaction of defensins with virions, which can lead to the inactivation of virions during transcytosis. Furthermore, secretion of HBD2 and HBD3 from polarized cells may also inactivate extracellular virus. Defensins may directly bind to the HIV envelope (Cole et al., 2002; Wang et al., 2003; Wang et al., 2004; Weinberg, Quinones-Mateu, and Lederman, 2006), and HBD2 and HBD3 are known to inactivate both X4 and R5 viruses (Sun et al., 2005). Furthermore, defensins bind to biological membranes and form multimeric pores, leading to disruption of membranes (Ganz et al., 1985; Hancock and Lehrer, 1998; Lehrer and Ganz, 2002; Nizet et al., 2001; White, Wimley, and Selsted, 1995; Zasloff, 2002). Thus, it is possible that HIV inactivation in adult oral epithelial cells could be due to HBD2- and HBD3-mediated disruption of viral membranes.
SLPI reduces infectivity of both X4- and R5-tropic viruses; however, it does not bind to virions directly, but rather interacts with HIV-susceptible cells (McNeely et al., 1995; McNeely et al., 1997; Wahl et al., 1997). Secretion of SLPI from the basolateral membranes of polarized cells into the lower chambers of our filter inserts may have enabled binding of SLPI to PBMCs leading to reduced entry of virus into target cells. Therefore, in the adult oral epithelium, the combined effects of HBD2 and HBD3 on intracellular and extracellular virus, and of SLPI on target cells, may inactivate virions and reduce the susceptibly of target PBMCs, respectively.
In summary, our data show that the resistance to HIV transmission via the adult oral epithelium could be due to high levels of innate protein expression that reduce infectivity of virus during its migration within the oral mucosal epithelial compartment. Fetal and infant epithelia, which lack expression of innate proteins, may allow transepithelial transmigration of infectious virions and therefore can be vulnerable to HIV transmission. Approaches designed to increase the levels of innate defense proteins in such epithelia may be of value in reducing the risk of HIV transmission by the oral route.
Material and methods
Viruses
The laboratory-adapted dual (X4-R5)-tropic HIV-1SF33 (Levy and Shimabukuro, 1985), R5-tropic HIV-1SF170, and X4-tropic HIV-192UG029 primary clinical isolates were grown in peripheral blood mononuclear cells (PBMCs) from HIV-negative individuals. PBMCs were isolated from buffy coats by Ficoll-Hypaque gradient centrifugation and were activated prior to infection with 2.5 µg/ml phytohemagglutinin (Sigma) and 1 µg/ml interleukin-2 (BD Bioscience) for 3 days. Green fluorescent protein (GFP)-expressing virions were produced by co-transfection of 293T cells with proviral DNA of X4-tropic HIV-1NL4-3 and R5-tropic HIV-181A, and an expression vector encoding a GFP-Vpr fusion protein, as described (Schaeffer, Geleziunas, and Greene, 2001). After 48 h, the released virions were concentrated by ultracentrifugation at 20,000 rpm in an SW41 rotor for 2 h at 4°C. The viral stocks were quantitated for p24 antigen using a p24 ELISA assay and stored in aliquots at −80°C.
