Abstract
Background
TSLP is expressed at sites of allergic inflammation, including eczematous skin. This cytokine has been reported to exert its Th2-inducing properties through dendritic cells. Expression of TSLP receptor on the surface of activated Th2 cells could amplify Th2 responses at inflamed sites through the direct actions of TSLP.
Objective
To rigorously test whether Th2 cells induced by “pro-allergic” factors express TSLP receptor and characterize these cells using an experimental platform that combines flow cytometry with microscopic capabilities.
Methods
CD4+ T cells isolated from patients with atopic dermatitis or normal healthy controls were co-cultured with autologous dendritic cells in the presence of Th2-promoting stimuli (TSLP±allergen and staphylococcal enterotoxin B±TSLP). Surface expression of TSLP receptor was analyzed by image-based flow cytometry and responsiveness of purified T cells to TSLP was assessed by phosphorylation of STAT5 and cytokine secretion.
Results
Th2-promoting stimuli induced a robust population of activated Th2 cells (CD25+IL-4+). Regardless of the nature of the stimulus, flow cytometry imaging confirmed that T cells expressing TSLP receptor were rare, constituting a minor fraction of the IL-4+ T cell pool; however, TSLP-responsiveness was nonetheless detectable. Analysis of cell size and nuclear morphology revealed preferential expression of TSLP receptor on IL-4-expressing cells undergoing mitosis. Analysis of lesional skin in atopic dermatitis supported the view that rare IL-4+ T cells expressing TSLP receptor are present at inflamed sites.
Conclusion
In a “pro-allergic” milieu, TSLP receptor is preferentially expressed on rare actively dividing Th2 cells. The direct action of TSLP on T cells could amplify Th2 responses at sites of allergic inflammation.
Keywords: TSLP, TSLP receptor, atopic dermatitis, Th2 cells, flow cytometry imaging
Introduction
The cytokine, thymic stromal lymphopoietin (TSLP) plays a key role in the pathogenesis of a variety of allergic diseases, including asthma and atopic dermatitis (AD).1–5 TSLP exhibits potent Th2-promoting properties based on its ability to differentiate Th2 cells from naïve CD4+ T cell precursors in humans.6, 7 High expression of TSLP is a feature of keratinocytes in AD skin lesions.6 and TSLP-priming of dendritic cells (DCs) in situ may serve to induce or enhance Th2 responses within the skin, as well as systemically. Consistent with this viewpoint, TSLP was originally reported to exert its Th2-promoting properties through a DC-mediated pathway in humans that involved induction of OX40L on DCs.7
Disruption of the skin barrier in AD provides a portal of entry for antigens that drive Th2 responses associated with this disease.8, 9 Skin DCs also likely play a pivotal role in this process by sampling antigens at the subepithelial interface.10 We recently reported that co-priming DCs with TSLP and an allergen variant targeted to DCs enhanced Th2 responses in vitro as compared with either stimulus alone in cultures from atopic subjects.11 This suggested that TSLP and allergen provide a “pro-allergic” milieu that favors the induction of a robust Th2 response.
TSLP receptor is a heterodimer comprising the IL-7 receptor α chain and the TSLP receptor chain (TSLPr).12, 13 While TSLPr was originally thought to be a key feature of CD11c+ myeloid DCs, this receptor was recently reported on activated human T cells and these cells had the capacity to respond to TSLP.14 This observation made us question whether a T cell-mediated TSLP pathway could contribute to Th2 responses in an inflammatory milieu that includes TSLP. In accordance with this theory, TSLP has been shown to enhance Th2 cytokine secretion by skin-infiltrating CD4+ T cells in a mouse model of allergic skin inflammation in a manner dependent upon TSLPr expression on T cells, but not DCs.15
It is not known whether TSLPr is expressed on human Th2 cells. The present study was designed to rigorously test whether TSLPr is expressed on these cells in the presence of a “pro-allergic” milieu. Using flow cytometry imaging, we found that TSLPr is preferentially expressed on rare actively dividing Th2 cells. The functional relevance and implications for allergic inflammation are addressed.
Materials and Methods
Human Subjects
Subjects were recruited from the University of Virginia Allergic Diseases Clinic, or else by advertisement. Patients with AD reported physician-diagnosed eczema and fulfilled the diagnostic criteria of Hanifin and Rajka for AD.16 All patients had moderate-to-severe disease based on SCORAD index,17 markedly elevated serum titers of total IgE (>400 IU/ml) and high titer IgE ab to dust mite allergen (CAP ≥0.7 IU/ml). Dust mite-allergic subjects without AD had high titer IgE ab to dust mite allergen (CAP ≥0.7 IU/ml). Healthy non-atopic controls had no measurable serum IgG or IgE antibodies to common allergens. All studies were approved by the University of Virginia Human Investigations Committee.
Cells and Reagents
See the Methods Section in the Online Repository.
