Abstract
Human T-cell leukemia virus type 1 (HTLV-1) basic leucine zipper factor (HBZ) is transcribed from the antisense genomic DNA strand and functions differently in its RNA and protein forms. To distinguish between the roles of hbz mRNA and HBZ protein, we generated mutants in a proviral clone that specifically disrupt the hbz gene product. A proviral clone with a splice acceptor mutation that disrupts expression of the predominant hbz mRNA resulted in lower levels of tax mRNA. Heterologous hbz expression restored Tax activity in cells expressing this mutant clone. In contrast, proviral mutants that disrupt HBZ protein did not affect levels of tax mRNA. Expression of hbz resulted in lower levels of p30II mRNA. Mutation of p30II overcame the effects of the splice acceptor mutation of hbz, and restored tax expression. Thus, there is a complex interplay of viral regulatory proteins controlling levels of HTLV-1 gene expression.
Keywords: hbz, tax, HTLV-1, p30II, antisense
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is a complex retrovirus belonging to the delta retrovirus family. It is the etiologic agent of adult T cell leukemia lymphoma (ATLL) (Hinuma et al., 1981; Poiesz et al., 1980), a malignancy of CD4+ T lymphocytes, and a chronic neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). In addition to the gag, env, and pol genes encoding structural and enzymatic proteins common to all retroviruses, the HTLV-1 genome includes several accessory proteins, which facilitate virus transmission in vivo by establishing persistent infection (Collins et al., 1998; Hiraragi et al., 2006; Nicot et al., 2004; Silverman et al., 2004; Ye et al., 2003). The tax gene product is a potent activator of viral transcription (Boxus and Willems, 2009; Felber et al., 1985; Yin and Gaynor, 1996). In addition, through its involvement in a number of cellular transcription pathways, Tax stimulates the proliferation, survival, and transformation of HTLV-1-infected T cells (Boxus and Willems, 2009; Matsuoka and Jeang, 2007; Peloponese, Kinjo, and Jeang, 2007). Another regulatory protein, p30II, down-regulates Tax expression by interaction with, and nuclear retention of tax mRNA (Nicot et al., 2004). Other HTLV-1 regulatory proteins include Rex, p12I, p27I, and p13II (Fig. 1) (Albrecht and Lairmore, 2002).
Fig. 1. Schematic representation of the HTLV-1 genome.
The viral mRNAs, their direction of transcription and location of the open reading frames (black boxes) are shown. hbz SP1 and SP2 are two spliced variants of hbz mRNA, while hbz US is the unspliced transcript. The dotted lines represent the introns removed after mRNA splicing. The arrows represent the primers used to amplify the viral mRNAs for the RT-PCR assays. The white cross in p30 represents the location of the p30 stop codon, introduced to generate the Δp30 mutants. The black line above hbz SP1 represents the location of the stem-loop structure in hbz.
Recently, another HTLV-1 regulatory gene was identified on the antisense genomic DNA strand; since its protein product includes a basic region and a leucine zipper, it was designated HTLV-1 bZIP factor, or HBZ (Cavanagh et al., 2006; Gaudray et al., 2002; Larocca et al., 1989). The hbz mRNA is expressed in primary ATLL cells, despite repression of other viral transcripts (Matsuoka and Green, 2009; Satou et al., 2006). Multiple hbz mRNA initiation sites have been identified in the 3′-LTR (Cavanagh et al., 2006; Yoshida et al., 2008), which result in three transcripts; two are spliced (hbz SP1 and SP2) while the other is unspliced (hbz US) (Fig. 1) (Cavanagh et al., 2006; Murata et al., 2006; Satou et al., 2006). The SP1 spliced variant is the most abundant form in ATLL cell lines and infected lymphocytes from ATLL patients (Satou et al., 2006; Usui et al., 2008). The promoters for hbz SP1 and hbz US have been identified, and studies suggest that the levels of antisense transcription are 20–50 fold lower (Arnold et al., 2006; Larocca et al., 1989) than sense transcription (Yoshida et al., 2008). The hbz SP1 and hbz US mRNAs encode protein products that differ only in their N-terminal seven amino acids, whereas the SP2 transcript does not code for a protein product, and its function is undefined. The hbz transcripts include sequences complementary to the other HTLV-1 transcripts encoded from the sense genomic DNA strand.
HBZ has been reported to be a negative modulator of Tax. Upon exogenous over-expression, HBZ binds to and inhibits CREB-2, an essential transcription factor for Tax-mediated trans-activation of the viral promoter (Basbous et al., 2003; Gaudray et al., 2002). However, HBZ expression does not affect the ability of HTLV-1 to immortalize T lymphocytes in culture (Arnold et al., 2006); in fact, HBZ enhances virus infectivity and persistence in vivo. Another study identified a distinct activity of hbz mRNA, and demonstrated enhanced T cell proliferation in culture and transgenic mice (Satou et al., 2006). They identified a domain of hbz mRNA, localized to the first stem-loop (SL), that mediated this activity. These data led to the hypothesis that the hbz gene has dual functionality: hbz mRNA promotes T-cell proliferation, while HBZ protein suppresses Tax-mediated viral transcription.
In order to delineate and differentiate the activities of hbz mRNA and HBZ protein, we used an infectious molecular proviral clone of HTLV-1 (Kimata et al., 1994) to assess mutations that specifically affect the structure and/or expression of the hbz gene product. This led to the unexpected identification of a third activity of hbz that promotes tax mRNA expression. These results have important implications for understanding the regulation of HTLV-1 gene expression.
