Abstract
Bovine papular stomatitis virus (BPSV) and orf virus (ORFV), members of the genus Parapoxvirus of the Poxviridae, are etiologic agents of worldwide diseases affecting cattle and small ruminants, respectively. Here we report the genomic sequences and comparative analysis of BPSV strain BV-AR02 and ORFV strains OV-SA00, isolated from a goat, and OV-IA82, isolated from a sheep. Parapoxvirus (PPV) BV-AR02, OV-SA00, and OV-IA82 genomes range in size from 134 to 139 kbp, with an average nucleotide composition of 64% G+C. BPSV and ORFV genomes contain 131 and 130 putative genes, respectively, and share colinearity over 127 genes, 88 of which are conserved in all characterized chordopoxviruses. BPSV and ORFV contain 15 and 16 open reading frames (ORFs), respectively, which lack similarity to other poxvirus or cellular proteins. All genes with putative roles in pathogenesis, including a vascular endothelial growth factor (VEGF)-like gene, are present in both viruses; however, BPSV contains two extra ankyrin repeat genes absent in ORFV. Interspecies sequence variability is observed in all functional classes of genes but is highest in putative virulence/host range genes, including genes unique to PPV. At the amino acid level, OV-SA00 is 94% identical to OV-IA82 and 71% identical to BV-AR02. Notably, ORFV 006/132, 103, 109, 110, and 116 genes (VEGF, homologues of vaccinia virus A26L, A33R, and A34R, and a novel PPV ORF) show an unusual degree of intraspecies variability. These genomic differences are consistent with the classification of BPSV and ORFV as two PPV species. Compared to other mammalian chordopoxviruses, PPV shares unique genomic features with molluscum contagiosum virus, including a G+C-rich nucleotide composition, three orthologous genes, and a paucity of nucleotide metabolism genes. Together, these data provide a comparative view of PPV genomics.
Parapoxviruses (PPVs) represent one of the eight genera within the chordopoxvirus (ChPV) subfamily of the Poxviridae and include orf virus (ORFV), bovine papular stomatitis virus (BPSV), pseudocowpoxvirus (PCPV), PPV of red deer in New Zealand, and PPV of the grey seal (6, 51, 57, 65). Tentative members of the genus cause disease in camels and red squirrels (14, 68). Features that distinguish PPVs from other poxvirus genera are the ovoid virion shape, the crisscross pattern on the particle surface, and the relatively small size and high G+C content of the genome (55, 86; this report).
PPVs cause nonsystemic, eruptive skin disease in domestic and wild mammals. ORFV, the prototype species of PPV, is responsible for contagious ecthyma, an acute disease of sheep and goats. The disease, also known as orf, contagious pustular dermatitis, or scabby mouth, is characterized by proliferative lesions in the skin of the lips, around the nostrils, and in the oral mucosa (27). Lesions progress through a typical pattern of erythema, papula, pustule, and scab and usually resolve in 1 to 2 months (45). Although considered a mild disease, mortality rates up to 93% have been reported in kids (41). High mortality rates occur when lesions in lips and udders prevent infected animals from suckling and grazing, resulting in rapid emaciation (13, 41, 58). Sheep can be repeatedly infected with ORFV, albeit with shorter times to recovery and less pronounced pathological changes than in a primary infection (45). A Th1-type immune response has been implicated in host immunity to ORFV infection (reviewed in reference 32). Attenuated orf vaccines can limit the severity of the infection but they are unable to completely prevent the disease (30).
BPSV infects cattle of all ages but clinical signs are usually seen in calves. The disease has a worldwide distribution and is characterized by papules, often mildly erosive, on the muzzle, oral mucosa, and udder and occasionally in the esophagus and forestomach (40). Like ORFV in sheep and goats, reinfection of cattle with BPSV is commonly observed, suggesting that virus infection does not confer significant immunity. Because of its clinical resemblance to foot-and-mouth disease, BPSV infections are reported to veterinary authorities for differential diagnosis.
Cocirculation of BPSV and ORFV in wild ruminants has been described (35), and PPV isolates from wild ruminants have been experimentally transmitted to sheep, goats, and cattle (59, 60). Both ORFV and BPSV cause occupational infections in humans with lesions characterized by large, painful nodules in the hands and, less frequently, the face (8, 47, 69).
