Abstract
Previously, we identified a naturally processed and presented measles virus (MV) 19-amino-acid peptide, ASDVETAEGGEIHELLRLQ (MV-P), derived from the phosphoprotein and eluted from the human leukocyte antigen (HLA) class II molecule by using mass spectrometry. We report here the identification of a 14-amino-acid peptide, SAGKVSSTLASELG, derived from the MV nucleoprotein (MV-N) bound to HLA-DRB1*0301. Peripheral blood mononuclear cells (PBMC) from 281 previously vaccinated measles-mumps-rubella II (MMR-II) subjects (HLA discordant) were studied for peptide recognition by T cells. Significant gamma interferon (IFN-γ) responses to MV-P and MV-N peptides were observed in 55.9 and 15.3% of subjects, respectively. MV-P- and MV-N-specific interleukin-4 (IL-4) responses were detected in 19.2 and 23.1%, respectively, of PBMC samples. Peptide-specific cytokine responses and HLA-DRB1 allele associations revealed that, for the MV-P peptide, the allele with the strongest association with both IFN-γ (P = 0.02) and IL-4 (P = 0.03) secretion was DRB1*0301. For MV-N, the allele with the strongest association with IFN-γ secretion was DRB1*1501 (P = 0.04), and the alleles with the strongest associations with IL-4 secretion were DRB1*1103 and DRB1*1303 (P = 0.01). These results indicate that HLA class II MV proteins can be processed, presented, and identified, and the ability to generate cell-mediated immune responses can be demonstrated. This information is promising for new vaccine design strategies with peptide-based vaccines.
Measles is one of the most transmissible diseases affecting humans and remains the leading cause of vaccine-preventable deaths in children worldwide (7). Since the introduction of immunization against measles with live attenuated measles virus (MV) vaccine, measles morbidity and mortality has been reduced worldwide (5). However, despite the availability of an effective vaccine, measles outbreaks occur even in highly vaccinated populations. A total of 20 to 40% of individuals who contracted measles in 1989-to-1991 U.S. outbreaks had previously been immunized against measles (32). Rates of primary and secondary vaccine failure are estimated to be 2 to 10% and <0.25%, respectively (31). In addition, the presence of passively acquired maternal antibody prevents effective immunization of young infants (1). Finally, live MV vaccines cannot be used in the presence of immunosuppressing illnesses or treatments. Hence, further vaccine developments are needed that will effectively circumvent these problems and control and prevent measles outbreaks. The development of a synthetic peptide vaccine based on the stimulatory epitopes from MV proteins may provide an important step to solving these problems (27).
A peptide-based immunization approach against viral pathogens is advantageous because of the lack of genetic material, the relative ease of production, the ability to administer the vaccine to persons with immunosuppressive disorders, and the ability to be quality controlled. Moreover, synthetic peptides are thermostable, biologically safe, and inexpensive to manufacture (9, 35). Synthetic peptides representing specific regions of MV proteins must induce a strong immune response specific to those proteins, and such epitope identification is important for potentially developing immunotherapeutic and diagnostic reagents and for the rational design of new vaccines. However, the large degree of human leukocyte antigen (HLA) polymorphisms, which differentially restrict peptide binding, are a major barrier to achieving this goal (6). Further, identifying immunogenic Th cell epitopes that would be restricted by multiple HLA alleles (i.e., a “promiscuous peptide”) is one of the critical aspects to designing such efficacious peptide-based vaccines.
Peptides derived from MV-processed antigens are usually presented by HLA class I molecules, and only a few such MV-derived class I epitopes have been reported in the literature (14, 48). However, class II HLA molecules, like class I molecules, are able to bind endogenously processed antigens, including nonmembrane MV antigens via the endoplasmic reticulum pathway (26). Studies on HLA class II-restricted presentation of cytoplasmic measles matrix and nucleocapsid antigens to cytotoxic T lymphocytes confirmed that endogenously synthesized MV proteins can be efficiently presented by HLA class II antigens (16, 17). Although the involvement of CD4+ T lymphocytes in the immune response to MV has been well established, naturally processed MV-derived peptides, presented in the context of HLA class II molecules, had not yet been identified (11).
Recently, we reported the identification of a naturally processed peptide, ASDVETAEGGEIHELLRLQ, derived from the MV phosphoprotein (P) and presented by class II HLA-DRB1*0301 molecules on MV-infected B-lymphoblastoid cells (28). This MV-P peptide induced a recall lymphoproliferative response in 17% of HLA-mismatched individuals (n = 95) previously immunized with measles vaccine.
In the present study, we report the discovery of another HLA class II peptide, SAGKVSSTLASELG, derived from the MV nucleoprotein (MV-N). This peptide was also identified from the population of peptides presented by class II HLA-DRB1*0301 molecules. In addition, we describe the results of the ability of these MV P and N peptides to stimulate MV-specific T cells from the blood of previously immunized subjects. Finally, we determine which alleles of the HLA-DRB1 locus are most strongly associated with these HLA class II epitopes.
MATERIALS AND METHODS
Donor cells, cell lines, and virus infection.
Our methods for donor cell preparation and MV infection have been previously described (28). In brief, we generated an Epstein-Barr virus (EBV)-transformed B-cell line from peripheral blood mononuclear cells (PBMC) of an HLA-DRB1 homozygous individual by using the B95-8 strain of EBV (American Type Culture Collection, Manassas, Va.) in RPMI medium containing 1 μg of cyclosporine/ml (25). We obtained a heparinized venous blood (20 U of heparin/ml) sample from a single EBV-seronegative subject (16-year-old female, DRB1*0301, A*1/3, B*8/44, C*7) who had been immunized with two doses of live attenuated MV vaccine (Attenuvax; Merck, West Point, Pa.). The subject had no previous history of MV infection. The circulating MV-specific immunoglobulin G (IgG) antibody titer in the subject's sera was determined by an IgG whole virus-specific enzyme immunoassay (MeasleELISA; BioWhittaker, Walkersville, Md.). The subject was characterized as a MV vaccine responder (enzyme immunoassay MV antibody titer = 2.43 U/ml).
The Edmonston-Enders vaccine strain of MV was cultured in African green monkey kidney cells in Dulbecco modified Eagle medium, supplemented with 5% fetal calf serum (FCS) (virus stocks of 2 × 107 PFU/ml). Subsequently, EBV-B cells were infected with live MV at a multiplicity of infection (MOI) of 1 PFU/cell for 1 h and maintained for 24 to 36 h at 37°C in RPMI 1640 supplemented with 2% FCS (Life Technologies, Gaithersburg, Md.). Equal-sized batches of MV-infected and uninfected cells were washed in phosphate-buffered saline, pelleted, and stored at −80°C. Evidence for the infection of cells was monitored by flow cytometry using purified monoclonal antibody (MAb) specific for MV hemagglutinin protein tagged with fluorescein isothiocyanate (Virostat, Portland, Maine) (24).
Isolation of HLA-DRB1*0301 molecules and HLA-bound peptides.
Our methodological strategy has been previously published (34). We used the same cellular mass of uninfected and MV-infected cells for HLA-DRB1-peptide complex purification. DRB1-bound peptides were isolated from immunoaffinity-purified class II molecules as previously described (18). Briefly, 8-g cell pellets consisting of either infected or uninfected cells were lysed in 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 150 mM NaCl, 20 mM Tris-HCl (pH 8.0), and 1 mM Pefabloc SC (Boehringer Mannheim GmbH, Mannheim, Germany). The lysates were centrifuged at 100,000 × g for 2 h, and the HLA-peptide complexes were immunoprecipitated from the supernatants by using an anti-HLA-DR MAb specific for an HLA-DR monomorphic epitope (L227, IgG1) (20) covalently linked to CNBr-activated Sepharose 4B beads (Sigma-Aldrich Corp., St. Louis, Mo.). The column was washed sequentially with five separate washings, and the HLA-DRB1-peptide complexes were eluted from the affinity column with 0.1% deoxycholic acid and 50 mM glycine (pH 11.5). We neutralized the eluates with 2 M glycine and concentrated in a Centricon-10 (Amicon, Beverly, Mass.) before a second round of precipitation by 14% acetic acid to dissociate any bound peptides from DRB1 molecules. We determined protein concentration by BCA assay (Pierce, Rockford, Ill.). The peptides were concentrated in a spin vacuum to 100-μl aliquots (109 cells) and then stored at −80°C for later analysis by mass spectrometry (MS).
Identification of MV-derived P and N peptides by two-dimensional LC-MS/MS.