Results
Construction and characterization of pACH.HBZ mutant proviral clones
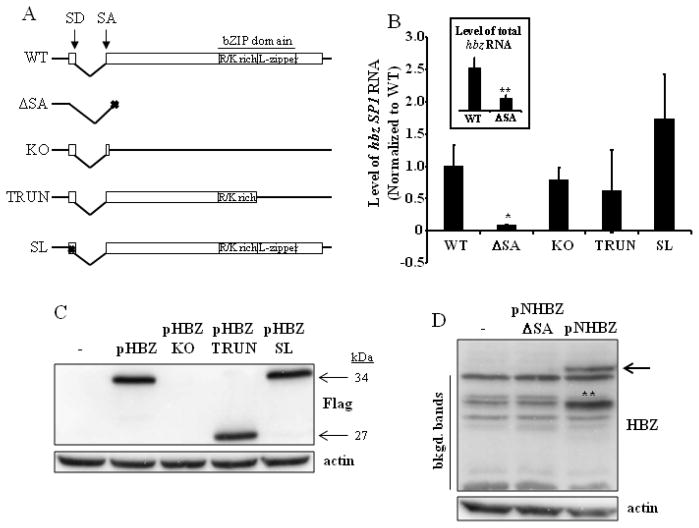
To investigate the role of HBZ in the context of an HTLV-1 provirus, we generated four HBZ mutant proviral clones (Fig. 2A). For this purpose, we used a well characterized infectious molecular clone, designated pACH (Kimata et al., 1994). pACH.HBZ-ΔSA has a mutation in the splice acceptor site of the hbz gene that prevents the proper splicing of the SP1 and SP2 hbz mRNAs (Fig. 1). pACH.HBZ-KO is a knockout mutant that terminates the predominant HBZ protein derived from the SP1 variant at amino acid seven, as well as the HBZ protein derived from the US variant at amino acid 10 (Fig. 2A). pACH.HBZ-TRUN is a truncation mutant that deletes the leucine zipper portion of the bZIP domain of variants SP1 and US, through insertion of a termination codon in place of codon 158 (SP1)/codon 161 (US) within the 206 (SP1)/209 (US) amino acid open reading frame. The leucine zipper portion of HBZ is required for the association of HBZ with CREB-2, JunB, and c-Jun (Basbous et al., 2003; Gaudray et al., 2002). pACH.HBZ-SL has a mutation that disrupts the stem-loop at the 5′ end of the hbz mRNA that is only found in the SP1 variant (Satou et al., 2006). Although mutants similar to pACH.HBZ-KO and pACH.HBZ-TRUN were constructed and tested by other investigators (Arnold et al., 2006), our studies were aimed at distinguishing the role of hbz mRNA versus HBZ protein at physiological levels.
Fig. 2. Analysis of the hbz mRNA transcript and HBZ protein.
(A) Schematic representation of wild-type HBZ (WT), with its splice donor (SD) and acceptor (SA) sites and bZIP domain indicated. The four HBZ mutants generated for this study are also depicted. These mutants were made in the context of the pACH proviral clone. ΔSA, splice-deficient mutant; KO, knockout mutant; TRUN, truncation mutant; SL, stem-loop mutant. (B) 293T cells were transfected with the pACH.HBZ-WT or mutant plasmids. Total RNA was isolated 96h post-transfection, and the hbz SP1 mRNA amplified by real-time RT-PCR using primers shown in Fig. 1. Background values were subtracted and data normalized to WT. GAPDH was amplified as an internal control, and did not differ significantly between samples. These results represent an average of at least three independent experiments. Inset, total hbz mRNA was amplified by real-time RT-PCR using the primers within exon 2 of the hbz gene shown in Fig. 1. Background values were subtracted and data normalized to WT (ΔSA value is 0.31). Student’s two-tailed t tests were performed to determine significant differences between samples (*, P < 0.05; **, P < 0.01). (C) 293T cells were transfected with pHBZ1 (pHBZ) or mutant HBZ expression plasmids. Western blot analysis was carried out to show HBZ-flag expression from these plasmids, using anti-Flag antibody. The size of the proteins are indicated to the right of the blot. Detection of actin was carried out as a loading control. (D) 293T cells were transfected with pNHBZ or pNHBZ-ΔSA expression plasmids. Western blot analysis was carried out to show HBZ expression (arrow) from these plasmids, using anti-HBZ antibody. Detection of actin was carried out as a loading control. The background bands (bkgd. bands) are indicated to the left of the blot. **, the intensity of this band (as compared to the others) is probably due to protein degradation. It cannot represent an HBZ protein translated using an internal ATG, since there is no start site that would produce a protein of that size.
We first determined the levels of hbz SP1 RNA expressed from the proviral mutant plasmids (Fig. 2B). 293T cells were transiently transfected with each of the various mutants, and total RNA isolated from these cells was subjected to real-time RT-PCR, using primers that spanned the hbz splice junction (Fig. 1). The results from an average of at least three independent experiments are shown in Fig. 2B. Mutating the splice acceptor site of the hbz gene significantly reduced the steady state levels of hbz SP1 RNA by greater than 90% (P = 0.03). As expected, mutants pACH.HBZ-KO and pACH.HBZ-TRUN, which were constructed to truncate the HBZ protein without affecting hbz mRNA, were found to have no significant effects on the levels of hbz RNA (pACH.HBZ-KO: P = 0.57; pACH.HBZ-TRUN: P = 0.15). Disrupting the stem-loop structure of hbz mRNA did not affect the stability of the hbz mRNA, since steady state levels were equivalent to those of cells expressing pACH.HBZ-WT (P = 0.35). We also measured levels of total hbz RNA in cells transfected with the pACH.HBZ-WT and pACH.HBZ-ΔSA plasmids (Fig. 2B, inset), and found that the levels were decreased by 69% in the presence of the splice acceptor mutation. These levels of total hbz most likely correspond to the hbz US transcript, as this level is consistent with previously reported levels of hbz US (Usui et al., 2008)
In order to examine the expression of HBZ protein from the various mutants, we generated HBZ wild-type (pHBZ) and mutant (pHBZ-KO, pHBZ-TRUN, and pHBZ-SL) expression plasmids, inserting a triple flag tag at the C-terminus of each predicted protein product. 293T cells were transiently transfected with these plasmids, and Western blot analysis was carried out (Fig. 2C). As expected, no HBZ expression was seen from cells transfected with the HBZ-KO mutant. The HBZ-TRUN mutant produced a truncated HBZ-flag protein of 27 kDa. Cells expressing wild-type HBZ and the HBZ-SL mutant both produced HBZ-flag proteins of 34 kDa. Levels of expression of WT, TRUN, and SL HBZ proteins were equivalent.
To confirm that pACH.HBZ- SA does not produce an unspliced gene product, we generated an HBZ- SA expression plasmid (pNHBZ-ΔSA) that contains both the exons of hbz SP1 and the sequence corresponding to the intronic region between them. 293T cells were transiently transfected with this plasmid and a HBZ wild-type expression plasmid, pNHBZ, and Western blot analysis was carried out with anti-HBZ antiserum (Fig. 2D). We did not see any HBZ expression from pNHBZ-ΔSA, confirming the lack of an unspliced gene product. Additionally, we did not see a difference in unspliced mRNA levels between cells transfected with the wild-type and ΔSA mutant proviral plasmids (data not shown).