Classification of PPVs has relied on natural host range, pathology, and viral DNA restriction enzyme analysis. The latter revealed considerable genomic heterogeneity, even between isolates of the same virus (26, 35, 63, 64). Hybridization analysis of viral DNA indicates that internal but not terminal genomic regions are conserved between PPVs (26). Data concerning PPV genomics, largely obtained by using ORFV strain NZ2, has revealed (i) colinearity between the ORFV and other poxvirus genera genomes (21, 49, 50), (ii) the presence of a set of genes mostly located at the termini of the viral genome with putative or known roles in virulence or immunomodulation (15, 23, 38, 42, 76), and (iii) the occurrence of genomic rearrangements of the termini upon serial virus passage in vitro (12, 22). Less is known about the gene complement and genomic organization of other PPV. DNA sequencing of the right end of the BPSV strain B177 genome indicated an organization similar to that of the right end of the ORFV genome, except for the lack of a vascular endothelial growth factor (VEGF) gene in BPSV (67). Here we present the complete DNA sequences of two ORFV isolates and one BPSV isolate, thus providing an overview of PPV genomic organization and gene content as well as a comparison between the two viruses.
MATERIALS AND METHODS
Virus strains.
ORFV strain SA00 (OV-SA00) was isolated in Texas from scab material collected from a kid with severe multifocal, proliferative dermatitis and propagated in Madin-Darby ovine kidney cells (29). ORFV strain IA82 (OV-IA82) is a field isolate obtained from nasal secretions of a lamb at the Iowa Ram Test Station during an orf outbreak in 1982 and was passaged in ovine fetal turbinate cells. BPSV strain AR02 (BV-AR02) was isolated from a 3-week-old calf with oral lesions in Arkansas and passaged in primary lamb kidney cells.
Viral DNA isolation, cloning, sequencing, and sequence analysis.
Viral genomic DNA was extracted from infected primary lamb kidney cell cultures as previously described (82). Random DNA fragments were obtained by incomplete enzymatic double digestion with AciI and TaqI endonucleases (New England Biolabs, Beverly, Mass.), and DNA fragments larger than 1.0 kbp were cloned and used in dideoxy sequencing reactions as previously described (2). Reaction products were analyzed on an ABI Prism 3700 automated DNA sequencer (Applied Biosystems, Foster City, Calif.). Sequence data were assembled with the Phrap and CAP3 software programs (18, 19, 33), and gaps were closed as described previously (1). The final DNA consensus sequences for each genome represented on average seven- to ninefold redundancy at each base position and a Consed estimated error rate of 0.01 per 10 kbp (18, 19, 28).
Genome DNA composition, structure, repeats, and restriction enzyme patterns were analyzed as previously described (1) with the Genetics Computer Group GCG version 10 software package (16). Pairwise genomic alignments were done with WABA (http://www.cse.ucsc.edu/∼kent/), and multiple genomic alignments were done with Dialign (54) and Clustal (77) alignment programs. Open reading frames (ORFs) longer than 30 codons were evaluated for coding potential and ORFs greater than 60 codons were subjected to homology searches as previously described (1, 2). In addition, Framefinder (http://www.hgmp.mrc.ac.uk/∼gslater) was used to evaluate coding potential. Based on these criteria, 131 (BPSV) and 130 (ORFV) putative genes were annotated and orthologous ORFs were similarly numbered. Phylogenetic comparisons were done with the PHYLO_WIN software package (25) and Puzzle (75).
Nucleotide sequence accession number.
The genome sequences of ORFV strains IA82 and SA00 and BPSV strain AR02 have been deposited in GenBank under accession no. AY386263, AY386264, and AY386265, respectively.
RESULTS AND DISCUSSION
BPSV and ORFV genomes.
Genome sequences of BV-AR02, OV-SA00 and OV-IA82 were assembled in contiguous sequences of 134431, 139962, and 137241 bp, respectively. This agrees with previous restriction enzyme-based size estimates for both viruses (26, 48, 63). Variable genome sizes are common between PPV isolates, especially in BPSV, where differences up to 17 kb have been reported (26, 64). Hairpin loop sequences at the end of the genomes were not sequenced, and the left-most nucleotide of each assembled genome was arbitrarily designated base 1. Nucleotide composition averaged 64% G+C for each of the three isolates analyzed here. This content is not uniformly distributed along the entire genome, with a G+C content lower than 50% being found in both coding (e.g., ORFs 127 and 006/132) and intergenic regions.