The analytical methods used for identification of the MV P peptide have been described in detail (28). Briefly, the complex peptide pool dissociated from HLA class II molecules was separated by two dimensions of automated chromatography: strong cation exchange (SCX) on a 0.3 (inner diameter [i.d.])- by 5-mm column, followed by reversed-phase (RP) nanoscale liquid chromatography (nanoLC) with a 75-μm (i.d.)- by 6-cm (length) PicoFrit column (New Objective, Inc., Woburn, Mass.). Prior to the cation-exchange separation, the peptide mixture was desalted on a 1-mm (i.d.)- by 10-mm (length) RP PeptideTrap column (Michrom BioResources, Inc., Auburn, Calif.). The eluant from the desalting cartridge was concentrated to dryness on a vacuum-centrifuge and reconstituted in SCX mobile phase A (5 mM KH2PO4 [pH 3.0] containing 1% n-propanol and 4% acetonitrile) before peptides were loaded onto the SCX column. The SCX separation was performed by using step elutions of increasing KCl strength in SCX mobile-phase A. As peptides eluted from the SCX column as a function of their positive charge, they were reconcentrated on an RP precolumn within the LC 10-port sampling valve. The precolumn was then washed with mobile phase A from the RP separation (water-acetonitrile-n-propanol-formic acid [98:1:1:0.2, vol/vol/vol/vol]) before the precolumn was placed in line with the nanoscale LC (nano-LC) column for separation in the second dimension by RP nano-LC-tandem MS (MS/MS).
Nano-LC-MS/MS experiments were performed on a quadrupole time-of-flight mass spectrometer (Waters, Milford, Mass.). MS/MS spectra were acquired in an automated data-dependent manner by using survey scans to select doubly, triply, or quadruply charged ions. Argon was used as the target collision gas. Collision energies were automatically chosen as a function of m/z and charge (z). For the MV-N peptide identified in this report, a collision energy of 26 V was used for the doubly charged ion at m/z 653.8 for both the naturally processed peptide and the synthesized peptide.
For increased MS/MS coverage of class II peptides, the m/z range for the survey scan was reduced from m/z 450 to 1,300 to smaller overlapping ranges. This enhanced our ability to identify minor peptides within the reduced m/z range, a technique referred to as gas-phase fractionation (46). Database searching of MS/MS spectra was conducted by using Sequest software (Thermo-Finnigan, San Jose, Calif.). Spectra were searched by using the NCBI database.
Peptide synthesis.
Identified peptides were subsequently synthesized by the Mayo Proteomics Research Center (Rochester, Minn.) by using N-(9-fluorenyl)methoxycarbonyl protection chemistry and carbodiimide-N-hydroxybenzotriazole activation on an MPS 396 multiple peptide synthesizer (Advanced Chemtech, Louisville, Ky.). Each peptide was purified by RP liquid chromatography and was verified by MS and amino acid analysis. The following peptides were synthesized: (i) an MV-derived 14-amino-acid peptide from MV nucleoprotein (MV-N, residues 372 to 385; SAGKVSSTLASELG) and (ii) an MV-derived 19-amino-acid peptide from the MV phosphoprotein (MV-P, residues 179 to 197; ASDVETAEGGEIHELLRLQ).
Study subjects.
Study participants were enrolled as part of a larger stratified random sampling study to assess associations between HLA class I and II genes and the immune response to rubella virus vaccine in healthy, school-age children and young adults in Rochester, Minn. To evaluate the immunogenicity of MV-derived peptides, 281 of these subjects (ages 12 to 18 years) were studied. All enrolled subjects had been previously immunized with two doses of MMR-II vaccine (Merck) containing the further attenuated Edmonston strain of MV (50% tissue culture infective dose of ≥1,000). In addition, all subjects resided in a geographic area where no wild-type MV had circulated in the community during the subjects' lifetimes. The Institutional Review Board of the Mayo Clinic granted approval for the study, and peripheral blood samples were drawn after written informed consent was obtained from each subject and/or guardian.
Molecular HLA typing.
Genomic DNA was extracted from frozen blood samples (5 ml of each) by conventional techniques by using the Pyregene extraction kit (Gentra Systems, Inc., Minneapolis, Minn.). DNA was used for class II HLA-DRB1 allele typing by high-resolution DRB96 sequence-specific primer Unitray typing kits with the entire locus on a single tray (Pel-Freez Clinical Systems, Brown Deer, Wis.) (3). Locus-specific primers were used to amplify the HLA-DRB1 locus. PCR products were separated on 2% agarose gels and stained with ethidium bromide. Any ambiguities were resolved by using the ABI DRB1 sequencing kit (Applied Biosystems, Foster City, Calif.). All PCR amplifications were carried out in a GeneAmp PCR system 9600 (Perkin-Elmer Cetus Instruments). All reactions were run with negative controls, and every 50th PCR was repeated for quality control.
Preparation of PBMC.
PBMC were separated from heparinized venous blood by Ficoll-Hypaque (Sigma) density gradient centrifugation and washed in complete RPMI 1640 medium (Celox Laboratories, Inc., St. Paul, Minn.) supplemented with 2 mM l-glutamine, 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 8% heat-inactivated FCS (Life Technologies). Cells were then counted, resuspended in a freezing medium containing 10% dimethyl sulfoxide, frozen at −80°C, and stored in liquid nitrogen until cultured. No significant differences in cellular viability estimated by trypan blue exclusion were observed between the same PBMC samples obtained before and after their storage in liquid nitrogen.
Measurement of IFN-γ and IL-4 supernatant cytokine responses to MV and synthetic MV peptides.
Cryopreserved PBMC were used to measure cytokine responses to MV-derived peptides. We thawed cryopreserved PBMC at a concentration of 107 cells/ml by a standard protocol. The vials were rapidly thawed in a 37°C water bath and then washed twice with a 10× volume of complete RPMI 1640 medium supplemented with 10% FCS at 700 rpm for 5 min. The final cell pellet was resuspended in complete RPMI medium containing penicillin-streptomycin (100 U/ml) and supplemented with 5% normal human AB sera (Ervin Sciences, Santa Ana, Calif.). For gamma interferon (IFN-γ) determination, thawed PBMC were cultured at a concentration of 2 × 105 in RPMI containing 5% normal human AB sera with or without MV peptides (10 μg/well) and MV (positive control) at an MOI of 0.5 for 6 days. For interleukin-4 (IL-4) determination in cell culture supernatants, we cultured thawed PBMC at a concentration of 4 × 105 in RPMI medium supplemented with 5% normal human AB sera. Cells were cultured in the presence of 2 μg of IL-4 receptor antibody (R&D Systems, Minneapolis, Minn.)/ml (8) with or without synthetic MV-derived peptides (10 μg/well) and MV (positive control) at an MOI of 0.1 for 6 days. Cell culture supernatants were collected in a volume of 150 μl/well for both IFN-γ and IL-4 and were frozen at −80°C until assayed. The culture supernatants were assayed by using a standard enzyme-linked immunosorbent assay (ELISA) protocol (OptiEIA Human IFN-γ and IL-4; Pharmingen, San Diego, Calif.) at a dilution of 1:1 in PBS containing 10% FCS. ELISA plates (Immulon-4; Dynex Technologies, Inc., Chantilly, Va.) were coated with capture IFN-γ or IL-4 MAb and incubated overnight at 4°C. The antibody-coated plates were incubated with diluted supernatant samples for 2 h at room temperature, followed by incubation with biotinylated mouse anti-human IFN-γ or IL-4 conjugated to avidin-horseradish peroxidase for 1 h at room temperature. The absorbance of the product was read by using a microplate reader (Molecular Devices, Sunnyvale, Calif.) at 450 nm. The IFN-γ and IL-4 concentration of test samples was calculated by reference to the standard curve. Unstimulated and MV-stimulated secretion measurements for IFN-γ were performed in triplicate, while all other secretion measurements for both IFN-γ and IL-4 were performed in duplicate. Individual-specific values were then summarized by taking means of the duplicate or triplicate values. Mean background levels of IFN-γ and IL-4 cytokine production in cultures not stimulated with MV peptides or MV was subtracted from the mean antigen-induced responses to produce “corrected” secretion values. Negative corrected values indicate that the unstimulated secretion levels were, on average, higher than the stimulated secretion levels.
Two criteria were established to define positive responses for secreted IFN-γ and IL-4 cytokines by ELISA. First, positive responses were identified if the mean cytokine level in peptide or MV-stimulated replicate cultures exceeded 1.645 standard deviations of the mean of control replicate samples from the same individual, a result akin to detecting a P value of 0.05 by using a one-sided test of hypothesis. Second, the difference between the mean of peptide or MV-stimulated cultures and that of control cultures was determined for each subject. Several cutoff values between 5 and 30 pg/ml were assessed for their ability to stratify subjects into positive and negative groups in accordance with the first statistical criterion described above. Hence, a minimum difference of 20 pg/ml for IFN-γ and 10 pg/ml for IL-4 between a stimulated and a control culture was selected as the optimal threshold to define a positive response. The data were analyzed by SoftMax-Pro (Molecular Devices).
Statistical analysis.
Six outcomes were of primary interest: two sets of in vitro cytokine production (both IFN-γ and IL-4), each induced separately by three stimuli (live MV and the MV-derived MV-N and MV-P peptides). The data were descriptively summarized by using frequencies and percentages for all categorical variables and medians and interquartile ranges for all continuous variables. Wilcoxon signed-rank tests were used to determine whether the centers of the cytokine secretion value distributions differed from zero. Spearman rank correlation coefficients were used to summarize the associations between secretion values, namely, those induced by MV versus those induced by the two MV-derived peptides. Cytokine levels are reported as median values with interquartile range in brackets (25th and 75th percentiles).