Cell Cultures for Flow Cytometry Imaging Studies
Blood DCs (1×105) were co-cultured in 24-well plates with autologous CD4+ T cells (4×105 cells per well) in complete medium containing 10% autologous serum.18 Cultures were stimulated with SEB (20ng/ml) ± TSLP (50ng/ml) for 3 days, or else with TSLP (50ng/ml) ± house dust mite allergen (20μg/ml of house dust mite extract containing 10μg/ml Der p 1) for 7 days. In some experiments, THP-1 cells were pulsed for 24 hours with H22-Fel d 1 (10μg/ml) in medium containing 10% autologous serum.
Flow Cytometry Imaging
Immunostaining of Cells
After culture, T cells or THP-1 cells were stained for surface markers and washed to remove excess antibody. Cells were then fixed and permeabilized using Caltag Fix and Perm kit (Invitrogen) followed by staining for intracellular IL-4 (T cells only) and DNA (T cells and THP-1 cells). Prior to staining for IL-4, T cells were restimulated with PMA (50ng/ml)(Fisher Scientific) and ionomycin (2μg/ml)(Invitrogen) on the final day of culture for 5 hours, followed by addition of Brefeldin A (BD Biosciences) for 4 hours. The purity of CD4+ T cells harvested from co-culture assays was determined to be 96–99% based on forward and side scatter gating in conjunction with CD3 staining using standard flow cytometry.
Analysis
Image-based flow cytometry was performed on an Imagestream100 (Amnis Corporation) using the manufacturer's collection program, INSPIRE.19 Fluorometric compensation was digitally calculated by analysis software (IDEAS: Amnis Corporation) based on single-stain controls. The data were analyzed according to the manufacturer's instructions by use of IDEAS software. Briefly, debris and cell aggregates were excluded and single cells were identified after compensation by gating on cell area and aspect ratio followed by visual inspection. After identifying single cells, cell integrity was confirmed by morphology and DNA staining. Identification of TSLPr+ cells was based on selection of distinct cell populations above background fluorescence and confirmed visually by the appearance of a circumferential fluorescence signal.
Analysis of Intracellular and Surface Markers by Standard Flow Cytometry
See the Methods Section in the Online Repository.
Analysis of the Direct Effects of TSLP on T Cells
See the Methods Section in the Online Repository.
Assay for Secreted Cytokines
See the Methods Section in the Online Repository.
Statistical Analysis
See the Methods Section in the Online Repository.
Results
Validation of Anti-TSLPr Monoclonal Antibodies by Image-Based Flow Cytometry
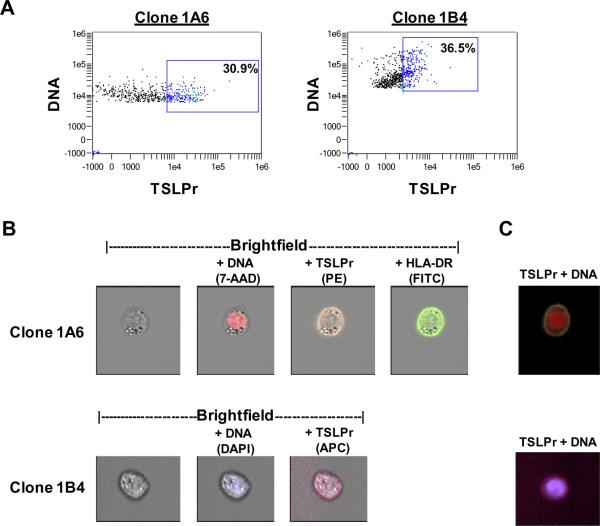
Initially, we compared the surface staining properties of two commercially available anti-TSLPr monoclonal antibodies. To do this, THP-1 monocytes were activated using an allergen variant that upregulates TSLPr on DCs.11 Cells were stained with different anti-TSLPr clones and analyzed by image-based flow cytometry. In dot plots, activated THP-1 cells staining for TSLPr were readily detectable (30.9% for clone 1A6 and 36.5% for clone 1B4)(Fig. 1A). For both clones, visual inspection of cells within the TSLPr+ gate confirmed circumferential staining for TSLPr similar to that for HLA-DR (Fig. 1B). Overlaying digital images for surface and nuclear markers revealed a circumferential band of TSLPr staining consistent with surface staining (Fig. 1C). The specificity of each antibody for TSLPr staining as judged microscopically was equivalent. Visual inspection of all THP-1 cells within the TSLPr+ gate revealed 82% and 88% true positives for cells stained with clones 1A6 and 1B4 respectively. These experiments validated surface staining using anti-TSLPr antibodies and demonstrated the ability to accurately enumerate TSLPr+ cells using image-based flow cytometry. All subsequent experiments were performed with anti-TSLPr clone 1A6 since this antibody, combined with other available fluorochrome conjugates, yielded an optimal quality image.