Establishment of polarized adult and fetal oral epithelial cells
Polarized HSC-3sort tongue epithelial cells were established in our laboratory (Tugizov, Berline, and Palefsky, 2003), and polarized HEC-1A endometrial epithelial cells were propagated as described (Bomsel, 1997). Two primary adult ectocervical epithelial cell lines (CERV#1 and CERV#2) were purchased from Lonza. To establish polarized cells from primary oral epithelial cells, we propagated primary keratinocytes from two adult tongue (ATNG#1, ATNG#2), three adult tonsil (ATNSL#1, ATNSL#2, ATNSL#3), three fetal tongue (FTNG#1, FTNG#2, FTNG#3), and three fetal oropharyngeal (FORPH#1, FORPH#2, FORPH#3) tissue samples. Each primary keratinocyte culture originated from independent, HIV-negative adult donors and fetuses. 1–2 mm pieces of adult and fetal epithelial tissues were placed on Matrigel-coated plates in KGM-2 media (Clonetics) and incubated at 37°C in a humidified incubator containing 5% CO2. Approximately 2–3 weeks later, keratinocyte outgrowths formed around the tissue explants. They were trypsinized and expanded as a primary culture in KGM-2 media and frozen at passage 3 or 4. The purity of epithelial cells was examined by detection of pankeratin using a cocktail of anti-keratin antibodies containing Ab-1 and Ab-2 (Thermo Scientific), and only those epithelial cell populations that were 100% positive for keratin were used. Polarized cells were established in 0.45-µm Transwell two-chamber filter inserts (12 well inserts) as described in our previous work (Tugizov et al., 1998; Tugizov, Berline, and Palefsky, 2003). The polarity of epithelial cells was verified by immunodetection of the tight junction proteins ZO-1 and occludin and measurement of paracellular permeability and transepithelial resistance (TER), as described previouly (Tugizov, Maidji, and Pereira, 1996; Tugizov et al., 1998; Tugizov, Berline, and Palefsky, 2003). Briefly, TER was measured with an epithelial Millicell-ERS voltohmmeter. To measure paracellular permeability, radioactive 3H-inulin (0.74 Ci/mmol, Amersham) was added to the upper filter chamber. After 10 min, medium from the lower chamber was collected and radioactivity was measured. To induce depolarization, cells were treated with 10 mM EDTA for 30 min prior to measurements, and this served as a positive control for the disruption of cell junctions.
Collection of tissues from adult, fetal and infant oral mucosal epithelium
Approval for collection of oral biopsies was obtained from the Institutional Review Board at the University of California, San Francisco. Biopsies of buccal mucosa were obtained from healthy HIV-seronegative adult volunteers (age range 30–41 years) who had no inflammation in the oral cavity (Department of Orofacial Sciences, UCSF). Fetal buccal and oropharyngeal tissue explants were obtained from fetuses 18 to 24 weeks old that had been aborted from HIV-negative women (Department of Obstetrics, Gynecology and Reproductive Sciences, San Francisco General Hospital). Infant tonsil tissues from 3 infant cadavers (1 day old, 3 days old, and 3 months old) were provided by Dr. Urcell (Department of Pathology). These tissues were used for immunostaining of innate proteins.
Confocal immunofluorescence and flow cytometry assays
Immunostaining of tissue sections and polarized epithelial cells was performed as described (Tugizov et al., 2007; Tugizov, Berline, and Palefsky, 2003). The following antibodies were used for detection of receptors, tight junctions, and innate immune proteins: mouse anti-CD4 (5 µg/ml) (BD Bioscience); anti-CXCR4 (5µg/ml) and anti-CCR5 (5 µg/ml) (both from R&D and provided by the NIH AIDS Reagent program); rabbit anti-GalCer (1:50) (Chemicon) and mouse anti-HSPG 10E4 (1:50) (Seikagaku); rabbit anti-ZO-1 (1 µg/ml), -occludin (1 µg/ml), and -claudin-1 (5 µg/ml) (all from Zymed); rabbit anti-human beta-defensin 1 (HBD1) (1 µg/ml) (Abcam), goat anti-HBD 2(R&D) (10 µg/ml), and mouse anti-HBD3 (Abcam) (10 µg/ml); and mouse anti-secretory leukocyte protease inhibitor (5 µg/ml) (R&D). For detection of HIV in the vesicular compartment we used rabbit antibodies against the early endosome marker EEA1 (5 µg/ml) (Abcam). Secondary antibodies labeled with fluorescein isothiocyanate (FITC), tetramethyl rhodamine isothiocyanate (TRITC), or cyanine 5 (Cy5) were purchased from Jackson Immunoresearch. The specificity of each antibody was confirmed by negative staining with the corresponding primary isotype control antibody. Cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes) (blue). Immunostaining and quantitative analysis were evaluated by two independent investigators (RH and ST). Cells were analyzed using a krypton-argon laser coupled with a Bio-Rad MRC2400 confocal head. The data were analyzed using Laser Sharp software.