Descriptive associations of the continuously distributed cytokine secretion values with HLA-DRB1 alleles were evaluated on an allelic level. Each person contributed two observations to this descriptive analysis: one for each allele. Alleles were grouped by HLA-DR type and summarized by using medians and interquartile ranges. After the descriptive comparisons, associations were more formally evaluated by using linear regression analyses. In contrast to the descriptive comparisons, each subject contributed one observation to the regression analysis, based on his or her genotype. Regression variables were created for each allele and were coded as 0, 1, or 2, according to the number of copies of the allele that a subject carried. Rare alleles, defined as occurring fewer than five times among all subjects, were pooled into a category labeled “other.” Due to data skewing, the original secretion values were replaced with corresponding rank values. Global differences in cytokine secretion levels among all alleles were first carried out by simultaneously including all but one of the allele variables in a multivariate linear regression model. After these global tests, we examined individual allele effects on cytokine secretion levels. This series of tests was performed in the spirit of the Fisher protected least-significant-difference test; individual allele associations were not considered statistically significant in the absence of global significance. Each allele variable was included in a separate univariate linear regression analysis, effectively comparing secretion levels for the allele of interest against all other alleles combined. Two sets of allele variables were analyzed. We first evaluated each distinct observed allele subtype (for instance, we separately evaluated the effects of DRB1*0401, DRB1*0402, DRB1*0404, and DRB1*0407). We then pooled specific subtypes into more general groupings (for instance, we pooled all DR4 alleles into one overall category). All global and univariate regression analyses included the design variable, age, as a covariate.
Subsequent to the linear regression analyses, we assessed the association between categorized cytokine positivity values and DRB1 alleles by using logistic regression analyses. Cut points for IFN-γ and IL-4 positivity were defined as 20 and 10 pg/ml, respectively, as described above. Univariate and multivariate analyses were carried out by using the same general outline as the linear regression analyses. All statistical tests were two-sided, and all analyses were carried out by using the SAS software system (SAS Institute, Inc., Cary, N.C.).
RESULTS
Identification of an MV-derived HLA-DRB1*0301 N peptide by nano-LC-MS/MS.
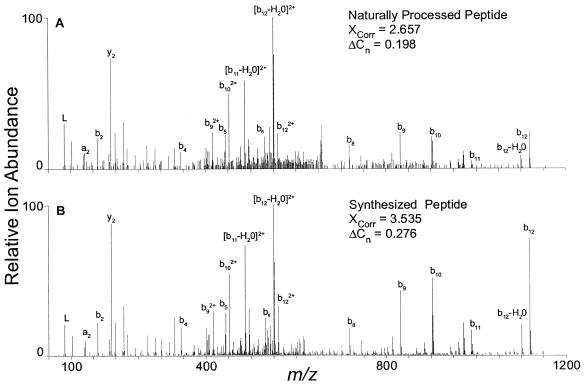
From a data set using a reduced survey scan range of m/z 590 to 720, a peptide, SAGKVSSTLASELG, was the top match by Sequest database searching (XCorr = 2.657, ΔCn = 0.198) of the MS/MS spectrum from a doubly charged precursor ion at m/z 653.82. This sequence is present in the NCBI database as multiple entries annotated as the nucleoprotein or nucleocapsid protein of MV. To confirm this identification, the peptide was synthesized and analyzed by nano-LC-MS/MS. Figure 1A shows the MS/MS spectrum of the naturally processed peptide identified as SAGKVSSTLASELG, whereas Fig. 1B shows the MS/MS spectrum of the synthesized peptide. The top-ranked Sequest database search result for the synthesized authentic peptide was also SAGKVSSTLASELG (XCorr = 3.535, ΔCn = 0.276). Sequest scoring parameters of an XCorr of >2.5 and a ΔCn of >0.1 are commonly used as thresholds for determining the uniqueness of database search results. Thus, the close agreement between fragment ions observed in the two spectra, as well as their discriminating Sequest scores, confirms our identification of the naturally processed doubly charged peptide at m/z 653.82 as originating from the MV nucleoprotein.
FIG. 1.
MS/MS product ion spectra from doubly charged precursor ions at m/z 653.8, a naturally processed HLA class II peptide identified as SAGKVSSTLASELG from the nucleoprotein of MV (A), and products of m/z 653.8 from the synthesized peptide SAGKVSSTLASELG (B). The naturally processed spectrum was generated by gas-phase fractionation in conjunction with two-dimensional LC-MS/MS. The synthesized peptide was analyzed by one-dimensional nano-LC-MS/MS. The Sequest scoring parameters XCorr and ΔCn are shown and are described in the text.
Cytokine responses of vaccinated donors to MV, MV-P, and MV-N peptides.
We examined the ability of these MV-derived peptides to induce in vitro production of cytokines (IFN-γ and IL-4) by the PBMC of 281 healthy subjects previously immunized with MMR-II vaccine. Cytokine secretion results revealed large inter-individual variation among the 281 tested subjects. An overall summary of the frequency and magnitude of the MV and peptide-specific induction of IFN-γ and IL-4 from vaccinated subjects' PBMC is shown in Table 1. Both MV and MV-P peptide were able to induce a recall peptide-specific IFN-γ response (>20 pg/ml) from the PBMC of previously immunized subjects. MV-specific IFN-γ responses were generally higher than MV-P or MV-N peptide-specific IFN-γ responses (P < 0.001). Specifically, MV-, MV-P-, and MV-N-specific IFN-γ responses were detected in 185 (65.8%), 157 (55.9%), and 43 (15.3%) of the 281 subjects, respectively. With regard to HLA-DRB1*0301, we found a marginally significant (P = 0.08) increase in the frequency of the *0301 allele among the subjects who produced IFN-γ in response to the MV-P peptide (12.4%) compared to those with low (<20 pg/ml) MV-P specific IFN-γ levels (8.1%, odds ratio [OR] = 1.7; 95% confidence interval [CI] = 0.93 to 2.99). In contrast, the frequency of *0301 alleles (OR = 0.4; CI = 0.13 to 1.09, P = 0.07) was lower in subjects with significant MV-N-specific IFN-γ levels (4.6%) than in individuals with low levels of IFN-γ to the MV-N peptide (11.5%).
TABLE 1.
MV- and peptide-specific cytokine responsesa in healthy subjects
| Antigen | IFN-γ
|
IL-4
|
||||||
|---|---|---|---|---|---|---|---|---|
| % Response (>20 pg/ml) | Median level (pg/ml) | Interquartile range of response (pg/ml) | Pb | % Response (>10 pg/ml) | Median level (pg/ml) | Interquartile range of response (pg/ml) | Pb | |
| MV | 65.8 | 57.0 | 11.3, 214.7 | <0.0001 | 50.9 | 10.7 | 3.9, 24.9 | <0.0001 |
| MV-P | 55.9 | 28.0 | 1.9, 106.1 | <0.0001 | 19.2 | −0.4 | −6.5, 6.3 | 0.8701 |
| MV-N | 15.3 | 1.9 | −6.6, 12.4 | 0.0039 | 23.1 | 2.8 | −2.8, 9.4 | <0.0001 |
Stimulated cells minus unstimulated cells.
As determined by Wilcoxon signed-rank test, testing whether cytokine responses differ from zero.
Associations of IFN-γ MV secretion levels with those of MV-derived peptides were modest; the observed Spearman correlations were 0.20 and 0.32 for the MV-P and MV-N peptides, respectively. Among the 185 subjects who responded to MV, 110 (59.5%) demonstrated IFN-γ production in response to the MV-P peptide and 39 (21.1%) responded to MV-N peptide, thereby suggesting a higher frequency of MV-P-specific T cells in subjects after measles vaccination. However, among the 157 subjects who responded to the MV-P peptide, only 33 (21%) also produced IFN-γ to MV-N peptide.
Comparatively, MV-specific IL-4 responses were higher than for MV-N peptide-specific IL-4 responses (P < 0.001). MV-P peptide was able to induce only low levels of IL-4 production from the PBMC of immunized subjects. Using a cutoff value for significant cytokine responses of >10 pg/ml, MV-specific IL-4 responses were detected in 50.9% (143 of 281) of the subjects. In contrast, MV-P-specific IL-4 responses were detected in only 19.2% (54 of 281) of the subjects. Likewise, MV-N-specific IL-4 responses were detected in a total of 23.1% (65 of 281) of the subjects. We found no association between the *0301 allele and MV-P-specific IL-4 secretion level. With regard to the MV-N peptide, we found a marginally significant (P = 0.08) increase in the frequency of the *0301 allele among subjects who produced significant IL-4 levels to the MV-N peptide (14.6%) compared to those with low levels of (<10 pg/ml) MV-N-specific IL-4 secretion (9.3%, OR = 1.7; CI = 0.94 to 3.14).
Spearman correlations of IL-4 MV secretion levels with those for MV-N and MV-P peptides were 0.12 and 0.28, respectively. Among the 143 subjects who demonstrated IL-4 production from MV-stimulated PBMC, 33 (23.1%) subjects also responded to MV-P peptide, and 41 (28.7%) subjects responded to the MV-N peptide. Interestingly, of the 54 subjects who responded to the MV-P peptide, MV-N-specific IL-4 responses were also detected in half of these subjects. These data suggest that both MV-derived epitopes exhibited the capacity to stimulate MV-specific T cells; however, the MV-N peptide was less stimulatory than the MV-P peptide.