Like other poxviruses, BPSV and ORFV genomes contain a large central coding region bounded by two identical inverted terminal repeat (ITR) regions (12, 26, 48). Assembled ITRs of BV-AR02, OV-SA00, and OV-IA82 contain 1,161, 3,936, and 3,092 bp, respectively. The differences in length between the ITRs of OV-SA00 and OV-IA82 strains are in agreement with previous work, indicating natural intrastrain variations in this genomic region (64). Only one ORF (001), previously described for ORFV strains NZ2 and NZ7 (20, 24) and in BPSV strain B177 (67), initiates and is completely located within the ITRs in the three virus isolates. This ORF of unknown function is unique to BPSV and ORFV, sharing 63% amino acid identity. Putative transcription control elements similar to those described for the ORFV strain NZ2 homologue are found flanking BPSV 001, suggesting early gene expression, as is the case for ORFV NZ2 (20). A second ORFV gene of unknown function (002), not present in BPSV, initiates within the unique region and terminates within the ITR.
Despite the high G+C content and paucity of stop codons, which yield 362 and 345 methionine-initiated ORFs of at least 60 codons in BPSV and ORFV genomes, respectively, coding potential analysis and similarity to known proteins led us to conservatively predict 131 genes in BPSV and 130 genes in ORFV. These genes, which encode proteins of 53 to 1289 amino acids, represent an approximate coding density of 90% for BPSV and 95% for ORFV (Table 1). The central genomic core region (ORFs 009 to 111) contains homologues of conserved poxvirus genes involved in basic replicative mechanisms and structure and morphogenesis of intracellular mature and extracellular enveloped virions (EEV) (55) (Table 1). Homologues of vaccinia virus (VACV) F9L and F10L, which are located at the left end of the conserved core in most ChPVs, are located at the right end of PPV genomes (ORFs 130 and 131). Terminal genomic regions (ORFs 001 to 008 and 112 to 134) represent approximately 20% of the viral genome and contain genes likely affecting pathogenesis. These include genes potentially involved in host range (ankyrin repeat proteins; ORFs 003, 004, 008, 118, 123, 126, 128, and 129), immune evasion (ORF 127), and immune modulation (ORF 117) and genes affecting virulence (ORF 006/132). Notably, PPVs contain a dUTPase gene previously characterized in ORFV (22, 43) but lack homologues of other ChPV genes likely involved in nucleotide metabolism, making this class of genes underrepresented in PPVs.
TABLE 1.
ORFV and BPSV ORFs
| ORF | ORFV
|
BPSV BV-AR02
|
Predicted structure/functiond | Best hite
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OV-SA00
|
OV-IA82
|
Accession no.c | Nucleotide position | Lengtha | % Id vs. OV-SA00 | Accession no. | VACV
|
MOCV
|
||||||
| Nucleotide position | Lengtha | Lengtha | % Idb vs. OV-SA00 | ORF | % Id vs. OV-SA00 | ORF | % Id vs. OV-SA00 | |||||||
| 001 | 3611-3165 | 149 | 73 | AY186732 | 956-516 | 147 | 63 | AY186733 | Unknown | |||||
| 002 | 4125-3781 | 115 | 117 | 92 | M30023 | Not present | Unknown | |||||||