For flow cytometry, polarized cells were dissociated enzyme-free cell-dissociation buffer and incubated with antibodies to CCR5, CXCR4, GalCer, and HSPG in PBS (pH 7.4) containing 1% bovine serum albumin (BSA) for 1 h on ice. Cells were then washed, incubated with secondary antibodies for 30 min at 4°C, and analyzed in a FACScan cytometer (Becton-Dickinson and Company, San Jose, California).
Assay for HIV transcytosis across polarized adult and fetal oral epithelial cells
Transcytosis assays were performed as described previously (Bomsel, 1997), with minor modifications. Prior to each experiment, the polarization of cells was confirmed by measuring TER. For transcytosis assays with cell-free virus, 20 ng of p24 HIV-1 cell-free virions was added to apical surfaces in 500 µl of media (10 ng p24/ml). To propagate cell-associated HIV-1, activated PBMCs were infected with 20 ng of p24 virus per 106/PBMCs. The dual tropic HIV-1SF33 strain was highly infectious and therefore infected PBMCs were maintained for 3–5 days. The R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 primary clinical isolates were less infectious, and PBMCs infected with these viruses were cultured for 7–10 days. Infection was confirmed by detection using an ELISA for p24 of virus released from PBMCs, and cells were used for transcytosis if the p24 amount was approximately 20 ng/ml from 106 cells. Thus, the amounts of cell-free (20 ng/insert) and cell-associated virus (10–20 ng/ml/106 cells) in transcytosis experiments were similar. HIV-1-infected PBMCs (106 PBMCs/per filter insert with approximately 2×105 epithelial cells) were added to the apical membranes of polarized cells. To the lower chamber we added 1 ml of medium containing 106 activated, HIV-uninfected target PBMCs. Cells were incubated at 37°C in CO2, and after transcytosis, the filter inserts were removed and one third of the culture medium with PBMCs from the lower chamber was collected for detection of HIV-1 p24 using an ELISA, according to the manufacturer’s instructions (Coulter). The remaining PBMCs in the lower chamber were cultured for 7, 10 or 14 days to evaluate replication of transcytosed virions using a reverse transcriptase assay (RT), as described previously (Hoffman, Banapour, and Levy, 1985).
Analysis of innate protein expression and secretion from oral epithelial cells and their functional activity
For quantitative analysis of innate protein expression polarized epithelial cells were immunostained for HBD2, HBD3 and SLPI. Cells were analyzed by confocal microscopy and positive cells were counted.
To measure the secretion of HBD2, HBD3 and SLPI from polarized and non-polarized cells, equal numbers of cells were seeded in filter inserts with 12 mm diameters (2×105 cells/insert) or in 24-well plates (2×105 cells/well). Cells were grown for 10 days and the polarity of cells on filter inserts was confirmed by measuring their TER. Cells were then washed and fresh media were added. After overnight incubation (16h), media from the apical and basolateral compartments of polarized cells were collected separately. At the same time, media from non-polarized cells were also collected. Culture media from both polarized and non-polarized cells were filtered through 0.22 µm filters and analyzed using HBD2, HBD3 and SLPI ELISA kits (Phoenix Pharmaceutical Inc and R&D, respectively).
For Western blot detection of human HBD3 and SLPI adult tonsil epithelial cells, cells were grown under polarized conditions in 6-well Transwell filter inserts. Polarized cells were extracted, proteins were separated on 16% SDS-polyacrylamide gel and blotted with mouse anti-HBD3 (2 µg/ml) (Abcam) and mouse SLPI (2 µg/ml) (R&D) antibodies. The protein bands were visualized using ECL detection reagents (Amersham) and equal protein load was confirmed by detection of β-actin (Ambio).
To determine the functional role of innate proteins in HIV inactivation, expression of HBD1, HBD2, HBD3 and SLPI in polarized adult oral epithelial cells were inhibited using an siRNA approach. Polarized tongue cells were treated with lipid-based transfection reagents containing specific siRNAs (Santa Cruz Biotechnology Inc) against the mRNAs of each protein, as described by the manufacturer. As controls, unrelated (scrambled) siRNAs were used (Santa Cruz Biotechnology Inc). The efficiency of siRNA transfection was confirmed using FITC-labeled control siRNA: after 24 h, about 70–80% cells were positive for siRNA-FITC. After 3 days of siRNA transfection, polarized cells were used for HIV-transcytosis assays using cell-associated HIV-1SF33. The RT activity of input virus was evaluated by measuring the RT activity of HIV-infected PBMCs before transcytosis. After 10 days the target PBMCs from the lower chambers were used for evaluation of RT activity.