Expression of HLA-DR alleles in study subjects previously immunized with MV.
All 281 subjects were HLA-typed, and associations between recall peptide-specific cytokine responses and HLA-DR type and MV-P and MV-N peptides were assessed. The most prevalent alleles in the study population (Table 2) were expressed at frequencies similar to the HLA-DRB1 frequencies published elsewhere (6, 45).
TABLE 2.
Phenotypic frequency of the 281 study subjectsa
| HLA-DRB1 locus | Allele | No. of alleles | Phenotype frequency (%)
|
|
|---|---|---|---|---|
| % Allele | % HLA-DRB1 locus | |||
| DR1 | DRB1*0101 | 46 | 8.19 | 9.08 |
| DRB1*0102 | 5 | 0.89 | ||
| DR103 | DRB1*0103 | 6 | 1.07 | 1.07 |
| DR2 | DRB1*1501 | 75 | 13.35 | 14.25 |
| DRB1*1502 | 2 | 0.36 | ||
| DRB1*1503 | 2 | 0.36 | ||
| DRB1*1601 | 1 | 0.18 | ||
| DR3 | DRB1*0301 | 59 | 10.50 | 11.93 |
| DRB1*03011 | 7 | 1.25 | ||
| DRB1*0302 | 1 | 0.18 | ||
| DR4 | DRB1*0401 | 49 | 8.72 | 17.09 |
| DRB1*0402 | 7 | 1.25 | ||
| DRB1*0403 | 4 | 0.71 | ||
| DRB1*0404 | 25 | 4.45 | ||
| DRB1*0405 | 4 | 0.71 | ||
| DRB1*0407 | 5 | 0.89 | ||
| DRB1*0408 | 2 | 0.36 | ||
| DR5 | DRB1*1101 | 24 | 4.27 | 12.11 |
| DRB1*1102 | 1 | 0.18 | ||
| DRB1*1103 | 5 | 0.89 | ||
| DRB1*1104 | 16 | 2.85 | ||
| DRB1*1106 | 1 | 0.18 | ||
| DRB1*1111 | 1 | 0.18 | ||
| DRB1*1121 | 1 | 0.18 | ||
| DRB1*1201 | 15 | 2.67 | ||
| DRB1*1202 | 4 | 0.71 | ||
| DR6 | DRB1*1301 | 43 | 7.65 | 17.99 |
| DRB1*1302 | 29 | 5.16 | ||
| DRB1*1303 | 7 | 1.25 | ||
| DRB1*1305 | 2 | 0.36 | ||
| DRB1*1310 | 1 | 0.18 | ||
| DRB1*1315 | 1 | 0.18 | ||
| DRB1*1401 | 15 | 2.67 | ||
| DRB1*1406 | 1 | 0.18 | ||
| DRB1*1410 | 1 | 0.18 | ||
| DRB1*1424 | 1 | 0.18 | ||
| DR7 | DRB1*0701 | 56 | 9.96 | 9.96 |
| DR8 | DRB1*0801 | 17 | 3.02 | 3.74 |
| DRB1*0802 | 1 | 0.18 | ||
| DRB1*0803 | 2 | 0.36 | ||
| DRB1*0804 | 1 | 0.18 | ||
| DR9 | DRB1*0901 | 10 | 1.78 | 1.78 |
| DR10 | DRB1*1001 | 5 | 0.89 | 0.89 |
| DR12 | DRB1*1208 | 1 | 0.18 | 0.18 |
Each subject is represented twice—once for each allele.
Associations between HLA-DRB1 alleles and MV-derived HLA class II peptides.
Analyses were performed for each of the 22 observed HLA-DRB1 alleles with allele frequencies greater than five. Tables 3, 4, and 5 present the results of the linear regression analysis of association with MV, MV-P, and MV-N peptides and individual comparison of HLA-DRB1 alleles across the IFN-γ and IL-4 secretion status. Table 3 relates the HLA-DRB1 allelic associations with recall MV (positive control)-specific IFN-γ and IL-4 cytokine responses. The global test reveals a significant association between MV-specific IFN-γ secretion and the HLA-DRB1 locus (P = 0.005). Allelic subtyping of HLA-DRB1 revealed that four subtypes, *0301 (P = 0.02), *0701 (P = 0.01), *1501 (P = 0.004), and *0801 (P = 0.05), which share largely overlapping peptide-binding repertoires (45), were the predominant alleles and were significantly associated with MV-specific IFN-γ response in previously immunized study subjects. The less-common subtypes, such as *0102 (P = 0.08), *0404 (P = 0.07), and *1103 (P = 0.09), showed a trend toward IFN-γ response, although these were not statistically significant. In contrast, the global test for association failed to find a statistically significant association with MV-specific IL-4 cytokine responses (Table 3). Examining HLA-DRB1 alleles individually, we found suggestive associations with alleles *0103 (P = 0.03), *0701 (P = 0.02), and *1303 (P = 0.04); however, these associations should be interpreted with caution due to the nonsignificance of the global test.
TABLE 3.
HLA-DRB1 allelic associations with MV-specific cytokinea responses
| HLA-DRB1 allele | No. of alleles | IFN-γ
|
IL-4
|
||
|---|---|---|---|---|---|
| Median secretion value (Q1, Q3) (pg/ml) | Pb | Median secretion value (Q1, Q3) (pg/ml) | Pb | ||
| DR1 | 51 | 0.51 | 0.21 | ||
| *0101 | 46 | 72.6 (15.7, 192.7) | 0.93 | 15.8 (6.6, 25.3) | 0.41 |
| *0102 | 5 | 281.1 (66.9, 565.7) | 0.08 | 18.9 (17.7, 33.1) | 0.16 |
| DR103 | 6 | 0.38 | 0.03 | ||
| *0103 | 6 | 17.5 (−1.3, 124.3) | 0.38 | −0.7 (−4.9, 7.3) | 0.03 |
| DR2 | 80 | 0.003 | 0.69 | ||
| *1501 | 75 | 120.8 (36.9, 325.8) | 0.004 | 15.5 (4.3, 25.3) | 0.47 |
| DR3 | 67 | 0.02 | 0.80 | ||
| *0301 | 59 | 18.4 (3.2, 110.6) | 0.02 | 8.6 (5.4, 24.5) | 0.96 |
| *003011 | 7 | 18.6 (8.9, 25.9) | 0.42 | 7.0 (−3.4, 14.2) | 0.46 |
| DR4 | 96 | 0.49 | 0.30 | ||
| *0401 | 49 | 44.0 (6.6, 118.0) | 0.13 | 9.0 (2.8, 20.0) | 0.41 |
| *0402 | 7 | 58.4 (−19.2, 187.2) | 0.60 | 8.7 (4.6, 83.1) | 0.61 |
| *0404 | 25 | 123.5 (18.6, 343.1) | 0.07 | 11.1 (4.7, 21.7) | 0.76 |
| *0407 | 5 | 188.3 (66.8, 565.7) | 0.32 | 40.7 (8.4, 68.4) | 0.30 |
| DR5 | 68 | 0.14 | 0.21 | ||
| *1101 | 24 | 88.4 (12.6, 333.1) | 0.54 | 13.4 (6.3, 42.7) | 0.16 |
| *1103 | 5 | 188.2 (114.8, 347.4) | 0.09 | 21.4 (5.9, 22.6) | 0.78 |
| *1104 | 16 | 111.7 (31.2, 637.6) | 0.11 | 17.2 (8.4, 26.6) | 0.28 |
| *1201 | 15 | 44.1 (8.4, 119.1) | 0.89 | 13.6 (4.7, 24.3) | 0.56 |
| DR6 | 101 | 0.44 | 0.46 | ||
| *1301 | 43 | 111.5 (12.6, 306.7) | 0.31 | 14.2 (4.5, 30.5) | 0.25 |
| *1302 | 29 | 45.1 (3.6, 301.4) | 0.50 | 8.1 (1.5, 18.5) | 0.26 |
| *1303 | 7 | 129.6 (13.6, 485.3) | 0.36 | 1.8 (−0.4, 7.3) | 0.04 |
| *1401 | 15 | 67.1 (36.9, 172.6) | 0.71 | 9.6 (0.6, 26.9) | 0.56 |
| DR7 | 56 | 0.01 | 0.02 | ||
| *0701 | 56 | 33.5 (2.8, 113.0) | 0.01 | 5.6 (0.9, 22.5) | 0.02 |
| DR8 | 21 | 0.04 | 0.46 | ||
| *0801 | 17 | 10.2 (2.5, 41.1) | 0.05 | 11.8 (4.4, 33.5) | 0.49 |
| DR9 | 10 | 0.40 | 0.68 | ||
| *0901 | 10 | 26.8 (2.8, 53.4) | 0.40 | 15.7 (0.6, 26.7) | 0.68 |
| DR10 | 5 | 0.26 | 0.27 | ||
| *1001 | 5 | 47.3 (3.4, 96.6) | 0.26 | 6.6 (0.1, 9.0) | 0.27 |
| All other allelesc | 36 | 53.4 (29.8, 233.9) | 0.36 | 11.1 (−0.3, 41.0) | 0.76 |
| Overall | 281 | 57.0 | 10.7 | ||
That is, the mean value of antigen-stimulated cells minus the mean value of control cells. Q1 and Q3 represent the first and third quartiles, respectively.