Loss of hbz leads to reduction in tax expression
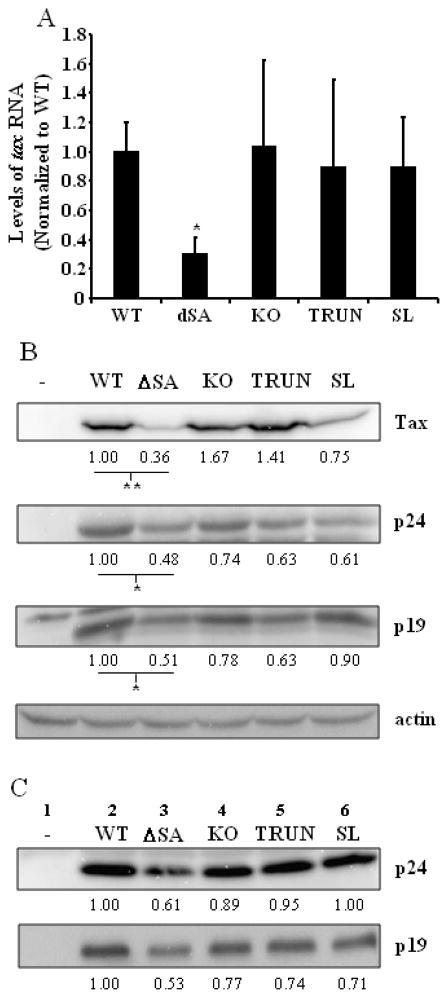
The pACH.HBZ mutants were used to determine the effects of hbz mRNA and HBZ protein on expression of other HTLV-1 genes. Total RNA isolated from 293T cells transiently transfected with the HBZ proviral mutants was subjected to real-time RT-PCR to measure the levels of tax transcript. The primers used to amplify tax spanned the second splice junction (Fig. 1). The results from an average of four independent experiments are shown in Fig. 3A. There was a 70% decrease in the steady state level of tax mRNA expressed from the pACH.HBZ-ΔSA mutant, as compared to the level of tax mRNA expressed by pACH.HBZ transfected cells. Jurkat cells transfected with the pACH.HBZ-WT plasmid expressed 0.12 ± 0.07 pg Tax mRNA, while those transfected with the pACH.HBZ-ΔSA mutant plasmid expressed 0.0 ± 0.02 pg Tax mRNA (P = 0.03) (data not shown). No significant changes were seen in tax mRNA expressed from the other pACH mutants (Fig. 3A). The levels of tax mRNA, relative to that of pACH.HBZ-WT expressing cells, with pACH.HBZ-KO, pACH.HBZ-TRUN, and pACH.HBZ-SL were 87%, 104%, and 73%, respectively (P = 0.78, 0.96, and 0.51, respectively).
Fig. 3. HTLV-1 gene expression in the absence of hbz mRNA or HBZ protein.
(A) 293T cells were transfected with the pACH.HBZ-WT or mutant plasmids. Total RNA was isolated 96h post-transfection, and the tax mRNA amplified by real-time RT-PCR, using primers shown in Fig. 1. GAPDH was amplified as an internal control, and did not exhibit significant differences between samples. These results represent an average of four independent experiments. Student’s two-tailed t tests were performed to determine significant differences between samples (*, P < 0.05). (B and C) Western blot analysis was carried out to measure levels of Tax and Gag (p19 and p24) proteins in the (B) lysates and (C) viral supernatant. Detection of actin was carried out as a loading control. The numbers above the blots represent the lane numbers, while the numbers below the blots represent the densitometry signal of the bands. A representative experiment is shown from a total of three experiments. Student’s two-tailed t tests were performed to determine significant differences between samples (B; *, P < 0.05; **, P < 0.01). Significant differences were seen between the WT and ΔSA samples (Tax: P = 0.0002, p24: P = 0.0462, p19: P = 0.0488), whereas no differences were seen between the WT and KO (Tax: P = 0.76, p24: P = 0.81, p19: P = 0.82), WT and TRUN (Tax: P = 0.58, p24: P = 0.53, p19: P = 0.38), or WT and SL (Tax: P = 0.08, p24: P = 0.11, p19: P = 0.79) samples.
Levels of viral proteins were also assessed, and normalized to levels of actin, used as a loading control (Fig. 3B). There was a 64% decrease in the amount of Tax protein expressed from the pACH.HBZ-ΔSA mutant, compared to Tax protein expressed from pACH.HBZ transfected cells. No significant decrease in Tax protein was seen with the other mutants.
Since Tax is responsible for the trans-activation of viral transcription, the levels of the viral structural protein, Gag were measured in the cellular lysate and virus particles in the culture supernatant. As expected, levels of Gag p24 and p19 were also decreased in cells transfected with pACH.HBZ-ΔSA compared to pACH.HBZ-WT (Fig. 3B, lane 3), as well as in virus particles released into the supernatant from those cells (Fig. 3C, lane 3). These results suggest that the effect of hbz mRNA on the levels of tax mRNA also influences the levels of Gag expression in transfected cells and the levels of released virus particles.