Figure 1. Validation of Anti-TSLP Receptor Monoclonal Antibodies by Image-Based Flow Cytometry.
Activated THP-1 monocytes were stained for TSLPr, HLA-DR and DNA. (A) Dot plots representing gated focused single cells with DNA negative cells excluded. Boxes denote TSLPr+ gates determined by visual inspection of single cells inside the gate versus cells outside the gate. Green crosshairs denote single cells shown in panels B and C. (B) Single cell images of TSLPr+ cells using different anti-TSLPr clones. Images for each stain are overlayed on the brightfield image. (C) Composite images corresponding to cells shown in (B).
Th2-Promoting Factors Induce Activated Th2 Cells
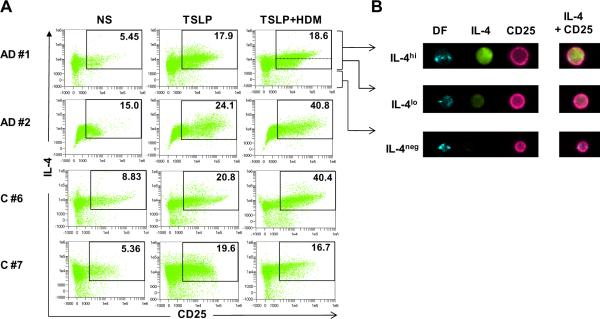
Initial experiments using standard flow cytometry showed that co-stimulating T cells from atopic subjects with TSLP and house dust mite allergen selectively amplified the induction of IL-4+ T cells as compared with either stimulus alone. By contrast, there was no effect on IFN-γ+ or IL-10+ T cells. Moreover, only a small fraction of IL-4+ T cells co-expressed IFN-γ (Fig. E1A). This confirmed our previous findings which indicated that co-stimulating T cells with TSLP and allergen provides a potent Th2-promoting milieu.11 Next, we tested the capacity for TSLP and house dust mite allergen to induce Th2 cells using image-based flow cytometry. Subjects selected for study were highly atopic patients with AD and healthy non-atopic controls (Table EI). Stimulation of T cells with TSLP alone induced a robust population of cells in all subjects that expressed IL-2R alpha chain (CD25), a molecule expressed on activated T cells (Fig. 2A). Co-stimulation with TSLP and allergen amplified the response, though this effect was variable and not related to atopic status (Fig. 2A). Upon visual inspection, the majority of CD25+ T cells induced under Th2-promoting conditions expressed IL-4; however, the intensity of cytoplasmic staining for IL-4 varied considerably within the CD25+IL-4+ gate as evidenced by the presence of clearly distinguishable IL-4hi and IL-4lo cells (Fig. 2B). Analysis of secreted cytokines confirmed a Th2-dominated cytokine profile in cultures stimulated with TSLP+allergen as judged by 6 to >100 fold levels of IL-5 or IL-13 as compared with IFN-γ (Fig. E1B). Interestingly, IL-10 was also induced; however, intracellular staining of T cells coupled with production of peak levels at day 3 indicated that DCs were the source of this cytokine (Fig. E1B and data not shown).
Figure 2. Th2-Promoting Factors Induce Activated Th2 Cells.
(A) CD4+ T cells from DC/T cell co-cultures analyzed for expression of CD25 and IL-4 by image-based flow cytometry. Values denote percentages of CD25+IL-4+ T cells for each condition. Dotted line indicates the gate used to delineate IL-4hi from IL-4lo cells. NS, not stimulated; HDM, house dust mite allergen. (B) Images of 3 representative T cells showing different staining intensity for IL-4 (green). Right panel shows composite images. DF, darkfield. Data is representative of 5 subjects.
TSLPr+ T Cells are Rare Under Th2-Promoting Conditions
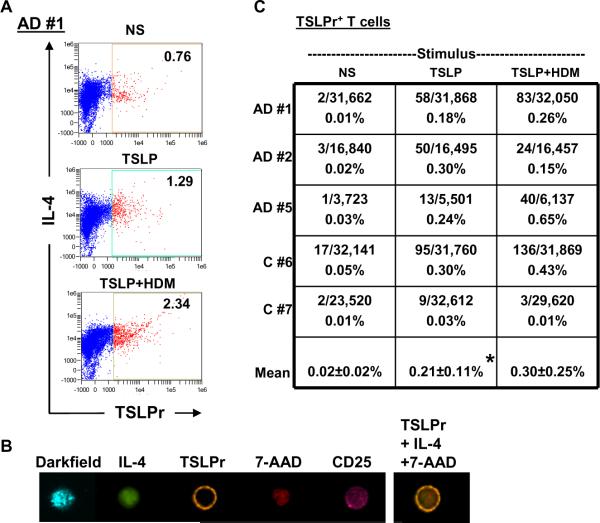
Next, TSLPr expression was analyzed on T cells. The strategy involved setting a TSLPr+ gate for total CD4+ T cells by identifying a threshold of staining intensity based on visual confirmation of bona fide TSLPr+ cells with a circumferential pattern of staining (Figs. 3A & 3B). Each cell within this gate was then visually inspected to confirm whether surface TSLPr was expressed in order to accurately enumerate rare TSLPr+ T cells. By this approach, analysis of unstimulated cells on day 7 revealed a very low frequency of TSLPr+ cells (mean=0.02±0.02%). Stimulation with TSLP or TSLP+allergen enhanced the frequency of these cells in all subjects tested (mean=0.21±0.11% (p=0.015) and 0.30±0.25% (p=0.061) respectively)(Fig. 3C). Nonetheless, TSLPr+ cells accounted for <0.7% of total T cells. Notably, TSLPr+ cells constituted only a small fraction of cells within the TSLPr+ gate (<15%), with false positive events accounting for the remainder (Fig. E2). These observations confirmed that TSLPr+ T cells are rare under Th2-promoting conditions.