As determined by linear regression analysis, accounting for the design variable age. Genotypes were modeled as ordinal variables, with values ranging from 0 to 2, reflecting the number of copies possessed by an individual. Due to data skewing, all secretion values were rank transformed. The P values compare the genotype of interest to all of the other genotypes combined. The global P values for IFN-γ and IL-4 were 0.005 and 0.21, respectively.
“Other” includes the following DRB1 alleles: *0302 (n = 1), *0403 (n = 4), *0405 (n = 4), *0408 (n = 2), *0802 (n = 1), *0803 (n = 2), *0804 (n = 1), *1102 (n = 1), *1106 (n = 1), *1111 (n = 1), *1121 (n = 1), *1202 (n = 4), *1208 (n = 1), *1305 (n = 2), *1310 (n = 1), *1315 (n = 1), *1406 (n = 1), *1410 (n = 1), *1424 (n = 1), *1502 (n = 2), *1503 (n = 2), and *1601 (n = 1).
TABLE 4.
HLA-DRB1 allelic associations with naturally processed MV-derived P peptide-specific cytokinea responses
| HLA-DRB1 allele | No. of alleles | IFN-γ
|
IL-4
|
||
|---|---|---|---|---|---|
| Median secretion value (Q1, Q3) (pg/ml) | Pb | Median secretion value (Q1, Q3) (pg/ml) | Pb | ||
| DR1 | 51 | 0.15 | 0.45 | ||
| *0101 | 46 | 51.2 (6.6, 132.2) | 0.08 | 1.1 (−4.7, 7.5) | 0.61 |
| *0102 | 5 | 17.6 (6.4, 25.5) | 0.43 | 5.6 (2.8, 10.0) | 0.42 |
| DR103 | 6 | 0.07 | 0.37 | ||
| *0103 | 6 | 116.2 (63.2, 659.6) | 0.07 | −1.5 (−3.9, 0.3) | 0.37 |
| DR2 | 80 | 0.99 | 0.52 | ||
| *1501 | 75 | 32.8 (−3.0, 131.6) | 0.97 | −0.7 (−9.0, 6.0) | 0.70 |
| DR3 | 67 | 0.09 | 0.05 | ||
| *0301 | 59 | 40.8 (11.9, 121.2) | 0.02 | 3.5 (−3.6, 11.3) | 0.03 |
| *03011 | 7 | 18.7 (1.8, 46.2) | 0.33 | 0.2 (−1.8, 2.6) | 0.96 |
| DR4 | 96 | 0.56 | 0.45 | ||
| *0401 | 49 | 33.5 (2.7, 75.7) | 0.50 | 0.9 (−4.8, 9.5) | 0.51 |
| *0402 | 7 | 0.1 (−6.7, 107.8) | 0.48 | 3.8 (−2.5, 26.1) | 0.20 |
| *0404 | 25 | 52.2 (14.6, 128.1) | 0.06 | 0.3 (−6.4, 6.4) | 0.99 |
| *0407 | 5 | 8.6 (6.0, 16.2) | 0.32 | −2.1 (−6.3, 10.0) | 0.92 |
| DR5 | 68 | 0.12 | 0.40 | ||
| *1101 | 24 | 48.5 (−3.1, 111.4) | 0.94 | −3.7 (−9.6, 7.5) | 0.29 |
| *1103 | 5 | 65.3 (37.3, 95.7) | 0.50 | −3.5 (−4.6, 9.3) | 0.99 |
| *1104 | 16 | 15.4 (−3.8, 88.3) | 0.28 | −1.3 (−5.7, 1.7) | 0.43 |
| *1201 | 15 | 14.6 (−11.2, 65.0) | 0.21 | −0.4 (−3.4, 3.5) | 0.69 |
| DR6 | 101 | 0.48 | 0.06 | ||
| *1301 | 43 | 27.4 (−10.0, 75.7) | 0.10 | −3.0 (−8.7, 2.7) | 0.15 |
| *1302 | 29 | 14.2 (−0.7, 142.1) | 0.89 | 1.2 (−7.4, 9.6) | 0.71 |
| *1303 | 7 | 63.9 (−11.7, 290.1) | 0.79 | −4.2 (−18.4, 0.2) | 0.09 |
| *1401 | 15 | 22.5 (11.5, 125.0) | 0.59 | −0.8 (−7.2, 6.0) | 0.75 |
| DR7 | 56 | 0.21 | 0.53 | ||
| *0701 | 56 | 18.6 (−3.1, 85.1) | 0.21 | −0.6 (−7.2, 12.4) | 0.53 |
| DR8 | 21 | 0.45 | 0.74 | ||
| *0801 | 17 | 27.2 (3.3, 32.4) | 0.25 | −1.4 (−8.0, 6.1) | 0.69 |
| DR9 | 10 | 0.86 | 0.77 | ||
| *0901 | 10 | 19.9 (4.6, 121.8) | 0.86 | −0.9 (−4.1, 16.9) | 0.77 |
| DR10 | 5 | 0.50 | 0.85 | ||
| *1001 | 5 | 46.9 (−9.9, 54.4) | 0.50 | 0.9 (−0.9, 2.8) | 0.85 |
| All other allelesc | 36 | 26.7 (9.9, 98.2) | 0.59 | −1.3 (−7.9, 1.5) | 0.17 |
| Overall | 281 | 28.0 | −0.4 | ||
See Table 3, footnote a.
As determined by linear regression analysis, accounting for the design variable age. Genotypes were modeled as ordinal variables, with values ranging from 0 to 2, reflecting the number of copies possessed by an individual. Due to data skewing, all secretion values were rank transformed. The P values compare the genotype of interest to all of the other genotypes combined. The global P values for IFN-γ and IL-4 were 0.20 and 0.746, respectively.
See Table 3, footnote c.
TABLE 5.
HLA-DRB1 allelic associations with naturally processed MV-derived N peptide-specific cytokinea responses
| HLA-DRB1 allele | No. of alleles | IFN-γ
|
IL-4
|
||
|---|---|---|---|---|---|
| Median secretion value (Q1, Q3) (pg/ml) | Pb | Median secretion value (Q1, Q3) (pg/ml) | Pb | ||
| DR1 | 51 | 0.87 | 0.94 | ||
| *0101 | 46 | −1.8 (−6.1, 11.6) | 0.67 | 4.3 (−1.6, 8.9) | 0.94 |
| *0102 | 5 | 7.6 (1.8, 8.4) | 0.45 | 4.6 (−0.7, 4.6) | 0.66 |
| DR103 | 6 | 0.97 | 0.36 | ||
| *0103 | 6 | 2.4 (−12.6, 33.6) | 0.97 | 1.3 (−1.7, 5.5) | 0.36 |
| DR2 | 80 | 0.36 | 0.59 | ||
| *1501 | 75 | 4.1 (−5.1, 18.8) | 0.04 | 3.2 (−3.5, 11.1) | 0.84 |
| DR3 | 67 | 0.37 | 0.36 | ||
| *0301 | 59 | 1.8 (−5.8, 6.2) | 0.20 | 2.8 (−2.8, 14.4) | 0.26 |
| *03011 | 7 | 9.1 (4.5, 13.0) | 0.45 | 1.7 (−1.0, 2.2) | 0.71 |
| DR4 | 96 | 0.76 | 0.89 | ||
| *0401 | 49 | −0.7 (−9.1, 9.5) | 0.12 | 4.3 (0.4, 9.7) | 0.15 |
| *0402 | 7 | 10.6 (−2.9, 46.6) | 0.27 | −1.0 (−4.6, 7.0) | 0.54 |
| *0404 | 25 | 2.8 (−8.2, 9.7) | 0.81 | 0.0 (−4.2, 6.8) | 0.48 |
| *0407 | 5 | 8.4 (8.2, 12.8) | 0.15 | 4.6 (4.0, 13.7) | 0.50 |
| DR5 | 68 | 0.13 | 0.03 | ||
| *1101 | 24 | −4.4 (−11.0, 16.2) | 0.31 | 4.4 (−0.1, 11.9) | 0.51 |
| *1103 | 5 | 3.0 (−3.8, 6.7) | 0.87 | 15.5 (8.8, 17.5) | 0.01 |
| *1104 | 16 | 3.1 (−5.9, 25.6) | 0.68 | 4.4 (0.1, 11.2) | 0.43 |
| *1201 | 15 | −4.5 (−11.1, 7.5) | 0.07 | 5.4 (1.5, 15.9) | 0.20 |
| DR6 | 101 | 0.56 | 0.20 | ||
| *1301 | 43 | 2.2 (−5.3, 13.0) | 0.55 | 3.6 (−3.2, 6.7) | 0.71 |
| *1302 | 29 | −1.9 (−10.5, 13.0) | 0.62 | 2.2 (−3.6, 6.4) | 0.81 |
| *1303 | 7 | 13.1 (−9.4, 37.5) | 0.19 | −12.9 (−19.2, 1.8) | 0.01 |
| *1401 | 15 | 0.2 (−10.1, 10.2) | 0.47 | 3.7 (−5.6, 12.7) | 0.95 |
| DR7 | 56 | 0.23 | 0.71 | ||
| *0701 | 56 | −1.1 (−7.8, 10.3) | 0.23 | 1.7 (−3.0, 10.2) | 0.71 |
| DR8 | 21 | 0.62 | 0.84 | ||
| *0801 | 17 | 4.1 (−7.9, 9.5) | 0.89 | 1.7 (0.1, 8.2) | 0.80 |
| DR9 | 10 | 0.43 | 0.29 | ||
| *0901 | 10 | 3.1 (0.2, 14.8) | 0.43 | −1.6 (−7.0, 17.6) | 0.29 |
| DR10 | 5 | 0.14 | 0.90 | ||
| *1001 | 5 | −1.8 (−7.1, −1.7) | 0.14 | 2.1 (0.6, 5.7) | 0.90 |
| All other allelesc | 36 | 4.1 (0.2, 18.6) | 0.02 | −0.5 (−6.0, 8.4) | 0.15 |
| Overall | 281 | 1.9 | 2.8 | ||
See Table 3, footnote a.