Trans-complementation of hbz increases Tax activity in pACH.HBZ-ΔSA cells to wild-type levels
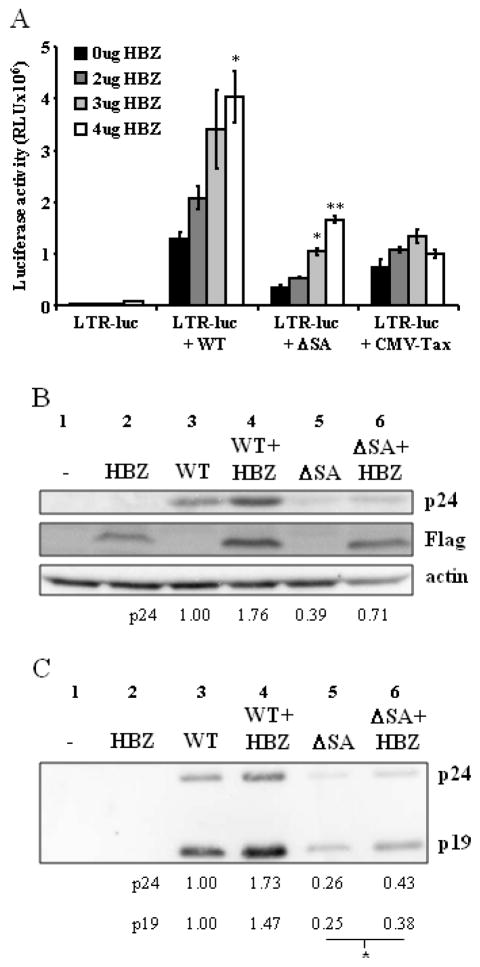
To ensure that the effects on steady state levels of Tax in pACH.HBZ-ΔSA transfected cells were due to loss of hbz mRNA, we measured levels of Tax-mediated LTR gene expression in the presence of increasing amounts of hbz. Co-transfection of pACH.HBZ-WT or pACH.HBZ-ΔSA, as a source of Tax, together with an LTR-luciferase reporter plasmid, provided a sensitive quantitative measure of Tax activity. It has previously been shown that Tax expressed from the pACH plasmid is able to trans-activate the HTLV-1 LTR (Robek and Ratner, 1999). In this study, the specific role of Tax in transcriptional transactivation was confirmed using the M47 Tax mutation in pACH, a mutation which prevents Tax from upregulating transcription through CREB/ATF (Smith and Greene, 1990). This mutant had 50-fold lower levels of HTLV-1 LTR activation, using the LTR-luciferase reporter plasmid, compared to wild-type pACH.
In the trans-complementation experiment with hbz, increasing quantities of a plasmid were utilized in which hbz was expressed under the regulation of a CMV promoter, pHBZ (Fig. 4A). In cells transfected with pACH.HBZ-ΔSA, together with increasing amounts of the hbz expression plasmid, the levels of LTR-directed gene expression increased. It should be noted that in the presence of 4μg pHBZ, the level of Tax activity in pACH.HBZ-ΔSA transfected cells increased to levels seen in pACH.HBZ-WT transfected cells. The levels of Tax activity also increased in cells transfected with pACH.HBZ-WT in the presence of pHBZ. However, there was no significant increase in Tax activity with increasing amounts of pHBZ in cells transfected with a Tax expression plasmid (CMV-Tax). One explanation for this result is that another viral gene, expressed from the proviral plasmid, is required for the effect of hbz on tax expression.
Fig. 4. Trans-complementation with heterologous hbz rescues the defect seen with the mutation of hbz in the pACH infectious clone.
(A) 293T cells were co-transfected with LTR-luc, and with the pACH.HBZ-WT or pACH.HBZ-ΔSA plasmids, or CMV-Tax, in the presence of increasing amounts of pHBZ. Luciferase assays were carried out 48h post-transfection. Student’s two-tailed t tests were performed to determine significant differences between samples (*, P < 0.05; **, P < 0.01). A representative experiment is shown from three total experiments. (B and C) 293T cells were transfected with the pACH.HBZ-WT or pACH.HBZ-ΔSA plasmids in the presence or absence of pHBZ. At 96h post-transfection Western blot analysis was carried out to measure levels of Gag (p19 and p24) proteins in the (B) lysates and (C) viral supernatant. Detection of actin was carried out as a loading control. The numbers above the blots represent the lane numbers, while the numbers below the blots represent the densitometry signal of the bands. Student’s two-tailed t tests were performed to determine significant differences between samples (*, P < 0.05).
As in the previous experiment (Fig. 3), we examined the levels of Gag protein in the cellular lysate and viral supernatant, as a further measure of Tax activity. As expected, the levels of Gag p24 in the lysate increased in the presence of exogenous hbz (Fig. 4B, lanes 4 and 6, compared to lanes 3 and 5, respectively), and significantly more viral particles were released into the supernatant (Fig. 4C, lanes 4 and 6, compared to lanes 3 and 5, respectively).
hbz represses p30II to indirectly promote tax expression
The HTLV-1 accessory protein p30II is a nuclear protein that retains the doubly spliced tax mRNA in the nucleus, leading to a reduction in the amount of Tax protein (Nicot et al., 2004). Since p30II is a negative regulator of tax, we hypothesized that hbz may negatively regulate p30II in order to promote tax mRNA expression. Since hbz mRNA is transcribed from the (−) strand of the HTLV-1 provirus, it is a natural antisense RNA to genes expressed from the (+) proviral DNA strand. Since the majority of the hbz transcript overlaps the p30II transcript, we hypothesized that the hbz mRNA binds to the p30II mRNA to promote its degradation or inhibit its translation.
To test our hypothesis, we co-transfected 293T cells with pMHp30IIHA1, a p30II expression plasmid and pHBZ, and carried out real-time RT-PCR on total RNA from the cells (Fig. 5A). In the presence of increasing amounts of HBZ, we saw a significant decrease in the levels of p30II mRNA. To determine whether this effect was dependent on HBZ protein or hbz mRNA, we generated a protein mutant of pHBZ, pmHBZ, mutating the initiator ATG to TTG. In the presence of increasing amounts of mutant pHBZ, we also found a significant decrease in the levels of p30II mRNA, suggesting that the hbz mRNA, not the HBZ protein, is responsible for the decreased levels of p30II mRNA. The p30II protein levels are also reduced in the presence of wild-type HBZ and the protein mutant of HBZ, mHBZ, whereas the p30II protein levels remained unchanged in the presence of the RNA mutant of HBZ, pNHBZ-ΔSA (Fig. 5B), confirming that hbz mRNA inhibits synthesis of p30II protein.
Fig. 5. Expression of hbz depresses p30II levels, and loss of p30II compensates for loss of hbz.
(A) 293T cells were transfected with pMHp30IIHA1 (p30) and increasing amounts of either pHBZ2 (HBZ) or pmHBZ (mHBZ). Total RNA was isolated 48h post-transfection, and the p30 mRNA amplified by real-time RT-PCR. GAPDH was amplified as an internal control, and did not exhibit significant differences between samples. A representative experiment is shown from two experiments. Student’s two-tailed t tests were performed to determine significant differences between samples (**, P < 0.01). (B) 293T cells were transfected with pMHp30IIHA1 (p30) and either HBZ, mHBZ, pNHBZ, or pNHBZ-ΔSA. Western blot analysis was carried out 48h post-transfection to measure levels of HBZ and p30 protein. Detection of actin was carried out as a loading control. The numbers below the blots represent the densitometry signal of the bands, normalized to levels of actin. (C and D) 293T cells were transfected with the pACH.HBZ-WT or pACH.HBZ-ΔSA plasmids with or without the p30II mutation, WTΔp30 and ΔSAΔp30. At 48h post-transfection, Western blot analysis was carried out on the (C) lysates and (D) viral supernatant for levels of viral Gag (p19 and p24). *, indicates background bands. Detection of actin was carried out as a loading control. The numbers above the blots represent the lane numbers, while the numbers below the blots represent the densitometry signal of the bands.