For intracellular neutralization of HBD2, HBD3, and SLPI in polarized adult oral epithelial cells, specific antibodies to these proteins and nonspecific isotype antibodies were allowed to be internalized by polarized adult tongue epithelial cells using the antibody delivery reagent Ab-DeliverIN™ as described by the manufacturer (OZ Biosciences). The quantity of antibody added to each insert – which contained about 2×105 cells each – were as follows: 2 µg/insert of goat anti-HBD2 IgG, 6 µg/insert of monoclonal mouse anti-HBD3 IgG, and 3µg/insert mouse monoclonal anti-SLPI IgG1. The following isotype antibodies were used as controls: 2 µg/insert of goat IgG and 6µg/insert of mouse IgG. After 16 h these cells were used for cell-associated transcytosis of HIV-1SF33 virus. The RT activity of input virus was evaluated by measuring activity in HIV-infected PBMCs before transcytosis. After 7 and 14 days the target PBMCs from the lower chambers were examined for RT activity.
Statistical analysis
Differences in RT values of PBMCs infected with trancytosed HIV were determined by calculation of median values using the Student's t test.
Acknowledgments
We thank Dr. Richard Jordan for histological evaluation of tissues, Dr. Phil Ursell for providing the infant tissues, Jennifer Berline and Samantha Soriano for propagation of primary oral epithelial cells and immunostaining of tissues and cells, and Dr. Matthew Petitt for editorial assistance. This project was supported by National Institutes of Health grants R01 DE14894 and R21 DE016009, and the UCSF ARI Carl L. Gaylord Estate fund, NIH/UCSF CFAR P30-AI027763 and NCI/UCSF HIV-Associated Malignancies Pilot Project grants (to ST).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alfsen A, Bomsel M. HIV-1 gp41 envelope residues 650–685 exposed on native virus act as a lectin to bind epithelial cell galactosyl ceramide. J Biol Chem. 2002;277(28):25649–25659. doi: 10.1074/jbc.M200554200. [DOI] [PubMed] [Google Scholar]
- Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell. 2005;16(9):4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 24(2):163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Trichel AM, An L, Liska V, Martin LN, Murphey-Corb M, Ruprecht RM. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272(5267):1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81(1):395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature Medicine. 1997;3(1):42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9(2):277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272(5258):104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Carreno MP, Krieff C, Irinopoulou T, Kazatchkine MD, Belec L. Enhanced transcytosis of R5-tropic human immunodeficiency virus across tight monolayer of polarized human endometrial cells under pro-inflammatory conditions. Cytokine. 2002;20(6):289–294. doi: 10.1006/cyto.2002.2009. [DOI] [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci U S A. 2002;99(4):1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, O'Neal R, Valore EV, Ganz T, Anderson GM, Weinberg A. Localized antimicrobial peptide expression in human gingiva. J Periodontal Res. 2001;36(5):285–294. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, Alnwick DJ, Rogers M, Shaffer N. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. Jama. 2000;283(9):1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- del Romero J, Marincovich B, Castilla J, Garcia S, Campo J, Hernando V, Rodriguez C. Evaluating the risk of HIV transmission through unprotected orogenital sex. Aids. 2002;16(9):1296–1297. doi: 10.1097/00002030-200206140-00017. [DOI] [PubMed] [Google Scholar]
- Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, Kimani J, Lopalco L, Piconi S, Bwayo JJ, Plummer F, Clerici M, Hinkula J. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165(9):5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- Dunsche A, Acil Y, Dommisch H, Siebert R, Schroder JM, Jepsen S. The novel human beta-defensin-3 is widely expressed in oral tissues. Eur J Oral Sci. 2002;110(2):121–124. doi: 10.1034/j.1600-0722.2002.11186.x. [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117(2):359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14(5):503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- Ganor Y, Zhou Z, Tudor D, Schmitt A, Vacher-Lavenu MC, Gibault L, Thiounn N, Tomasini J, Wolf JP, Bomsel M. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. doi: 10.1038/mi.2010.32. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16(2):82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocini H, Becquart P, Bouhlal H, Chomont N, Ancuta P, Kazatchkine MD, Belec L. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J Virol. 2001;75(11):5370–5374. doi: 10.1128/JVI.75.11.5370-5374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocini H, Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. Journal of Infectious Diseases. 1999;179(1) Suppl 3:S448–S453. doi: 10.1086/314802. [DOI] [PubMed] [Google Scholar]