| 003 | Not present | 2587-1100 | 496 | Ankyrin repeat protein | M1L | 28 | ||||||||
| 004 | Not present | 4215-2659 | 519 | Ankyrin repeat protein | M1L | 27 | ||||||||
| 005 | 5110-4874 | 79 | 71 | 90 | M30023 | 4627-4334 | 98 | 45 | Unknown | |||||
| 006 | Present in RT | 5362-4907 | 152 | 38 | VEGF | |||||||||
| 007 | 5700-5194 | 169 | 159 | 90 | AF056304 | 5949-5461 | 163 | 72 | dUTPase | F2L | 57 | |||
| 008 | 7331-5742 | 530 | 516 | 91 | S78516 | 7581-6028 | 518 | 62 | Ankyrin repeat protein | B4R | 22 | |||
| 009 | 8829-7474 | 452 | 453 | 96 | U34774 | 9110-7716 | 465 | 56 | Unknown | F11L | 29 | MC018L | 25 | |
| 010 | 10785-8857 | 643 | 98 | U34774 | 11078-9150 | 74 | Actin tail, EEV maturation | F11L | 28 | MC019L | 34 | |||
| 011 | 11993-10860 | 378 | 97 | U06671 | 12291-11158 | 83 | EEV phospholipase | F13L | 44 | MC021L | 45 | |||
| 012 | 12291-12025 | 89 | 87 | AY231125 | 12569-12315 | 85 | 45 | Unknown | ||||||
| 013 | 12601-12837 | 79 | 93 | 12910-13128 | 73 | 50 | Unknown | |||||||
| 014 | 13163-12885 | 93 | 93 | 13493-13215 | 68 | Modified RING finger | MC026L | 46 | ||||||
| 015 | 14785-13169 | 539 | 96 | 15110-13503 | 536 | 61 | Unknown | MC027L | 23 | |||||
| 016 | 15633-14857 | 259 | 91 | AY283523 | 15947-15201 | 249 | 57 | Unknown | F16L | 22 | MC029L | 24 | ||
| 017 | 15893-16207 | 105 | 93 | U30337 | 16259-16573 | 71 | DNA-binding phosphoprotein | F17R | 49 | MC030R | 48 | |||
| 018 | 17652-16237 | 472 | 98 | 18040-16598 | 481 | 81 | Poly(A) polymerase catalytic subunit | E1L | 42 | MC031L | 47 | |||
| 019 | 19837-17663 | 725 | 97 | 20228-18054 | 73 | Unknown | E2L | 28 | MC032L | 32 | ||||
| 020 | 20458-19910 | 183 | 93 | AF380126 | 20876-20292 | 195 | 54 | dsRNA-binding PKR inhibitor | E3L | 29 | ||||
| 021 | 21069-20491 | 193 | 97 | AY299390 | 21482-20886 | 199 | 79 | RNA polymerase subunit RPO30 | E4L | 51 | MC034L | 48 | ||
| 022 | 21156-22856 | 567 | 98 | 21585-23282 | 566 | 84 | Unknown | E6R | 48 | MC037R | 55 | |||
| 023 | 22880-23695 | 272 | 100 | 23315-24130 | 88 | Membrane protein | E8R | 58 | MC038R | 57 | ||||
| 024 | 23758-24633 | 292 | 288 | 92 | AY283522 | 24187-24867 | 227 | 63 | Unknown | |||||
| 025 | 27675-24640 | 1,012 | 99 | U49979 | 27901-24875 | 1,009 | 86 | DNA polymerase | E9L | 56 | MC039L | 53 | ||
| 026 | 27693-27980 | 96 | 100 | 27931-28221 | 97 | 89 | IMV redox protein, virus assembly | E10R | 62 | MC040R | 65 | |||
| 027 | 28393-27983 | 137 | 98 | 28634-28224 | 82 | Virion core protein | E11L | 38 | MC041L | 40 | ||||
| 028 | 30509-28383 | 709 | 718 | 95 | AY231124 | 30723-28624 | 700 | 66 | Unknown | O1L | 22 | MC042L | 27 | |
| 029 | 32972-30555 | 806 | 811 | 95 | AY267341 | 33190-30770 | 807 | 65 | Unknown | MC043L | 25 | |||
| 030 | 34128-33166 | 321 | 99 | 34360-33395 | 322 | 78 | DNA-binding protein | I1L | 47 | MC044L | 44 | |||
| 031 | 34350-34141 | 70 | 100 | 34578-34372 | 69 | 72 | Unknown | I2L | 34 | MC045L | 40 | |||
| 032 | 35217-34363 | 285 | 95 | AY231127 | 35451-34588 | 288 | 67 | DNA-binding phosphoprotein | I3L | 40 | MC046L | 43 | ||