To investigate the role of p30II, we generated two p30II mutant proviral clones. Both pACHΔp30 and pACH.HBZ-ΔSAΔp30 include a termination codon in place of amino acid eight of the p30II open reading frame, in the context of the hbz wild-type and hbz splice-deficient expression plasmids, respectively (Fig. 1). 293T cells were transiently transfected with these plasmids and the cellular lysate and supernatant evaluated for levels of Gag (p24 and p19) protein. In the absence of p30II, there was a 1.95-fold increase in the levels of Gag protein in the cellular lysate (Fig. 5C, lane 3, compared to lane 2) and a four-fold increase over wild-type in the viral supernatant (Fig. 5D, lane 3, compared to lane 2), which corresponds, as expected, to an increase in the level of Tax protein. As seen previously (Fig. 3), loss of hbz leads to a decrease in the amount of Gag protein in the cellular lysate and supernatant (Fig. 5C and 5D, lane 4). However, the loss of hbz was overcome by deleting p30II, as evidenced by an increase in the levels of Gag protein in the cellular lysate (Fig. 5C, lane 5) and viral supernatant (Fig. 5D, lane 5) from cells transfected with pACH.HBZ-ΔSAΔp30. Taken together, these results suggest that hbz promotes expression of tax indirectly through p30II.
Discussion
Since the discovery of the HTLV-1 antisense hbz gene (Gaudray et al., 2002), numerous studies have been carried out to establish its role in HTLV-1 replication and pathogenesis. Though these studies have been informative and have established a foundation for hbz in the HTLV-1 field, the function of hbz in vivo is still unclear. HBZ was identified as a binding partner to CREB-2; it binds CREB-2 through its basic leucine zipper (bZIP) domain (Gaudray et al., 2002). Since CREB-2 has been shown to cooperate with Tax in trans-activating the HTLV-1 LTR (Ching et al., 2004), studies have shown that exogenously over-expressed HBZ protein down-regulated Tax-mediated viral transcription by binding CREB-2 (Gaudray et al., 2002; Lemasson et al., 2007). Furthermore, Tax has been shown to activate a number of cellular pathways, such as the activator protein-1 (AP-1) pathway (Fujii et al., 1991; Fujii, Sassone-Corsi, and Verma, 1988; Hooper et al., 1991). Studies with exogenous HBZ showed that, through its bZIP domain, HBZ could bind to members of the AP-1 pathway (cJun and JunB) and down-regulate Tax-mediated AP-1 transcription (Basbous et al., 2003; Clerc et al., 2009; Hivin et al., 2007; Matsumoto et al., 2005). However, since these studies employed over-expression of HBZ in the absence of other viral proteins, a more physiologically relevant study was carried out using HBZ expressed from a molecular clone of HTLV-I (Arnold et al., 2006). Data from this study showed that HBZ protein enhanced viral infectivity and persistence in vivo. In addition to this function of HBZ protein, the hbz mRNA has been shown to promote T-cell proliferation (Arnold et al., 2008; Satou et al., 2006).
Since previous studies suggested that hbz has dual functionality, we examined the effects of various proviral hbz mRNA and protein mutants on viral gene expression. We showed that a loss of hbz mRNA leads to a significant reduction in the levels of tax mRNA (Fig. 3). This reduction of tax mRNA leads, as expected, to lower Tax protein levels, and a reduction in the levels of Gag protein and virus particle production. These data are in contrast to previous work that showed that hbz-specific short hairpin (sh)-RNAs, which down-regulated the levels of hbz in an HTLV-1 T-cell line, did not affect levels of gag/pol and tax/rex mRNAs or p19 Gag and Tax protein (Arnold et al., 2008). A possible reason for this discrepancy is discussed below.
To confirm that the reduction in Tax levels was a consequence of the loss of hbz mRNA, a complementation study was carried out in which increasing amounts of exogenous hbz were expressed. In contrast to previous work (Arnold et al., 2006), we found that when Tax was expressed from a proviral plasmid, the levels of Tax activity increased with increasing amounts of pHBZ (Fig. 4). Of particular note is that, in the presence of 4ug of pHBZ, Tax activity in cells transfected with the splice-deficient hbz mutant increased to wild-type levels.
Another interesting finding was that when Tax was expressed from an expression plasmid rather than a proviral expression plasmid, there was no increase in Tax activity in the presence of pHBZ (Fig. 4). We concluded that a viral gene, expressed from the proviral plasmid, was required for hbz to have its effect on tax. Alternatively, there is the possibility that there are differences in the cis-sequences of the RNA expressing Tax encoded by the proviral plasmid compared to that encoded by the Tax expression plasmid. However, this would imply that hbz has a direct effect on tax, but we do not favor this possibility, as discussed below. Possible explanations for the result seen in Figure 4 are: (1) hbz represses an inhibitor of tax, (2) hbz promotes an activator of tax, or (3) hbz has a direct effect on tax. The first hypothesis is more attractive for the following reasons. First, since hbz is transcribed from the (−) strand of the HTLV-1 proviral genome, it is a natural antisense RNA to other viral transcripts; it could form a double stranded (ds)-RNA structure with other viral transcripts, which may be degraded by cellular nucleases or merely inhibit their translation. Second, it is notable that the HTLV-1 gene encoding p30II has a repressive function on tax (Nicot et al., 2004), similar to that manifested by loss of hbz. The p30II protein retains doubly spliced tax mRNA in the nucleus, thereby decreasing the levels of Tax protein. It is notable that the hbz transcript overlaps those encoding pX open reading frames I and II, which encode p12I and p30II and p13II, respectively (Fig. 1). Of the three accessory proteins encoded by these regions of the viral genome, only p30II has been shown to directly regulate tax expression (Albrecht and Lairmore, 2002). Hence, we hypothesized that hbz inhibits p30II mRNA, and the reduction in p30II protein expression leads to an increase in the levels of Tax protein. Moreover, steady state levels of p30II mRNA are low compared to the level of hbz mRNA, in contrast to levels of tax and gag mRNAs, thus p30II mRNA would be predicted to be exquisitely sensitive to inhibition by a complementary RNA sequence.