Figure 3. TSLPr+ T Cells are Rare Under Th2-Promoting Conditions.
(A) CD4+ T cells were stimulated and analyzed for TSLPr expression by image-based flow cytometry. Values denote the percentage of cells within a gate that captured all TSLPr+ events by visual inspection. NS, non-stimulated. Data is representative of 5 subjects. (B) Image of a single representative TSLPr+ T cell with corresponding composite image. (C) Table of actual frequencies of TSLPr+ T cells confirmed by inspection of cells (data from 5 subjects, *p=0.015).
In order to recapitulate the in vivo microenvironment, TSLP was present for the duration of culture in vitro. Since cytokine receptor expression may be downregulated by the presence of its ligand, it was important to exclude receptor modulation in our system. No TSLPr was detected on CD4+ T cells isolated directly ex vivo from AD or non-AD subjects (data not shown). Time course studies performed using standard flow cytometry showed that co-stimulation with TSLP+allergen induced TSLPr expression as early as 24 hours. Notably, TSLPr+ cells were predominantly CD25neg or CD25lo early in culture with a trend towards increased expression of CD25 at day 7 (Fig. E3). These findings indicate that TSLPr expression precedes upregulation of CD25 on activated T cells and persists in the presence of its ligand.
TSLPr is a Feature of Actively Dividing Th2 Cells
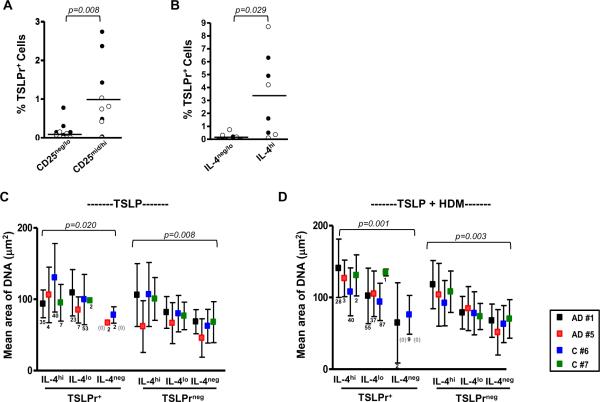
Surface TSLPr was further analyzed in the context of CD25 and IL-4 in order to determine whether this molecule was preferentially expressed on activated Th2 cells. Under Th2-promoting conditions, visual inspection confirmed that TSLPr+ cells were enriched within T cell subsets expressing increased levels of CD25 (p=0.008, Figs. 4A & E4). In addition, the majority of TSLPr+ cells induced under Th2-promoting conditions expressed IL-4 (>93%), and these cells were enriched within the IL-4hi subset, comprising up to 8.7% of these cells (p=0.029, Figs. 4B & E4).
Figure 4. TSLPr+ T Cells are Enlarged and Express High Levels of CD25 and IL-4.
(A, B) Percentage of TSLPr+ T cells within CD25mid/hi and CD25neg/lo subsets (A) and IL-4hi and IL4neg/lo subsets (B) after stimulation with TSLP (closed symbols) or TSLP+HDM (open symbols). Bars represent the mean (n=5 subjects). (C, D) Area of nuclear staining for IL-4hi, IL-4lo, and IL-4neg subsets induced by TSLP (C) or TSLP+HDM (D) (mean ± SD). Numbers of TSLPr+ cells for each observation are shown. Data for TSLPr-negative subsets was compiled using 200 cells randomly selected from each subset (n=4 subjects).
Since dividing T cells will stain more brightly for IL-4 owing to increased cytoplasmic content, we postulated that IL-4hi cells induced by Th2-promoting factors represented dividing cells. Analysis of the area of nuclear staining confirmed a trend towards larger cell size with higher expression of IL-4 (p=0.008 for TSLP alone and p=0.003 for TSLP+allergen). A similar trend was observed for TSLPr+ cells (Figs. 4C & D). Collectively, these findings indicate that a large proportion of TSLPr+ cells are blasting T cells.