As determined by linear regression analysis, accounting for the design variable age. Genotypes were modeled as ordinal variables, with values ranging from 0 to 2, reflecting the number of copies possessed by an individual. Due to data skewness, all secretion values were rank transformed. The P values compare the genotype of interest to all of the other genotypes combined. The global P values for IFN-γ and IL-4 were 0.22 and 0.37, respectively.
See Table 3, footnote c.
MV-derived peptide (MV-P and MV-N)-specific cytokine responses and HLA-DRB1 allele associations are summarized in Tables 4 and 5. Global tests revealed no significant associations of HLA-DRB1 alleles with peptide-specific cytokine levels. However, univariate analyses revealed intriguing results. For the MV-P peptide, the allele with the strongest association with both IFN-γ (P = 0.02) and IL-4 (P = 0.03) responses was DRB1*0301 (Table 4), confirming the DRB1*0301 origin of this class II MV-derived peptide and suggesting that MV-P contains both Th1 and Th2 cell epitopes. In examining alleles individually for recall IFN-γ MV-P responses, we found that only alleles *0101, *0103, and *0404 provide evidence suggestive of an association. There were no strong associations (except for the *0301 allele) between the MV-P-specific IL-4 levels and the frequency of other DRB1 alleles.
For the MV-N peptide, the allele providing the strongest evidence of an association with IFN-γ secretion was DRB1*1501 (P = 0.04), and the alleles providing the strongest evidence of an association with IL-4 secretion were DRB1*1103 and DRB1*1303 (P = 0.01), respectively, suggesting that MV-N is a promiscuous T-cell epitope (Table 5). Although the global tests for the association of MV-N- and MV-P-specific cytokine responses and the DRB1 locus were not statistically significant, allele-specific analyses provide some hints for possible associations that would require confirmation in larger studies.
Sensitivity analyses.
All primary comparisons of cytokine response and DRB1 alleles use continuously distributed cytokine secretion values. We explored the categorization of subjects into positive versus negative responses for both IFN-γ (positivity cut point of 20 pg/ml) and IL-4 (positivity cut point of 10 pg/ml) by using logistical regression analysis. The results were very similar to results obtained by linear regression analysis (data not shown).
Cytokine secretion was defined as the mean response of stimulated cells (measured either in duplicate or triplicate) minus the mean response of unstimulated cells (also measured either in duplicate or triplicate). We were concerned that simply taking mean values would fail to account for inherent variability observed within an individual. Thus, secondary models were fit by using repeated-measures analysis-of-variance techniques that accounted for intrasubject variability. The P values produced by these methods were similar to those presented in Tables 3 to 5.
DISCUSSION
The characterization of highly stimulatory Th epitopes generated from foreign pathogens has traditionally been used to better understand the requirements for a protective immune response (47). However, the isolation and identification of naturally processed and presented pathogen-derived antigenic peptides have historically been extremely difficult. In our study we identified a new class II HLA-DRB1*0301 MV-specific epitope, the 14-amino-acid SAGKVSSTLASELG, which is encoded by the MV N gene, by using nano-LC-MS/MS methods. In addition, we demonstrated that an HLA class II-derived MV-N peptide and a previously identified 19-amino-acid ASDVETAEGGEIHELLRLQ peptide derived from MV P protein led to recall cytokine responses in immune individuals in vitro. In addition, we have shown that these peptides can be recognized by human CD4+ T cells in association with different HLA-DRB1 alleles.
MV-derived immunogenic peptides are generated in extremely low levels, and experimental data have shown the importance of peptide abundance in the development of an immune response (40, 48, 49). Nevertheless, these low levels of class II peptides are sufficient to elicit an effective CD4+ T-cell response against foreign peptides (47). We demonstrated the biologic functional significance of the MV-derived peptides that we identified by directly comparing specific T-cell cytokine responses induced by these peptides in HLA-DRB1*0301-positive and HLA-DRB1*0301-negative (HLA discordant) healthy subjects who had been previously immunized against measles during childhood. The immunologic relevance of the MV-P and MV-N peptides was established by performing lymphocyte stimulation of each subject's PBMC in vitro. IFN-γ was studied because it may be considered a signature marker of Th1-type immune responses and because IFN-γ plays a significant role in the control and resolution of MV infection (10, 41). IL-4 cytokine was studied as a signature marker of Th2-type immune responses and because the production of IL-4 by CD4+ T lymphocytes is essential for the development of MV-specific antibody production (21, 50). We demonstrated that PBMC required only one round of peptide stimulation in vitro to produce effector cytokines, such as IFN-γ and IL-4, suggesting that these MV-P and MV-N peptides were recognized by HLA class II-restricted memory T cells in healthy subjects previously immunized against MV. We detected IFN-γ and IL-4 responses to single MV-P and MV-N epitopes in PBMC samples. These recall cytokine responses elicited by MV and MV-derived peptides were antigen specific because only very low levels of cytokine production were detected in the absence of MV or peptide stimulation. Furthermore, these responses were induced in a setting of subjects previously vaccinated against measles because MV- or MV peptide-specific IFN-γ and IL-4 responses theoretically could not be generated from naive subjects. In addition, it is important to realize that these two peptides, presented in vivo, failed to induce recall cytokine immune responses in some of the human subjects. Because these peptides were eluted from an HLA-DRB1*03 “nonresponder” allele, it would be surprising if a large percentage of HLA discordant subjects could respond to these peptides, especially since these peptides are not universal degenerate peptides that bind to all HLA-DR molecules.
The ability of individual peptides to bind to multiple HLA-DRB1 alleles and be presented to DR-restricted T cells is defined as promiscuous T-cell recognition and has been previously described for some antigenic epitopes such as influenza virus hemagglutinin-derived peptide, tetanus toxoid, and T-cell epitopes from malarial Plasmodium falciparum (4, 6, 29, 36, 39, 44). A promiscuous T-cell epitope from the MV F protein (residues 288 to 302) that binds to several isotypic and allotypic forms of human HLA class II molecules has also been described (19, 30).
Since polymorphic residues of HLA-DRB1 molecules are distributed within the peptide-binding grooves, different DR molecules are able to bind peptides with different structural motifs, and this contributes to the HLA-linked polymorphism of immune responses or susceptibility to immunity-related diseases (23). In the current study we tested naturally processed and presented MV peptides bound to HLA-DRB1*0301, which is one of the HLA molecules associated with low levels of MV antibody after immunization (33). HLA-DRB1*03 molecules are not highly polymorphic and have been considered minor antigens (12). The HLA-DRB1*03 primary amino acid sequence motif is characterized by four conserved anchor positions (1, 4, 6, 9) similar to those found for DRB1*01 and DRB1*05 motifs (22). Sidney et al. (43) report that DRB1*01, DRB1*03, and DRB1*04 molecules recognize a structural motif for binding peptides distinct from the one recognized by most HLA-DRB1 alleles; however, relatively few immune responses in humans have been demonstrated to be HLA allele specific (13, 23).
The present study demonstrated that an MV-N epitope is recognized by memory T cells in association with several class II DRB1 molecules. For the MV-P peptide, the allele with the strongest association with IFN-γ and IL-4 responses was DRB1*0301. Further peptide-binding studies with purified human HLA-DRB1 molecules containing specific HLA-DRB1 binding motifs could fully address the question of whether MV-P is restricted via HLA-DRB1*0301. Because HLA-DRB1*03 alleles are linked to DRB1*01 alleles, this linkage disequilibrium with DRB1*01 could contribute to findings associated with DRB1*0301 (12). For the MV-N peptide, the alleles providing strongest evidence of an association with IFN-γ and IL-4 secretion were DRB1*1501 and DRB1*1103/*1303, respectively. These results might imply that naturally processed, single MV peptides are capable of inducing cytokine T-lymphocyte responses in individuals of several class II HLA-DRB1 types and that an active T-cell repertoire exists for these two epitopes.