The discrepancy between the currently reported studies and those reported previously based on studies of the effects of shRNAs to hbz (Arnold et al., 2008), can be explained by the fact that the expression of p30II may have been suppressed in the transformed cell lines used in the previous study. Furthermore, it is unclear whether the shRNAs were able to reduce the levels of hbz RNA effectively enough to repress its effect on p30II. Another difference between the current study with an infectious proviral clone and the previous study using transformed cell lines may be related to differences in levels of hbz mRNA and its mechanism of gene expression. It is unclear whether hbz expression in transformed cell lines is derived predominantly from intact proviruses or deleted proviruses (Matsuoka and Green, 2009). Expression of hbz mRNA from a deleted provirus in ATLL cell lines may repress tax expression from intact proviruses in the same cell, which may resemble the results in the current study in which trans-complementation effects of hbz were examined (Fig. 4). Although (+) strand transcriptional occlusion of the hbz promoters in an intact provirus may inhibit its expression, we found only four-fold lower levels of hbz mRNA generated from the full provirus compared to that generated from the 3′ half of the provirus, under our transient transfection conditions (data not shown). Thus, promoter occlusion is not likely a major restrictive mechanism for hbz expression except when the 5′ LTR promoter is highly upregulated (Greger et al., 1998; Palmer et al., 2009).
To examine the effect of hbz on p30II, we measured levels of p30II mRNA in the presence of increasing amounts of wild-type HBZ and an HBZ protein mutant. We found that levels of p30II mRNA were significantly reduced in either case, implicating hbz mRNA for this function (Fig. 5). Furthermore, we generated two p30II mutants in the context of the hbz wild-type and hbz splice-deficient mutant plasmids, to examine the effect of p30II on Tax activity. We observed that in the absence of p30II, there was an increase in the levels of Gag proteins, and virus particle production (Fig. 5). Further studies focusing on the relationship between hbz and p30II mRNA will further define the detailed molecular mechanism for this interaction.
The current studies implicate hbz mRNA rather than HBZ protein in promoting Tax expression and activity. Mutations that disrupt the coding capacity of hbz but do not affect hbz mRNA, pACH.HBZ-KO and pACH.HBZ-TRUN, did not perturb Tax expression (Figs. 2 and 3). It has been proposed that a stem-loop structure at the 5′-end of hbz mRNA is important for its growth-promoting activity (Satou et al., 2006). It is conceivable that this stem-loop RNA sequence may bind specific cellular proteins or is processed into microRNA that could inhibit other viral genes. However, in the current studies, pACH.HBZ-SL exhibited similar activity to pACH.HBZ-WT with respect to Tax expression (Fig. 3). This suggests that the effects of hbz mRNA in promoting Tax expression are not specifically localized to the 5′ stem-loop structure.
An interesting recent observation is that the spliced hbz transcript (hbz SP1) and the pX region are present in all ATLL cell lines, regardless of levels of tax expression (Matsuoka and Green, 2009; Satou et al., 2006). Since it is known that Tax elicits a strong cytotoxic T-lymphocyte (CTL) response in vivo, it is conceivable that the virus has evolved intricate mechanisms to regulate the levels of Tax, in order to maximize its spread. It is possible that hbz and p30II work together to provide this tight regulation early in infection. A possible model of how the hbz mRNA controls tax expression, via p30II, is shown in Fig. 6. This model proposes that hbz mRNA down-regulates p30II mRNA and inhibits synthesis of the p30II protein (Fig. 6, step 1). Thus, in the presence of hbz mRNA, less p30II protein is present in the nucleus (step 2) to retard tax mRNA nucelo-cytoplasmic transport (step 3). Consequentially, tax mRNA is more readily transported to the cytoplasm, and translated (step 4). Therefore, in the presence of hbz mRNA, there is an increased level of Tax protein and thus, increased levels of other viral gene products. These hypotheses, testable in animal models and clinical studies, may provide new insights into HTLV-1 pathogenesis.
Fig. 6. Model for effect of hbz on tax.
Schematic representation of a possible mechanism for hbz’s control of tax: (1) hbz mRNA inhibits p30II mRNA, which (2) leads to a reduction in the amount of p30II protein. Thus, less p30II is translocated to the nucleus, and as a result, (3) less tax mRNA is retained in the nucleus. (4) This results in the synthesis of more Tax protein, leading to an increase in viral gene expression and virus production.
Materials and Methods
Cells
293T cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% FetalClone III (FCIII) serum (Hyclone), 2mM glutamine, 1mM sodium pyruvate, penicillin (100 U/mL), and streptomycin (100μg/mL).
Plasmids
The HTLV-1 molecular proviral clone pACH was used in this study (Kimata et al., 1994). Site directed mutagenesis was performed by a PCR overlap extension method (Higuchi, Krummel, and Saiki, 1988). pACH.HBZ-ΔSA was generated by introducing a T to G silent point mutation (nt. 7268), which abolishes the splice acceptor site of the hbz gene. pACH.HBZ-KO was generated by introducing a G to A point mutation (nt. 7258), which terminates the HBZ protein at amino acid seven (HBZ SP1 open reading frame). This mutation also results in an arginine to glutamine change in the p30II accessory protein. However, this does not affect the activity of p30II (Arnold et al., 2006). pACH.HBZ-TRUN was generated by introducing a C to A point mutation (nt. 6807), which terminates the HBZ protein at amino acid 158, and truncates the protein by deleting its leucine zipper domain. pACH.HBZ-SL was generated by mutating the sequence 8670GGC8672 to TTT. This change was based on a previously described mutant, which mutates the stem-loop structure in the hbz gene (Satou et al., 2006). pACHΔp30 and pACH.HBZ-ΔSAΔp30 were generated by introducing a C to G point mutation (nt. 6850) in pACH and pACH.HBZ-ΔSA, respectively. This mutation terminates the p30II protein at amino acid eight. The sequence numbers are based on that of pACH. To ensure that the mutations had no effect on the integrity of the proviral plasmids, we digested the plasmids with the PvuII restriction enzyme, to check for any gross deletions in the plasmids, and sequence analysis of the plasmids was performed. The HBZ expression vectors, pHBZ1 and pHBZ2, were generated by cloning the hbz SP1 cDNA into the SnaBI and XbaI and EcoRI and XbaI sites of p3xFLAG-CMV™ -14 (Sigma-Aldrich), respectively. The mutant HBZ expression plasmids, pHBZ-KO, pHBZ-TRUN, and pHBZ-SL, were generated in a similar manner to pHBZ1, by cloning the mutant hbz SP1 cDNAs. The modified HBZ expression vector, pNHBZ, was generated by cloning the hbz SP1 cDNA into the EcoRI and BamHI sites of p3xFLAG-CMV™-10 (Sigma-Aldrich). The pNHBZ-ΔSA plasmid was generated by amplifying the sequence corresponding to the two hbz SP1 exons and the intronic region from pACH, and cloning into the same plasmid as pNHBZ. The pmHBZ plasmid was generated by mutating the initiating ATG in pHBZ2 to TTG. The pMHp30IIHA1 plasmid was a generous gift from Dr. C. Nicot (Nicot et al., 2004). The LTR-luciferase (LTR-luc) reporter plasmid and the CMV-Tax plasmid have been described previously (Rauch et al., 2009; Smith and Greene, 1990).