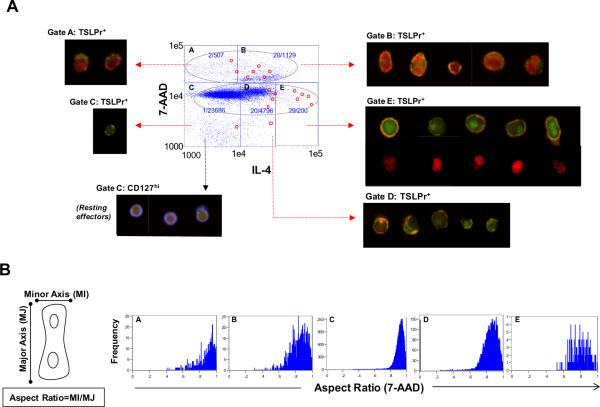
The relationship between IL-4 content, TSLPr expression, and cell division was further analyzed. A major advantage of flow cytometry imaging is that dividing T cells can be identified by the characteristic morphology of their mitotic nuclei. To avoid exclusion of dividing cells and thus, capture all TSLPr+ dividing T cell events, total cells were analyzed without first adjusting for cell area or nuclear aspect ratio. This strategy was adopted since elongated dividing cells with a lower nuclear aspect ratio than non-dividing cells spherical cells, would be excluded by preferentially selecting single cells based on a high nuclear aspect ratio. To avoid inclusion of doublets, which can result in artifactual single cells with high DNA content on scatter plot, cells with a low darkfield aspect ratio were excluded (≤0.4). This strategy excluded all doublets while retaining dividing cells. Two discrete populations were identified by dot plot based on DNA staining intensity (grey spheres, Fig. 5A). In the experiment shown (stimulus: TSLP+HDM) approximately one third of total TSLPr+ T cells (28/80) resided within a DNAhiIL-4+ subset (gate B, Fig. 5A) and visual inspection indicated that these cells were blasting or actively dividing, as evidenced by their large size and the presence of mitotic nuclei characteristic of cells in early or late anaphase (see corresponding cell images). Consistent with this, cells with high DNA staining intensity generally exhibited a lower nuclear aspect ratio (gates A and B, Fig. 5B). The majority of remaining TSLPr+ T cells showed lower DNA staining intensity; however, a disproportionately high number of these cells stained brightly for IL-4 and exhibited a lower nuclear aspect ratio indicating a mitotic phenotype (gate E, Fig. 5A & 5B). DNA imaging of these cells revealed intact nuclei suggesting that the high IL-4 content masked DNA staining (see representative images for gate E, Fig. 5A). The increased cell size of the majority of TSLPr+ T cells was readily evident upon microscopic exam as compared with resting effector cells identified by low DNA content and high expression of CD127 (gate C, Fig. 5A).20, 21 Collectively, these observations indicate that TSLPr is expressed on Th2 cells, and a large proportion of these cells are actively dividing.
Figure 5. TSLPr+ T Cells are Actively Dividing.
(A) Scatter plot showing T cells stimulated with TSLP+allergen analyzed for DNA (7-AAD) and IL-4. Values denote the frequency of TSLPr+ T cells within each gate and red circles denote TSLPr+ cells shown in corresponding composite images. DNA staining for cells in gate E is shown in bottom row of cell panel. Composite images of representative resting effector cells in gate C are shown for comparison (CD127 (blue) and DNA (red)). (B) Nuclear aspect ratios for gates in panel (A). Representative data is shown (AD #1).