- Hoffman AD, Banapour B, Levy JA. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Jana NK, Gray LR, Shugars DC. Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: a role for SLPI in innate mucosal immunity. J Virol. 2005;79(10):6432–6440. doi: 10.1128/JVI.79.10.6432-6440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspan HB, Robinson JE, Amedee AM, Van Dyke RB, Garry RF. Amniotic fluid has higher relative levels of lentivirus-specific antibodies than plasma and can contain neutralizing antibodies. J Clin Virol. 2004;31(3):190–197. doi: 10.1016/j.jcv.2004.03.010. [DOI] [PubMed] [Google Scholar]
- King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59(1):1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Krautz-Peterson G, Chapman-Bonofiglio S, Boisvert K, Feng H, Herman IM, Tzipori S, Sheoran AS. Intracellular neutralization of shiga toxin 2 by an a subunit-specific human monoclonal antibody. Infect Immun. 2008;76(5):1931–1939. doi: 10.1128/IAI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RB, Maher DM, Herzberg MC, Southern PJ. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: implications for HIV binding and primary oral infection. Virol J. 2006;3:25. doi: 10.1186/1743-422X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14(1):96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- Levy JA, Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K. Mother-to-child Transmission of HIV: A Global Perspective. Curr Infect Dis Rep. 2007;9(6):511–517. doi: 10.1007/s11908-007-0076-2. [DOI] [PubMed] [Google Scholar]
- Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci U S A. 2005;102(32):11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiques V, Garcia-Tejedor A, Perales A, Cordoba J, Esteban RJ. HIV detection in amniotic fluid samples. Amniocentesis can be performed in HIV pregnant women? Eur J Obstet Gynecol Reprod Biol. 2003;108(2):137–141. doi: 10.1016/s0301-2115(02)00405-0. [DOI] [PubMed] [Google Scholar]
- Mandelbrot L, Burgard M, Teglas JP, Benifla JL, Khan C, Blot P, Vilmer E, Matheron S, Firtion G, Blanche S, Mayaux MJ, Rouzioux C. Frequent detection of HIV-1 in the gastric aspirates of neonates born to HIV-infected mothers. AIDS. 1999;13(15):2143–2149. doi: 10.1097/00002030-199910220-00018. [DOI] [PubMed] [Google Scholar]
- McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96(1):456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90(3):1141–1149. [PubMed] [Google Scholar]
- Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, Shaw GM, Smith PD. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8(2):150–156. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Kawashima K, Gallup JM, Grubor B, Ackermann MR. Expression of select immune genes (surfactant proteins A and D, sheep beta defensin 1, and toll-like receptor 4) by respiratory epithelia is developmentally regulated in the preterm neonatal lamb. Dev Comp Immunol. 2006;30(11):1060–1069. doi: 10.1016/j.dci.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama A, Shimoya K, Ogata I, Kimura T, Nakamura T, Wada H, Ohashi K, Azuma C, Saji F, Murata Y. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Hum Reprod. 1999;5(7):656–661. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Nares S, Nikitakis N, Rangel Z, Wen J, Munson P, Sauk J, Wahl SM. Tonsil epithelial factors may influence oropharyngeal human immunodeficiency virus transmission. Am J Pathol. 2007;171(2):571–579. doi: 10.2353/ajpath.2007.061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy DC, Schinazi RF, Gerber AR, Nahmias AJ, Randall HW., Jr Human immunodeficiency virus isolated from amniotic fluid. Lancet. 1987;2(8556):459–460. doi: 10.1016/s0140-6736(87)91001-4. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Nussenblatt V, Lema V, Kumwenda N, Broadhead R, Neville MC, Taha TE, Semba RD. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int J STD AIDS. 2005;16(3):227–232. doi: 10.1258/0956462053420248. [DOI] [PubMed] [Google Scholar]