| 033 | 35486-35253 | 78 | 94 | 35725-35468 | 86 | 76 | IMV membrane protein | I5L | 36 | MC047L | 40 | |||
| 034 | 36656-35490 | 389 | 99 | 36900-35734 | 80 | Unknown | I6L | 31 | MC048L | 38 | ||||
| 035 | 37945-36656 | 430 | 99 | 38189-36900 | 85 | Virion core protease | I7L | 56 | MC049L | 61 | ||||
| 036 | 37951-39999 | 683 | 98 | 38195-40246 | 684 | 78 | NPH-II, RNA helicase | I8R | 47 | MC050R | 51 | |||
| 037 | 41788-39980 | 603 | 99 | 42032-40227 | 602 | 76 | Metalloprotease, virion morphogenesis | G1L | 44 | MC056L | 47 | |||
| 038 | 42125-42817 | 231 | 98 | AY254902 | 42376-43074 | 233 | 74 | Late transcription elongation factor | G2R | 32 | MC058R | 37 | ||
| 039 | 42131-41802 | 110 | 92 | 42382-42050 | 111 | 71 | Unknown | G3L | 33 | MC057L | 34 | |||
| 040 | 43158-42748 | 137 | 98 | 43412-42999 | 138 | 80 | Glutaredoxin 2, virion morphogenesis | G4L | 39 | MC059L | 44 | |||
| 041 | 43161-44516 | 452 | 96 | AY267343 | 43415-44737 | 441 | 67 | Unknown | G5R | 33 | MC060R | 36 | ||
| 042 | 44521-44709 | 63 | 100 | 44740-44928 | 84 | RNA polymerase subunit RPO7 | G5.5R | 68 | MC061R | 57 | ||||
| 043 | 44731-45285 | 185 | 97 | 44943-45518 | 192 | 70 | Unknown | G6R | 35 | MC062R | 36 | |||
| 044 | 46481-45288 | 398 | 98 | 46665-45493 | 391 | 66 | Virion core protein | G7L | 30 | MC065L | 36 | |||
| 045 | 46514-47311 | 266 | 100 | 46699-47496 | 93 | Late transcription factor VLTF-1 | G8R | 65 | MC067R | 69 | ||||
| 046 | 47322-48323 | 334 | 340 | 93 | 47511-48512 | 76 | Myristylated protein | G9R | 38 | MC068R | 37 | |||
| 047 | 48327-49058 | 244 | 98 | 48516-49247 | 87 | Myristylated IMV envelope protein | L1R | 59 | MC069R | 59/PICK> | ||||
| 048 | 49107-49376 | 90 | 98 | AY231128 | 49299-49565 | 89 | 62 | Unknown | L2R | 32 | MC070R | 37 | ||
| 049 | 50639-49389 | 417 | 418 | 98 | 50699-49572 | 376 | 65 | Unknown | L3L | 39 | MC072L | 43 | ||
| 050 | 50669-51445 | 259 | 97 | 50729-51496 | 256 | 83 | DNA-binding virion core protein VP8 | L4R | 50 | MC073R | 48 | |||
| 051 | 51471-51854 | 128 | 99 | 51530-51916 | 129 | 75 | Putative membrane protein | L5R | 38 | MC074R | 43 | |||
| 052 | 51811-52263 | 151 | 99 | 51873-52325 | 76 | Membrane protein, morphogenesis | J1R | 32 | MC075R | 38 | ||||
| 053 | 52336-53343 | 336 | 98 | AY254905 | 52414-53424 | 337 | 82 | Poly(A) polymerase small subunit VP39 | J3R | 55 | MC076R | 59 | ||
| 054 | 53261-53818 | 186 | 98 | 53342-53899 | 84 | RNA polymerase subunit RPO22 | J4R | 53 | MC077R | 54 | ||||
| 055 | 54275-53775 | 167 | 99 | 54356-53856 | 83 | Late membrane protein | J5L | 55 | MC078L | 52 | ||||
| 056 | 54358-58224 | 1,289 | 99 | 54446-58312 | 90 | RNA polymerase subunit RPO147 | J6R | 67 | MC079R | 67 | ||||
| 057 | 58816-58274 | 181 | 97 | 58851-58315 | 179 | 71 | Protein phosphatase, virus assembly | H1L | 41 | MC082L | 41 | |||
| 058 | 58446-59408 | 321 | 98 | 58863-59444 | 194 | 86 | Unknown | H2R | 50 | MC083R | 56 | |||
| 059 | 60442-59417 | 342 | 338 | 97 | AY040082 | 60479-59460 | 340 | 68 | IMV protein VP55, morphogenesis | H3L | 31 | MC084L | 29 | |
| 060 | 62857-60446 | 804 | 99 | S62819 | 62891-60483 | 803 | 86 | RNA polymerase-associated protein, RAP94 | H4L | 54 | MC085L | 55 | ||