Bacterial Superantigen Induces Rare Th2 Cells that Express TSLPr
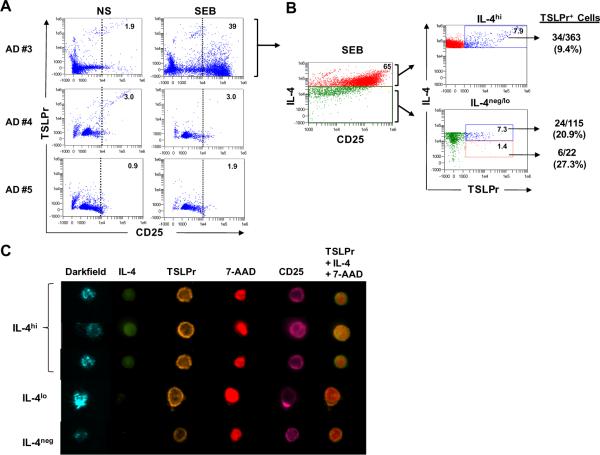
Many patients with AD are colonized with S. aureus, and IgE antibodies to staphylococcal enterotoxin B (SEB) are common.22, 23 We examined whether SEB had the capacity to induce TSLPr+ T cells. Interestingly, only 1 of 3 SEB-sensitized AD patients responded to SEB (AD #3, Table EI), as judged by increased CD25 (Fig. 6A). A similar frequency of TSLPr+ T cells was observed as compared with other Th2-promoting stimuli (64/9,850 (0.65%)) and cells expressing TSLPr were not amplified by co-stimulating with SEB+TSLP (32/12,326 (0.26%)). Stimulation with SEB induced a robust population of IL-4hi T cells that were predominantly CD25+ (Fig. 6B). Upon imaging, all TSLPr+ T cells were CD25+ and most cells (34/64) were IL-4hi. TSLPr was also visible on T cells expressing lower levels of IL-4, including IL-4neg cells (Figs. 6B &C). TSLPr+ cells exhibited a morphology similar to that induced by other Th2-promoting factors(Fig. 6C). Analysis of secreted cytokines showed that SEB induced a mixed Th1/Th2 profile in the responder subject (AD #3) which was in contrast to a Th2-dominated profile in a non-responder patient (AD#1)(Fig. E5A). Consistent with this, IL-4+ T cells from the responder subject co-expressed other cytokines including IFN-γ and IL-10, and to a lesser extent, IL-17 (Fig. E5B). These findings confirmed the capacity for SEB to induce TSLPr on rare IL-4-expressing T cells.
Figure 6. Bacterial Superantigen Induces Rare TSLPr+ Th2 Cells.
(A) Analysis of CD25 and TSLPr on CD4+ T cells stimulated with SEB (day 3). Percentage of T cells within the CD25+ gate (dotted line) is denoted. NS, nonstimulated. (B) Frequency of TSLPr+ T cells within IL-4hi, IL-4lo and IL-4neg subsets in SEB-stimulated cultures from a responder patient. Left scatter plot shows gating strategy for IL-4hi (red) and IL-4neg/lo cells (green). (C) Images of representative TSLPr+ cells.
T Cells Induced in a “Pro-Allergic” Milieu Respond Directly to TSLP
Formation of a functional TSLP receptor requires heterodimerization of the TSLPr chain with IL-7 receptor alpha chain (CD127).12, 13 Interestingly, CD127 expression was visible on only a minority of total TSLPr+ T cells induced by TSLP+allergen (34.1%±23.3%) and was generally absent on those induced by TSLP alone (Table EII, Fig. E6). By contrast, CD127 was readily detected on both unstimulated and stimulated T cells, including those expressing CD25, consistent with its expression on resting memory and activated T cells (Table EII & Fig. E7).24
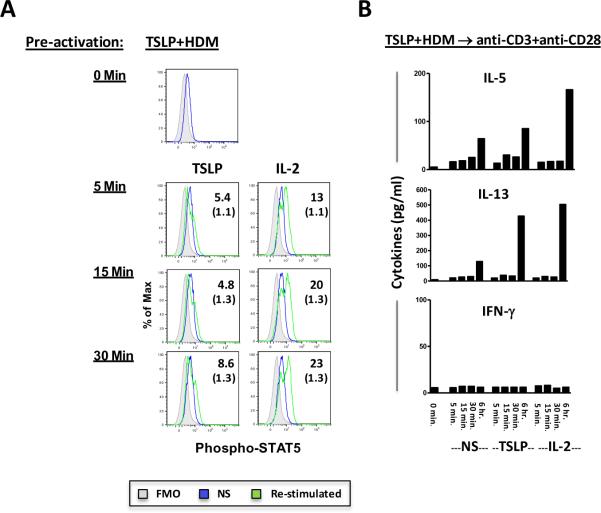
It was previously reported that T cells pre-activated with anti-CD3 and anti-CD28 respond directly to TSLP by activation of STAT5.14 In order to test whether T cells that had been pre-activated with TSLP+allergen could respond directly to TSLP, T cells activated with TSLP+allergen were isolated, rested for 1 day and then re-stimulated with TSLP. As expected, re-stimulation with IL-2 resulted in phosphorylation of STAT5, reflecting signaling through the IL-2 receptor pathway. By contrast, only a weak signal for phosphorylated STAT5 was detected upon re-stimulation with TSLP (Fig. 7A). Moreover, no secreted cytokines were detected for T cells pre-activated with TSLP+allergen followed by TSLP re-stimulation. However, Th2 cytokines were selectively induced upon re-stimulation with TSLP or IL-2 when T cells were sequentially pre-activated with TSLP+allergen and anti-CD3+anti-CD28 (Fig. 7B). Collectively, these findings suggest that Th2 cells induced by TSLP+allergen are weakly responsive to TSLP and re-activation by T cell receptor stimulation enhances this responsiveness.
Figure 7. Direct Effects of TSLP on Purified T Cells Induced in a “Pro-Allergic” Milieu.