Transfections and luciferase assay
To test the various mutants, 5 × 106 293T cells were transfected with 10ug of pACH, a mutant proviral plasmid, or an empty plasmid, in the presence or absence of 5ug of pHBZ1. At 96h post-transfection, the cells were washed from the plate and pelleted. The supernatants were filtered through 0.45μm filters (Corning) and subjected to ultracentrifugation in a Beckman-Coulter L7 Ultracentrifuge. These lysates were used for Western blot analysis. The cell pellets were washed with PBS; half of the cells were used for real-time RT-PCR, while the other half was used for Western blot analysis. 293T (3 × 106) cells were transfected with 5ug of pHBZ1 or pNHBZ, a mutant HBZ expression plasmid, or an empty plasmid. At 48h post-transfection, the cells pellets were lysed and used for Western blot analysis. 293T (3 × 106) cells were transfected with 3ug of pMHp30IIHA1, and either 0.5ug, 2ug, or 8ug of pHBZ2 or pmHBZ, or 5ug of pHBZ2, pmHBZ, pNHBZ, or pNHBZ-ΔSA. DNA levels were kept constant with empty vector. At 48h post-transfection the cell pellets were lysed, and used for real-time RT-PCR, or Western blot analysis. To test for Tax activity, 2.5 × 105 293T cells were transfected with 1ng LTR-luc, 1ug of pACH/mutant, pCMV-Tax, and 0, 2, 3, or 4ug of pHBZ. Empty vector was included to equalize the amount of DNA added, where necessary. At 48h post-transfection, the cell pellets were resuspended in lysis buffer and subjected to a luciferase assay (Luciferase Assay System, Promega). All transfections were carried out using TransIT-LT1 Transfection Reagent (Mirus) ransfected according to the manufacturer’s recommendations. Five million Jurkat cells were t with 15ug of pACH.HBZ-WT or pACH.HBZ-ΔSA proviral plasmids, using polyethylenimine (PEI) transfection reagent (Sigma-Aldrich).
Quantitative real-time RT-PCR
Total RNA was extracted from transfected 293T cells using the RNeasy kit (Qiagen). One microgram of RNA was subjected to reverse transcription using the SuperScript™ III First-Strand Synthesis System (Invitrogen). Gene-specific reverse primers were used to generate cDNAs; the primers used were as follows: tax(GCtaxR) – 7649CCATTTCGGAAGGGGGAGTATTTGC7625, hbz SP1 and total hbz (GChbzoR) – 6857TTGTCTCCACTTGCGCTCACGGCG6880, and p30 (GChbzoF) – ATGGTTAACTTTGTATCTGTAGGGC. Reactions were performed both in the presence and absence of reverse transcriptase to control for DNA contamination. The cDNAs (3μL) were subjected to real-time PCR using a 2X iQ SYBR Green Supermix (Bio-Rad) and gene-specific primer pairs (500nM each), as follows: Tax (GCtaxF) – 5117AGCTGCATGCCCAAGACCCGTCGGA5141 and GCtaxR, HBZ SP1 (GChbznF) – 8799TCTAAGGGAGCGCCGGACAAAG8778 and GChbzoR, total HBZ (GChbztotalF) – AAACGCATCGTGATCGGCAGC and GChbzoR, and p30 – GChbzoR and GChbzoF. The amount of each transcript present was determined based on a specific standard curve generated from log10 dilutions of plasmids containing cloned copies of the particular amplified sequences. Samples and standards were run in triplicate and the final values were averaged, after background values were subtracted.
Western blot analysis
Cell lysates were prepared using lysis buffer (50mM Tris-Cl, pH 6.8, 150mM NaCl, 1mM EDTA, 1% Triton X-100) plus protease inhibitors. Lysate (200μg) was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF; Millipore) membranes, blocked with 5% dry milk in TBS-Tween (10mM Tris-Cl, pH 7.5, 150mM NaCl, 0.05% Tween-20) and probed overnight with 1:100 HTLV-1 positive patient serum (p19, p24), 1:25 anti-Tax antibody (supernatant from hybridoma cell line 168A51-42; NIH AIDS Research & Reference Reagent Program) (Langton et al., 1988), 1:1000 anti-Flag antibody (Sigma-Aldrich), 1:500 anti-HBZ antiserum (a generous gift from Dr. P. L. Green), or 50 mU/mL horseradish peroxidase (HRP)-conjugated anti-HA antibody (3F10, Roche) or for 30 minutes with an HRP-conjugated anti-actin antibody (1:2000; Santa Cruz Biotechnology, Inc.). After washing with TBS-Tween, the appropriate HRP-labeled secondary antibody was added (1:3000 anti-human antibody [Amersham], 1:5000 anti-mouse antibody [Amersham], or 1:2000 anti-rabbit antibody [Pierce]). The blots were developed using SuperSignal West Femto substrate (Pierce) and the proteins were visualized with an Alpha Innotech imager (Model: ChemiImager).