| 061 | 62968-63648 | 227 | 228 | 91 | AY231123 | 62996-63721 | 242 | 51 | Late transcription factor VLTF-4 | H5R | 30 | MC086R | 30 | |
| 062 | 63676-64629 | 318 | 99 | U12401 | 63754-64713 | 320 | 87 | DNA topoisomerase I | H6R | 56 | MC087R | 54 | ||
| 063 | 64625-65038 | 138 | 97 | U12401 | 64709-65125 | 139 | 64 | Unknown | H7R | 24 | MC088R | 34 | ||
| 064 | 65076-67598 | 841 | 99 | 65164-67689 | 842 | 85 | mRNA capping enzyme, large subunit | D1R | 56 | MC090R | 59 | |||
| 065 | 68033-67566 | 156 | 98 | 68130-67657 | 158 | 73 | Virion protein | D2L | 32 | MC091L | 42 | |||
| 066 | 67807-68679 | 291 | 97 | 68111-68785 | 225 | 62 | Virion protein | D3R | 35 | MC092R | 35 | |||
| 067 | 68682-69374 | 231 | 98 | AY231122 | 68733-69473 | 247 | 86 | Uracil DNA glycosidase | D4R | 63 | MC093R | 63 | ||
| 068 | 69391-71751 | 787 | 98 | AY267342 | 69490-71853 | 788 | 88 | NTPase, DNA replication | D5R | 57 | MC094R | 58 | ||
| 069 | 71761-73665 | 635 | 99 | 71837-73786 | 650 | 92 | Early transcription factor VETFs | D6R | 67 | MC095R | 67 | |||
| 070 | 73705-74274 | 190 | 97 | 73827-74354 | 176 | 82 | RNA polymerase subunit RPO18 | D7R | 56 | MC097R | 58 | |||
| 071 | 74307-74978 | 224 | 99 | 74389-75057 | 223 | 79 | NPH-PPH downregulator | D10R | 35 | MC099R | 38 | |||
| 072 | 76887-74974 | 638 | 100 | 76966-75053 | 87 | NPH-I | D11L | 57 | MC100R | 58 | ||||
| 073 | 77518-76955 | 188 | 94 | 77580-76999 | 194 | 63 | Unknown | |||||||
| 074 | 78467-77568 | 300 | 98 | AY254904 | 78505-77636 | 290 | 86 | mRNA capping enzyme, small subunit | D12L | 57 | MC101L | 52 | ||
| 075 | 80112-78478 | 545 | 99 | 80184-78550 | 85 | Rifampin resistance, IMV assembly | D13L | 57 | MC102L | 55 | ||||
| 076 | 80585-80136 | 150 | 98 | 80657-80208 | 84 | Late transcription factor VLTF-2 | A1L | 40 | MC103L | 49 | ||||
| 077 | 81298-80627 | 224 | 100 | 81358-80687 | 91 | Late transcription factor VLTF-3 | A2L | 71 | MC104L | 71 | ||||
| 078 | 81546-81298 | 83 | 82 | 96 | 81608-81369 | 80 | 72 | Thioredoxin-like protein | A2.5L | 41 | MC105L | 44 | ||
| 079 | 83584-81560 | 675 | 98 | 83664-81616 | 683 | 74 | P4b precursor | A3L | 45 | MC106L | 44 | |||
| 080 | 84586-83603 | 328 | 324 | 89 | AY231126 | 84414-83683 | 244 | 44 | Virion core protein, virion assembly | A4L | 24 | MC107L | 45 | |
| 081 | 84625-85143 | 173 | 172 | 98 | AY254903 | 84455-84967 | 171 | 84 | RNA polymerase subunit RPO19 | A5R | 47 | MC108R | 49 | |
| 082 | 86288-85155 | 378 | 97 | 86126-84975 | 384 | 73 | Unknown | A6L | 42 | MC109L | 42 | |||
| 083 | 88849-86327 | 841 | 99 | 88287-86170 | 706 | 88 | Early transcription factor VETFL | A7L | 56 | MC110L | 60 | |||
| 084 | 88512-89420 | 303 | 99 | AY254900 | 88354-89274 | 307 | 83 | Intermediate transcription factor VITF-3 | A8R | 45 | MC111R | 46 | ||
| 085 | 89673-89395 | 93 | 97 | U30340 | 89527-89240 | 96 | 88 | Late virion membrane protein | A9L | 65 | MC112L | 53 | ||
| 086 | 92405-89691 | 905 | 98 | 92269-89546 | 908 | 76 | P4a precursor | A10L | 40 | MC113L | 47 | |||