(A) Purified T cells that had been pre-activated with TSLP+allergen were not stimulated or re-stimulated with TSLP or IL-2 for 0 to 30 minutes and analyzed for phosphorylated STAT5. Values represent the percentage of cells positive for phospho-STAT5 following re-stimulation (values for non-stimulated cells are shown in parentheses for each corresponding time point). Data is representative of 3 experiments. (B) Culture supernatants from purified T cells that had been pre-activated sequentially with TSLP+allergen followed by anti-CD3+anti-CD28 were assayed for cytokines following re-stimulation with TSLP or IL-2 for up to 6 hours. NS, not stimulated.
IL-4+ T Cells Expressing TSLP Receptor are Detectable in AD Skin
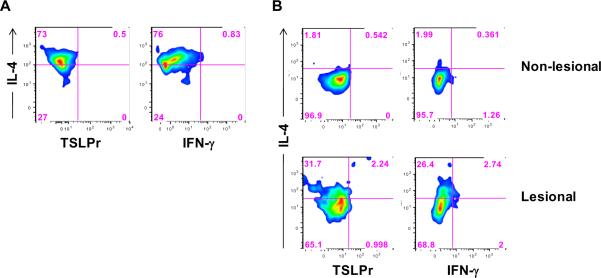
In order to determine the in vivo relevance of our observations, we examined whether IL-4+ T cells expressing TSLPr were detectable in T cells isolated from the skin. Stimulation of T cells from normal skin with TSLP+allergen induced Th2-like (IL-4+IFN-γ−) T cells; however, no TSLPr+ T cells were detected by standard flow cytometry (Fig. 8A). By contrast, direct ex vivo analysis of T cells from AD skin identified a low frequency of IL-4+TSLPr+ T cells in lesional but not in non-lesional skin (Fig. 8B). These findings supported our in vitro observations using image-based flow cytometry.
Figure 8. Analysis of TSLPr on Skin T Cells.
(A) Cells isolated from normal skin obtained from a non-atopic donor were cultured for 7 days with TSLP+allergen and analyzed by standard flow cytometry. (B) Cells isolated directly ex vivo from lesional or non-lesional skin from an AD patient (#1) were analyzed for TSLPr. Both panels show density plots for cells in the CD3+ T lymphocyte gate.
Discussion
Our findings confirm that TSLPr is preferentially expressed on rare actively dividing Th2 cells under Th2-promoting conditions. While a large proportion of cells expressing TSLPr did not co-express CD127, T cells that were pre-activated with TSLP+allergen alone did respond weakly to TSLP as judged by phosphorylation of STAT5. Alternate STATs implicated in TSLP signaling in human DCs and T cells, including STAT6, were not examined in our study.24, 25 Interestingly, sequential pre-activation of T cells with TSLP+allergen followed by anti-CD3+anti-CD28 resulted in secretion of TSLP-induced Th2 cytokines, while pre-activation with TSLP+allergen alone did not. Collectively, these findings suggest that Th2 cells induced in a “pro-allergic” milieu require re-activation by a potent TCR stimulus in order to respond optimally to TSLP. In terms of the biological relevance, it is tempting to speculate that Th2 cells respond to the direct actions of TSLP during the effector phase of the response, after Th2 differentiation has occurred. Such a scenario would equip Th2 cells primed in regional nodes with the capacity to respond to TSLP in inflamed tissues. Consistent with this theory, TSLPr expression on T cells, but not DCs, was required for enhanced Th2 cytokine secretion by skin-infiltrating CD4+ T cells in a mouse model of allergic skin inflammation.15 Analysis of cells isolated from AD skin supported the presence of T cells expressing TSLPr at inflamed sites and these cells constituted ~10% of IL-4+ T cells. While low expression of this receptor could reflect a transient event associated with Th2 differentiation or cell division in situ, its presence in lesional but not non-lesional skin suggests a functional role at the site of allergic inflammation.
It has been reported that IL-4-expressing T cells infiltrating AD skin, a site where TSLP is present, represent a heterogeneous population.26 Our findings indicate that expression of TSLPr on T cells may not be unique to classical Th2 cells since IL-4-expressing cells induced by SEB co-expressed a variety of other cytokines. Thus, further studies are warranted to elucidate whether TSLPr expression is exclusive to T cells with an IL-4-restricted cytokine profile.