A striking finding was a significant association of the *0301 allele with MV- and MV-P peptide-specific IFN-γ and IL-4 responses. We previously reported the role of class II HLA-DRB1*03 molecules in a measles vaccine virus antibody response (I. G. Ovsyannikova, Y. Sohni, R. M. Jacobson, R. Vierkant, D. Schaid, S. V. Pankratz, S. J. Jacobsen, and G. A. Poland, Keystone Symp., abstr. 145, 2000). Thus, the present results confirm this observation regarding the important role of class II HLA-DRB1*03 antigens in MV-induced immune responses.
Our findings are in concordance with other reports demonstrating that HLA-DRB1 promiscuous T-cell epitopes from pathogens could be restricted by multiple HLA class II alleles. For example, Hickman et al. (15) demonstrated that synthetic N peptides (20mers), based on the predicted amino acid sequences of the Edmonston strain of MV, were recognized by ∼70% of all tested donors. We confirmed this observation, supporting this earlier report that MV N peptides may be broadly recognized within an HLA-DRB1 diverse population (15). Importantly, identified naturally processed and presented MV-N peptide (residues 372 to 385) contains the amino acid sequence of the published MV predicted N peptide (residues 367 to 386) that induced significant proliferative responses (stimulation indices of ≥3) in approximately 67% of vaccinated and 100% of naturally infected donors (15).
In the present study, we used live attenuated plaque-purified MV (Edmonston-Enders vaccine strain) cultured in Vero cells to infect HLA-DRB1*0301 homozygous EBV-B cells and to isolate the HLA-bound peptides. MV-derived N and P peptide sequences were obtained from the protein sequences listed in the public NCBI database. Because the sequences of the Edmonston-derived vaccine strains are quite similar to the sequences of a low-passage seed of the wild-type Edmonston virus (38), it is likely that this degree of conservation between MV strains plays a positive role in the peptide identification. MV P, N, and F proteins were found to be antigenically more stable between strains than H and M proteins (42), and the phosphoprotein-binding sites are conserved between vaccine and wild-type MV (2). Rota et al. (38) demonstrated that sequences of H, F, and N coding genes were nearly identical to the H, F, and N sequences of wild-type Edmonston virus; however, genetic variations in the H and F genes were described within circulating wild-type MV relative to the vaccine strain Moraten (37). This suggests that the class II peptides identified here may be potentially incorporated in a vaccine designed to protect humans against wild-type MV infection.
There are several limitations to our study. First, the sample size is only moderate. Despite this, the results of our study demonstrate that MV-derived peptides were recognized in association with different HLA-DRB1 molecules and elicited cytokine responses in vitro in individuals expressing DRB1-encoded molecules. Second, the lack of racial diversity (93% of our subjects were Caucasian) does not allow us to project population coverage by these MV epitopes. Finally, further studies with larger population sample sizes to determine the kinetics of HLA binding capacity and HLA restriction of MV-derived peptides may help assess the role of HLA class II pathogen-derived peptides in antigen-directed immune responses.
In summary, we have described the direct elution and identification of a naturally processed peptide, SAGKVSSTLASELG, from the MV nucleoprotein through the HLA class II pathway. This peptide (MV-N) was identified by two-dimensional nano-LC-MS/MS by using gas-phase fractionation, which enhanced our ability to acquire MS/MS spectra of low-abundance peptides. These data, in conjunction with a previous report describing the identification of the peptide ASDVETAEGGEIHELLRLQ (MV-P) from the MV phosphoprotein, provide direct evidence that MV-processed proteins can be presented by class II HLA-DRB1 molecules. We have further established that both peptides are immunogenic, as assessed by their ability to stimulate IFN-γ and IL-4 cytokine responses from the PBMC of immune individuals. We observed that these peptides were recognized by HLA class II-restricted memory T cells in healthy subjects immunized against MV in association with different HLA-DRB1 alleles. These results are promising and provide experimental support for the development of novel immunization strategies with peptide-based vaccines against MV and other viral infections.
Acknowledgments
We thank the parents and children who participated in this study. We thank Diana Ayerhart for help in preparing the figures. We acknowledge the efforts of the fellows, nurses, and students from the Mayo Vaccine Research Group. We thank Rawleigh C. Howe, Neelam Dhiman, and Jenna E. Ryan for performing cell cultures and ELISAs and Nathan J. Easler and V. Shane Pankratz for assistance with statistical analysis. We gratefully acknowledge Kim S. Zabel for editorial assistance in preparing the manuscript.
This study was supported by NIH grants AI53592, AI33144, and AI48793.
REFERENCES
- 1.Albrecht, P., F. A. Ennis, E. J. Saltzman, and S. Krugman. 1977. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91:715-718. [DOI] [PubMed] [Google Scholar]
- 2.Bankamp, B., W. J. Bellini, and P. A. Rota. 1999. Comparison of L proteins of vaccine and wild-type measles viruses. J. Gen. Virol. 80:1617-1625. [DOI] [PubMed] [Google Scholar]
- 3.Büchler, T., D. Gallardo, M. Rodríguez-Luaces, J. M. Pujal, and A. Granena. 2002. Frequency of HLA-DPB1 disparities detected by reference strand-mediated conformation analysis in HLA-A, -B, and -DRB1 matched siblings. Hum. Immunol. 63:139-142. [DOI] [PubMed] [Google Scholar]
- 4.Chicz, R. M., R. G. Urban, J. C. Gorga, D. A. A. Vignali, W. S. Lane, and J. L. Strominger. 1993. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 178:27-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutts, F. T., and L. E. Markowitz. 1994. Successes and failures in measles control. J. Infect. Dis. 170:S32-S41. [DOI] [PubMed] [Google Scholar]
- 6.Doolan, D. L., S. Southwood, R. Chesnut, E. Appella, E. Gomez, A. Richards, Y. I. Higashimoto, A. Maewal, J. Sidney, R. A. Gramzinski, C. Mason, D. Koech, S. L. Hoffman, and A. Sette. 2000. HLA-DR promiscuous T-cell epitopes from Plasmodium falciparum ore-erythrocytic-state antigens restricted by multiple HLA class II alleles. J. Immunol. 165:1123-1137. [DOI] [PubMed] [Google Scholar]
- 7.Duke, T., and C. S. Mgone. 2003. Measles: not just another viral exanthem. Lancet 361:763-773. [DOI] [PubMed] [Google Scholar]
- 8.Ekerfelt, C., J. Ernerudh, and M. C. Jenmalm. 2002. Detection of spontaneous and antigen-induced human interleukin-4 responses in vitro: comparison of ELISPOT, a novel ELISA and a real-time RT-PCR. J. Immunol. Methods 260:55-67. [DOI] [PubMed] [Google Scholar]
- 9.El Kasmi, K. C., S. Fillon, D. M. Theisen, H. Hartter, N. H. C. Brons, and C. P. Muller. 2000. Neutralization of measles virus wild-type isolates after immunization with a synthetic peptide vaccine which is not recognized by neutralizing passive antibodies. J. Gen. Virol. 81:729-735. [DOI] [PubMed] [Google Scholar]
- 10.Finke, D., U. G. Brinckmann, V. ter Meulen, and U. G. Liebert. 1995. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J. Virol. 69:5469-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin, D. E., and B. J. Ward. 1993. Differential CD4 T-cell activation in measles. J. Infect. Dis. 168:275-281. [DOI] [PubMed] [Google Scholar]
- 12.Hader, S. L., T. W. Hodge, K. A. Buchacz, R. A. Bray, N. S. Padian, A. Rausa, S. A. Slaviniski, and S. D. Holmberg. 2002. Discordance at human leukocyte antigen-DRB3 and protection from human immunodeficiency virus type 1 transmission. J. Infect. Dis. 185:1729-1735. [DOI] [PubMed] [Google Scholar]
- 13.Hammer, J., P. Valsasnini, K. Tolbla, D. Bolin, J. Higelin, B. Takacs, and F. Sinigaglia. 1993. Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell 74:197-203. [DOI] [PubMed] [Google Scholar]
- 14.Herberts, C. A., K. J. Stittelaar, E. van der Heeft, J. van Gaans-Van den Brink, M. C. M. Poelen, P. J. M. Roholl, L. J. W. van Alphen, C. J. M. Melief, A. P. J. M. de Jong, and C. A. C. M. van Els. 2001. A measles virus glycoprotein-derived human CTL epitope is abundantly presented via the proteasomal-dependent MHC class I processing pathway. J. Gen. Virol. 82:2131-2142. [DOI] [PubMed] [Google Scholar]