| 087 | 92420-93427 | 336 | 99 | 92284-93318 | 345 | 87 | Unknown | A11R | 45 | MC114R | 48 | |||
| 088 | 94213-93434 | 260 | 261 | 91 | 93996-93328 | 223 | 56 | Virion core protein | A12L | 42 | MC115L | 38 | ||
| 089 | 94508-94233 | 92 | 97 | 94286-94053 | 78 | 71 | Virion membrane protein | A13L | 25 | MC117L | 33 | |||
| 090 | 94807-94535 | 91 | 90 | 97 | 94591-94322 | 90 | 76 | IMV phosphorylated membrane protein | A14L | 42 | MC118L | 46 | ||
| 091 | 94985-94827 | 53 | 100 | 94769-94611 | 84 | IMV membrane protein, virulence factor | A14.5L | 50 | MC119L | 40 | ||||
| 092 | 95255-94989 | 89 | 98 | 95037-94762 | 92 | 66 | Unknown | A15L | 22 | MC120L | 32 | |||
| 093 | 96318-95245 | 358 | 99 | 96100-95024 | 359 | 84 | Myristylated protein | A16L | 41 | MC121L | 47 | |||
| 094 | 96935-96348 | 196 | 100 | 96750-96148 | 201 | 81 | Phosphorylated IMV membrane protein | A17L | 37 | MC122L | 43 | |||
| 095 | 96950-98413 | 488 | 99 | 96765-98231 | 489 | 88 | DNA helicase, transcription elongation | A18R | 47 | MC123R | 52 | |||
| 096 | 98660-98391 | 90 | 91 | 96 | 98457-98209 | 83 | 71 | Unknown | A19L | 41 | MC124L | 43 | ||
| 097 | 98996-100282 | 429 | 98 | AY254901 | 98799-100076 | 426 | 71 | DNA polymerase processivity factor | A20R | 29 | MC126R | 36 | ||
| 098 | 98997-98674 | 108 | 97 | 98965-98474 | 164 | 77 | Unknown | A21L | 39 | MC125L | 42 | |||
| 099 | 100282-100719 | 146 | 100 | 100084-100521 | 95 | Holiday junction resolvase | A22R | 55 | MC127R | 54 | ||||
| 100 | 100745-101884 | 380 | 98 | AY283521 | 100548-101690 | 381 | 84 | Intermediate transcription factor VITF-3 | A23R | 51 | MC128R | 57 | ||
| 101 | 101912-105394 | 1,161 | 99 | U33419 | 101718-105200 | 92 | RNA polymerase subunit RPO132 | A24R | 69 | MC129R | 70 | |||
| 102 | 107099-105540 | 520 | 518 | 71 | 106895-105336 | 76 | A type inclusion protein | A26L | 24 | MC131L | 25 | |||
| 103 | 108692-107145 | 516 | 522 | 53 | 108480-106924 | 519 | 57 | A type inclusion protein | A26L | 25 | MC131L | 27 | ||
| 104 | 109004-108735 | 90 | 93 | P26654 | 108798-108532 | 89 | 74 | Fusion protein, virus assembly | A27L | 40 | MC133L | 35 | ||
| 105 | 109466-109047 | 140 | 99 | AY299389 | 109250-108831 | 89 | Unknown | A28L | 41 | MC134L | 46 | |||
| 106 | 110426-109485 | 314 | 98 | 110230-109274 | 319 | 79 | RNA polymerase subunit RP035 | A29L | 39 | MC135L | 49 | |||
| 107 | 110608-110429 | 60 | 95 | 110415-110230 | 62 | 75 | Virion morphogenesis | A30L | 36 | MC136L | 44 | |||
| 108 | 111601-110780 | 274 | 266 | 94 | 111404-110628 | 259 | 94 | DNA packaging, ATPase | A32L | 50 | MC140L | 71 | ||
| 109 | 111686-112177 | 164 | 159 | 56 | AY231121 | 111489-111974 | 162 | 49 | EEV glycoprotein | A33R | 29 | MC142R | 30 | |
| 110 | 112191-112691 | 167 | 165 | 49 | 112004-112504 | 50 | EEV glycoprotein | A34R | 25 | MC143R | 23 | |||
| 111 | 112723-113259 | 179 | 198 | 95 | AY231120 | 112527-113078 | 184 | 62 | Unknown | A35R | 26 | MC145R | 27 | |
| 112 | 113486-114349 | 288 | 286 | 82 | AY231119 | 113173-114063 | 297 | 41 | Putative chemokine binding protein | C23L | 21 | |||
| 113 | 114424-115023 | 200 | 211 | 81 | 114159-114755 | 199 | 41 | Unknown | 25 | |||||