- Page-Shafer K, Shiboski CH, Osmond DH, Dilley J, McFarland W, Shiboski SC, Klausner JD, Balls J, Greenspan D, Greenspan JS. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. Aids. 2002;16(17):2350–2352. doi: 10.1097/00002030-200211220-00022. [DOI] [PubMed] [Google Scholar]
- Page-Shafer K, Sweet S, Kassaye S, Ssali C. (C2) Saliva, breast milk, and mucosal fluids in HIV transmission. Adv Dent Res. 2006;19(1):152–157. doi: 10.1177/154407370601900127. [DOI] [PubMed] [Google Scholar]
- Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23(1):5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9(7):847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids. 2003;17(16):F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Ruprecht RM, Baba TW, Liska V, Ayehunie S, Andersen J, Montefiori DC, Trichel A, Murphey-Corb M, Martin L, Rizvi TA, Bernacky BJ, Buchl SJ, Keeling M. Oral SIV, SHIV, and HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14 Suppl 1:S97–S103. [PubMed] [Google Scholar]
- Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358(1):55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 202(3):337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75(6):2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C, Porter S. HIV topic update: oro-genital transmission of HIV. Oral Dis. 2000;6(2):92–98. doi: 10.1111/j.1601-0825.2000.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–162. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Semba RD, Neville MC. Breast-feeding, mastitis, and HIV transmission: nutritional implications. Nutr Rev. 1999;57(5 Pt 1):146–153. doi: 10.1111/j.1753-4887.1999.tb01795.x. [DOI] [PubMed] [Google Scholar]
- Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 184(7):3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83(7):3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Smythies LE, Clements RH, Novak L, Smith PD. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J Leukoc Biol. 87(4):663–670. doi: 10.1189/jlb.0909605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino-Demo A. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79(22):14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Williams G, Lyall EG. Mother to infant transmission of HIV. Curr Opin Infect Dis. 1999;12(1):21–26. doi: 10.1097/00001432-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein-Barr Virus (EBV)-Infected Monocytes Facilitate Dissemination of EBV within the Oral Mucosal Epithelium. J Virol. 2007;81(11):5484–5496. doi: 10.1128/JVI.00171-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77(Pt 1):61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Maidji E, Xiao J, Zheng Z, Pereira L. Human cytomegalovirus glycoprotein B contains autonomous determinants for vectorial targeting to apical membranes of polarized epithelial cells. J. Virol. 1998;72:7374–7386. doi: 10.1128/jvi.72.9.7374-7386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9(3):307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- Wahl SM, McNeely TB, Janoff EN, Shugars D, Worley P, Tucker C, Orenstein JM. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997;3 Suppl 1:S64–S69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170(9):4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- Wang W, Owen SM, Rudolph DL, Cole AM, Hong T, Waring AJ, Lal RB, Lehrer RI. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173(1):515–520. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Quinones-Mateu ME, Lederman MM. Role of human beta-defensins in HIV infection. Adv Dent Res. 2006;19(1):42–48. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- White SH, Wimley WC, Selsted ME. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5(4):521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- Wright A, Yan H, Lamm ME, Huang YT. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology. 2006;356(1–2):165–170. doi: 10.1016/j.virol.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N, Spitalnik SL, Stefano KA, De Micco P, Gonzalez-Scarano F, Fantini J. Interferon-gamma decreases cell surface expression of galactosyl ceramide, the receptor for HIV-1 GP120 on human colonic epithelial cells. Virology. 1994;204(2):550–557. doi: 10.1006/viro.1994.1568. [DOI] [PubMed] [Google Scholar]
- Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113(4):524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O'Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109(1):137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]