Acknowledgments
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 168A51-42 from Dr. Beatrice Langton. We thank Dr. C. Nicot for the pMHp30IIHA1 plasmid, and Dr. P. L. Green for the HBZ antiserum. We thank Dr. D. Rauch for critique of the manuscript and experimental advice. Supported by NIH grants CA94056, CA63417, and CA10073.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht B, Lairmore MD. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol Mol Biol Rev. 2002;66(3):396–406. doi: 10.1128/MMBR.66.3.396-406.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Yamamoto B, Li M, Phipps AJ, Younis I, Lairmore MD, Green PL. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107(10):3976–82. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112(9):3788–97. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, Mesnard JM. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J Biol Chem. 2003;278(44):43620–7. doi: 10.1074/jbc.M307275200. [DOI] [PubMed] [Google Scholar]
- Boxus M, Willems L. Mechanisms of HTLV-1 persistence and transformation. Br J Cancer. 2009;101(9):1497–501. doi: 10.1038/sj.bjc.6605345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh MH, Landry S, Audet B, Arpin-Andre C, Hivin P, Pare ME, Thete J, Wattel E, Marriott SJ, Mesnard JM, Barbeau B. HTLV-I antisense transcripts initiating in the 3′LTR are alternatively spliced and polyadenylated. Retrovirology. 2006;3:15. doi: 10.1186/1742-4690-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching YP, Chun AC, Chin KT, Zhang ZQ, Jeang KT, Jin DY. Specific TATAA and bZIP requirements suggest that HTLV-I Tax has transcriptional activity subsequent to the assembly of an initiation complex. Retrovirology. 2004;1:18. doi: 10.1186/1742-4690-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc I, Hivin P, Rubbo PA, Lemasson I, Barbeau B, Mesnard JM. Propensity for HBZ-SP1 isoform of HTLV-I to inhibit c-Jun activity correlates with sequestration of c-Jun into nuclear bodies rather than inhibition of its DNA-binding activity. Virology. 2009;391(2):195 –202. doi: 10.1016/j.virol.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91(12):4701–7. [PubMed] [Google Scholar]
- Felber BK, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis GN. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229(4714):675–9. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV- 1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6 (6):1023–9. [PubMed] [Google Scholar]
- Fujii M, Sassone-Corsi P, Verma IM. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1988;85(22):8526–30. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76(24):12813–22. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Demarchi F, Giacca M, Proudfoot NJ. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 1998;26(5):1294–301. doi: 10.1093/nar/26.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16(15):7351–67. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981;78(10):6476–80. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraragi H, Kim SJ, Phipps AJ, Silic-Benussi M, Ciminale V, Ratner L, Green PL, Lairmore MD. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo. J Virol. 2006;80(7):3469–76. doi: 10.1128/JVI.80.7.3469-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivin P, Basbous J, Raymond F, Henaff D, Arpin-Andre C, Robert-Hebmann V, Barbeau B, Mesnard JM. The HBZ-SP1 isoform of human T-cell leukemia virus type I represses JunB activity by sequestration into nuclear bodies. Retrovirology. 2007;4:14. doi: 10.1186/1742-4690-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper WC, Rudolph DL, Lairmore MD, Lal RB. Constitutive expression of c-jun and jun-B in cell lines infected with human T-lymphotropic virus types I and II. Biochem Biophys Res Commun. 1991;181(3):976–80. doi: 10.1016/0006-291x(91)92032-f. [DOI] [PubMed] [Google Scholar]
- Kimata JT, Wong FH, Wang JJ, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204(2):656–64. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- Langton BC, Sliwkowski M, Tran KV, Knapp S, Keitelman E, Smith C, Wallingford S, Liu HL, Ralston JS, Brandis J, Coates S. Development and characterization of monoclonal antibodies to the HTLV-I Tax (P40X) protein. Med Virol. 1988;8:295. [Google Scholar]
- Larocca D, Chao LA, Seto MH, Brunck TK. Human T-cell leukemia virus minus strand transcription in infected T-cells. Biochem Biophys Res Commun. 1989;163(2):1006–13. doi: 10.1016/0006-291x(89)92322-x. [DOI] [PubMed] [Google Scholar]
- Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thebault S, Barbeau B, Nyborg JK, Mesnard JM. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81(4):1543–53. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Ohshima T, Isono O, Shimotohno K. HTLV-1 HBZ suppresses AP-1 activity by impairing both the DNA-binding ability and the stability of c-Jun protein. Oncogene. 2005;24(6):1001–10. doi: 10.1038/sj.onc.1208297. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–80. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- Murata K, Hayashibara T, Sugahara K, Uemura A, Yamaguchi T, Harasawa H, Hasegawa H, Tsuruda K, Okazaki T, Koji T, Miyanishi T, Yamada Y, Kamihira S. A novel alternative splicing isoform of human T-cell leukemia virus type 1 bZIP factor (HBZ-SI) targets distinct subnuclear localization. J Virol. 2006;80(5):2495–505. doi: 10.1128/JVI.80.5.2495-2505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10(2):197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- Palmer AC, Ahlgren-Berg A, Egan JB, Dodd IB, Shearwin KE. Potent transcriptional interference by pausing of RNA polymerases over a downstream promoter. Mol Cell. 2009;34(5):545–55. doi: 10.1016/j.molcel.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloponese JM, Jr, Kinjo T, Jeang KT. Human T-cell leukemia virus type 1 Tax and cellular transformation. Int J Hematol. 2007;86(2):101–6. doi: 10.1532/IJH97.07087. [DOI] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77 (12):7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch D, Gross S, Harding J, Niewiesk S, Lairmore M, Piwnica-Worms D, Ratner L. Imaging spontaneous tumorigenesis: inflammation precedes development of peripheral NK tumors. Blood. 2009;113(7):1493–500. doi: 10.1182/blood-2008-07-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73(6):4856–65. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103(3):720–5. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, Phipps AJ, Montgomery A, Ratner L, Lairmore MD. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion. J Virol. 2004;78(8):3837–45. doi: 10.1128/JVI.78.8.3837-3845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Greene WC. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4(11):1875–85. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- Usui T, Yanagihara K, Tsukasaki K, Murata K, Hasegawa H, Yamada Y, Kamihira S. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology. 2008;5:34. doi: 10.1186/1742-4690-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Silverman L, Lairmore MD, Green PL. HTLV-1 Rex is required for viral spread and persistence in vivo but is dispensable for cellular immortalization in vitro. Blood. 2003;102(12):3963–9. doi: 10.1182/blood-2003-05-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Gaynor RB. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16(6):3156–68. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Satou Y, Yasunaga J, Fujisawa J, Matsuoka M. Transcriptional control of spliced and unspliced human T-cell leukemia virus type 1 bZIP factor (HBZ) gene. J Virol. 2008;82(19):9359–68. doi: 10.1128/JVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]