The lack of a functional TSLPr complex on some TSLPr+ T cells was a novel observation. The absence of CD127 on TSLPr+ T cells that were stimulated specifically with TSLP alone did not appear to be explained by downregulation of the TSLPr complex upon binding of TSLP since the ligand binding component (TSLPr chain) of the TSLPr complex12 was readily detectable on the cell surface. T cells were maintained in culture with TSLP in our system in order to reproduce a “pro-allergic” milieu. Several lines of evidence argue against receptor downregulation in the presence of TSLP. First, time course studies indicated a consistent signal for TSLPr following stimulation with TSLP. Second, TSLPr+ T cells were detected at low frequencies in SEB-stimulated cultures in the absence of TSLP. Third, work performed by other groups has demonstrated only low surface TSLPr and a modest increase in TSLPr mRNA in human T cells activated in the absence of TSLP.14, 27
In the present study, identification of TSLPr+ T cells using image-based technology was a labor intensive process requiring visual inspection of thousands of cells. This approach facilitated the enumeration of bona fide TSLPr+ T cells. The microscopy component of this platform also allowed us to elucidate the presence of a functional receptor by confirming whether CD127 was expressed on rare TSLPr+ cells at the single-cell level. Another advantage of the approach described was the ability to visualize mitotic cells based on a distinctive nuclear and surface morphology, thereby linking TSLPr to actively dividing T cells. The technology we describe allowed the simultaneous assessment of up to 5 cellular markers (CD127, CD25, IL-4, DNA, and TSLPr). In order to analyze the maximal number of markers related to TSLPr cells, the T cell marker, CD4, was not included. Thus, it could be argued that the rare TSLPr+ T cells identified in our system constituted a contaminating DC population. There are several key immunobiologic features of DCs that argue against this. Notably, expression of IL-4 is not an attribute of human DCs.28 To the contrary, secretion of the Th1-promoting cytokine IL-12, is a notable characteristic of DCs under Th2-promoting conditions.29, 30 Furthermore, DCs primed specifically with TSLP do not produce detectable amounts of IL-4.1 Initial studies performed using standard flow cytometry detected a TSLPr signal on CD4+ T cells under Th2-promoting conditions at a similar frequency to that obtained by image-based technology (data not shown). This provided further evidence that TSLPr expression was a feature of T cells in our system. Finally, when considered collectively, the morphologic attributes and staining properties of TSLPr+ cells were characteristic of a T cell phenotype.
In summary, our findings support the view that in a “pro-allergic” milieu, TSLPr is preferentially expressed on rare actively dividing Th2 cells. The capacity for these cells to respond directly to TSLP may contribute to amplification of Th2 responses at sites of allergic inflammation.
Key messages.
TSLP receptor is expressed on rare actively dividing Th2 effectors.
T cells induced in a “pro-allergic” milieu can respond directly to TSLP.
Supplementary Material
Acknowledgments
The authors thank: Holly Carper, MS and Deborah Murphy, RN (University of Virginia) for assistance with blood draws.
This work was supported by NIH grants AI-052196 and AI-070364. Martin Chapman is a co-founder and co-owner of Indoor Biotechnologies Inc and receives income and research support from the company.
Abbreviations
- AD
atopic dermatitis
- DC
dendritic cell
- HDM
house dust mite allergen
- SEB
staphylococcal enterotoxin B
- TSLP
thymic stromal lymphopoietin
- TSLPr
TSLP receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 3.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. 1. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka J, Watanabe N, Kido M, Saga K, Akamatsu T, Nishio A, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2008;39:89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 9.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–1891. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 11.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, et al. Targeting allergen to FcγRI reveals a novel Th2 regulatory pathway linked to TSLP receptor. J Allergy Clin Immunol. 2010;125:247–256. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 13.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochman I, Watanabe N, Ariam K, Liu YK, Leonard WJ. Cutting edge: direction action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 15.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockholm) 1980;92(Suppl.):44–47. [Google Scholar]
- 17.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 18.Reefer AJ, Satinover SM, Solga MD, Lannigan JA, Nguyen JT, Wilson BB, et al. Analysis of CD25hiCD4+ “regulatory” T-cell subtypes in atopic dermatitis reveals a novel Th2-like population. J Allergy Clin Immunol. 2008;121:415–422. doi: 10.1016/j.jaci.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 19.George TC, Basjii DA, Hall BE, Lynch DH, Ortyn WE, Perry DJ, et al. Distinguishing modes of cell death using the ImageStream® multispectral imaging flow cytometry. Cytometry A. 2004;59:237–245. doi: 10.1002/cyto.a.20048. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-yu Z, Szot GL, Ree MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320–322. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- 23.Reefer AJ, Satinover SM, Wilson BB, Woodfolk JA. The relevance of microbial allergens to the IgE antibody repertoire in atopic and nonatopic eczema. J Allergy Clin Immunol. 2007;120:156–163. doi: 10.1016/j.jaci.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 24.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omori M, Ziegler S. Induction of IL-4 expression in CD4+ T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–13404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 26.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: Linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Lu N, Wang Y-H, Arima K, Hanabuchi S, Liu Y-J. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 29.Hochrein H, O'Keeffe M, Luft T, Vandenabeele S, Grumont RJ, Maraskovsky E, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebner S, Ratzinger G, Krosbacher B, Schmuth M, Weiss A, Reider D, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–641. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.