- 15.Hickman, C. J., A. S. Khan, P. A. Rota, and W. J. Bellini. 1997. Use of synthetic peptides to identify measles nucleoprotein T-cell epitopes in vaccinated and naturally infected humans. Virology 235:386-397. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson, S., R. P. Sekaly, W. J. Bellini, C. L. Johnson, H. F. McFarland, and E. O. Long. 1988. Recognition of intracellular measles virus antigens by HLA class II restricted measles virus-specific cytotoxic T lymphocytes. Ann. N. Y. Acad. Sci. 540:352-353. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson, S., R. P. Sekaly, C. L. Jacobson, H. F. McFarland, and E. O. Long. 1989. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J. Virol. 63:1756-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschmann, D. A., K. L. Duffin, C. E. Smith, J. K. Welply, S. C. Howard, B. D. Schwartz, and S. L. Woulfe. 1995. Naturally processed peptides from rheumatoid arthritis associated and non-associated HLA-DR alleles. J. Immunol. 155:5655-5662. [PubMed] [Google Scholar]
- 19.Lairmore, M. D., A. M. DiGeorge, S. F. Conrad, A. V. Trevino, R. B. Lal, and P. T. P. Kaumaya. 1995. Human T-lymphotropic virus type 1 peptides in chimeric and multivalent constructs with promiscuous T-cell epitopes enhance immunogenicity and overcome genetic restriction. J. Virol. 69:6077-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampson, L. A., and R. Levy. 1980. Two populations of Ia-like molecules on human B-cell line. J. Immunol. 125:293-299. [PubMed] [Google Scholar]
- 21.Li, H., C. J. Hickman, R. F. Helfand, H. Keyserling, L. J. Anderson, and W. J. Bellini. 2001. Induction of cytokine mRNA in peripheral blood mononuclear cells of infants after the first dose of measles vaccine. Vaccine 19:4896-4900. [DOI] [PubMed] [Google Scholar]
- 22.Malcherek, G., K. Falk, O. Rötzschke, H.-G. Rammensee, S. Stevanovic, V. Gnau, G. Jung, and A. Melms. 1993. Natural peptide ligand motifs of two HLA molecules associated with myasthenia gravis. Int. Immunol. 5:1229-1237. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita, S., K. Takahashi, M. Motoki, K. Komoriya, S. Ikagawa, and Y. Nishimura. 1994. Allele specificity of structural requirement for peptides bound to HLA-DRB1*0405 and -DRB1*0406 complexes: implication for the HLA-associated susceptibility to methimazole-induced insulin autoimmune syndrome. J. Exp. Med. 180:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neitzel, H. 1986. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum. Genet. 73:320-326. [DOI] [PubMed] [Google Scholar]
- 26.Nuchtern, J. G., W. E. Biddison, and R. D. Klausner. 1990. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature 343:74-76. [DOI] [PubMed] [Google Scholar]
- 27.Obeid, O. E., C. D. Partidos, C. R. Howard, and M. W. Steward. 1995. Protection against morbillivirus-induced encephalitis by immunization with a rationally designed synthetic peptide vaccine containing B- and T-cell epitopes from the fusion protein of measles virus. J. Virol. 69:1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ovsyannikova, I. G., K. L. Johnson, S. Naylor, D. C. Muddiman, and G. A. Poland. 2003. Naturally processed measles virus peptide eluted from class II HLA-DRB1*03 recognized by T lymphocytes from human blood. Virology 312:495-506. [DOI] [PubMed] [Google Scholar]
- 29.Panina-Bordignon, P., A. Tan, A. A. M. Termijtelen, S. Demotz, G. Corradin, and A. Lanzavecchia. 1989. Universally immunogenic T-cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 79:2237-2242. [DOI] [PubMed] [Google Scholar]
- 30.Partidos, C. D., and M. W. Steward. 1990. Prediction and identification of a T-cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J. Gen. Virol. 71:2099-2105. [DOI] [PubMed] [Google Scholar]
- 31.Poland, G. A. 1999. Immunogenetic mechanisms of antibody response to measles vaccine: the role of the HLA genes. Vaccine 17:1719-1725. [DOI] [PubMed] [Google Scholar]
- 32.Poland, G. A., and R. M. Jacobson. 1994. Failure to reach the goal of measles elimination. Apparent paradox of measles infections in immunized persons. Arch. Intern. Med. 154:1815-1820. [PubMed] [Google Scholar]
- 33.Poland, G. A., I. G. Ovsyannikova, R. M. Jacobson, R. A. Vierkant, S. J. Jacobsen, V. S. Pankratz, and D. J. Schaid. 2001. Identification of an association between HLA class II alleles and low antibody levels after measles immunization. Vaccine 20:430-438. [DOI] [PubMed] [Google Scholar]
- 34.Poland, G. A., I. G. Ovsyannikova, K. L. Johnson, and S. Naylor. 2001. The role of mass spectrometry in vaccine development. Vaccine 19:2692-2700. [DOI] [PubMed] [Google Scholar]
- 35.Pütz, M. M., and C. P. Muller. 2003. The rationale of a peptide-conjugare vaccine against measles. Vaccine 21:663-666. [DOI] [PubMed] [Google Scholar]
- 36.Roche, P. A., and P. Cresswell. 1990. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J. Immunol. 144:1849-1856. [PubMed] [Google Scholar]
- 37.Rota, J. S., K. B. Hummel, P. A. Rota, and W. J. Bellini. 1992. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology 188:135-142. [DOI] [PubMed] [Google Scholar]
- 38.Rota, J. S., Z.-D. Wang, P. A. Rota, and W. J. Bellini. 1994. Comparison of sequences of the H, F, and N coding genes of measles virus vaccine strains. Virus Res. 31:317-330. [DOI] [PubMed] [Google Scholar]
- 39.Rothbard, J. B., R. I. Lechler, K. Howland, V. Bal, D. D. Eckels, R. Sekaly, E. O. Long, W. R. Taylor, and J. R. Lamb. 1988. Structural model of HLA-DR1 restricted T-cell antigen recognition. Cell 52:515-523. [DOI] [PubMed] [Google Scholar]
- 40.Santori, F. R., W. C. Kieper, S. M. Brown, Y. Lu, T. A. Neubert, K. L. Johnson, S. Naylor, S. Vukmanovic, K. A. Hogquist, and S. C. Jameson. 2002. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17:1001-1012. [DOI] [PubMed] [Google Scholar]
- 41.Schneider-Schaulies, J., S. Schneider-Schaulies, and V. ter Meulen. 1993. Differential induction of cytokines by primary and persistent measles virus infections in human glial cells. Virology 195:219-228. [DOI] [PubMed] [Google Scholar]
- 42.Sheshberadaran, H., S.-N. Chen, and E. Norrby. 1983. Monoclonal antibodies against five structural components of measles virus. Virology 128:341-353. [DOI] [PubMed] [Google Scholar]
- 43.Sidney, J., C. Oseroff, S. Southwood, M. Wall, G. Ishioka, F. Koning, and A. Sette. 1992. DRB1*0301 molecules recognize a structural motif distinct from the one recognized by most DRβ1 alleles. J. Immunol. 149:2634-2640. [PubMed] [Google Scholar]
- 44.Sinigaglia, F., M. Guttinger, J. Kilgus, D. M. Doran, H. Matile, H. Etlinger, A. Trzeciak, D. Gillessen, and J. R. Pink. 1988. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature 336:778-780. [DOI] [PubMed] [Google Scholar]
- 45.Southwood, S., J. Sidney, A. Kondo, M. F. del Guercio, E. Appella, S. Hoffman, R. T. Kubo, R. W. Chesnut, H. M. Grey, and A. Sette. 1998. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 160:3363-3373. [PubMed] [Google Scholar]
- 46.Spahr, C. S., M. T. Davis, M. D. McGinley, J. H. Robinson, E. J. Bures, J. Beierle, J. Mort, P. L. Courchesne, K. Chen, R. C. Wahl, W. Yu, R. Luethy, and S. D. Patterson. 2002. Towards defining the urinary proteome using liquid chromatography-tandem mass spectrometry. I. Profiling an unfractionated tryptic digest. Proteomics 1:93-107. [DOI] [PubMed] [Google Scholar]
- 47.Urban, R. G., R. M. Chicz, D. A. Vignali, and J. L. Strominger. 1993. The dichotomy of peptide presentation by class I and class II MHC proteins. Chem. Immunol. 57:197-234. [PubMed] [Google Scholar]
- 48.van Els, C. A. C. M., C. A. Herberts, E. van der Heeft, M. C. M. Poelen, J. A. M. van Gaans-van den Brink, A. van der Kooi, P. Hoogerhout, G. J. ten Hove, H. D. Meiring, and A. P. J. M. de Jong. 2000. A single naturally processed measles virus peptide fully dominates the HLA-A*00201-associated peptide display and is mutated at its anchor position in persistent viral strains. Eur. J. Immunol. 30:1172-1181. [DOI] [PubMed] [Google Scholar]
- 49.Velazquez, C., R. DiPaolo, and E. R. Unanue. 2002. Quantitation of lysozyme peptides bound to class II MHC molecules indicates very large differences in levels of presentation. J. Immunol. 166:5488-5494. [DOI] [PubMed] [Google Scholar]
- 50.Ward, B. J., and D. E. Griffin. 1993. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin. Immunol. Immunopathol. 67:171-177. [DOI] [PubMed] [Google Scholar]