| 114 | 115070-116101 | 344 | 346 | 94 | 114805-115797 | 331 | 64 | Unknown | MC149R | 25 | ||||
| 115 | 116225-116671 | 149 | 143 | 79 | 115859-116254 | 132 | 34 | Unknown | ||||||
| 116 | 116743-117360 | 206 | 234 | 54 | 116254-117027 | 258 | 29 | Unknown | ||||||
| 117 | 117539-118333 | 265 | 94 | AF192803 | 117203-117994 | 264 | 40 | GM-CSF/IL-2 inhibition factor | A41L | 25 | ||||
| 118 | 118588-118893 | 102 | 221 | 94 | AY231118 | Not present | Unknown | |||||||
| 119 | 119303-119920 | 206 | 204 | 93 | AY326433 | 118278-118625 | 116 | 56 | Unknown | |||||
| 120 | 120376-120957 | 194 | 199 | 81 | 118862-119281 | 140 | 34 | Unknown | ||||||
| 121 | 121089-121994 | 302 | 300 | 88 | AY231129 | 119398-120204 | 269 | 41 | Unknown | |||||
| 122 | 122050-123018 | 323 | 94 | AY283520 | 120301-121266 | 322 | 56 | Unknown | A51R | 23 | ||||
| 123 | 123113-124687 | 525 | 95 | AY186732 | 121371-122921 | 517 | 61 | Ankyrin repeat protein | M1L | 24 | ||||
| 124 | 124726-126321 | 532 | 96 | 122964-124481 | 506 | 57 | Unknown | |||||||
| 125 | 126418-126936 | 173 | 93 | AY231117 | 124623-125153 | 177 | 64 | Unknown | ||||||
| 126 | 127051-128541 | 497 | 96 | AY186732 | 125230-126747 | 506 | 58 | AY186733 | Ankyrin repeat protein | M1L | 31 | |||
| 127 | 128622-129173 | 184 | 186 | 94 | U60552 | 126898-127452 | 185 | 77 | AY186733 | IL-10 | ||||
| 128 | 129357-130859 | 501 | 527 | 95 | AY186732 | 127480-129030 | 517 | 57 | AY186733 | Ankyrin repeat protein | B4R | 21 | ||
| 129 | 130924-132471 | 516 | 520 | 91 | AY186732 | 129107-130651 | 515 | 59 | AY186733 | Ankyrin repeat protein | B4R | 22 | ||
| 130 | 132555-134048 | 498 | 98 | U29944 | 130698-132134 | 479 | 87 | AY186733 | Protein kinase | F10L | 52 | MC017L | 35 | |
| 131 | 134011-134688 | 226 | 225 | 90 | U29944 | 132100-132771 | 224 | 73 | AY186733 | Putative membrane protein | F9L | 33 | MC016L | 40 |
| 132 | 134777-135223 | 149 | 137 | 40 | S67522 | Present in LT | 37 | VEGF | ||||||
| 133 | Not present | 132821-133267 | 149 | AY186733 | Unknown | |||||||||
| 134 | 136352-136798 | 149 | 73 | AY186732 | 133476-133916 | 147 | 63 | Unknown | ||||||
Length of ORF in codons. OV-IA82 and BPSV lengths are presented only if different from lengths of OV-SA00 homologues. RT and LT, right and left terminal genomic regions, respectively.
% Id, percent amino acid identity.
GenBank database accession numbers of homologous PPV sequences.
Function was deduced from the degree of similarity to known genes and from Prosite signatures. PKR, protein kinase R; NPH, nucleophosphohydrolase; PPH, pyrophosphohydrolase; VLTF, vaccinia virus late transcription factor; VETF, vaccinia virus early transcription factor; VITF, vaccinia virus intermediate transcription factor.
Comparison of BPSV with ORFV.
At the genomic level, BPSV and ORFV genomes share 67 to 75% nucleotide identity (versus 94% between the two ORFV strains) and contain 127 genes with the same relative order and orientation, of which 15 are unique to PPVs. These features support the inclusion of BPSV and ORFV in the same genus. BV-AR02 and OV-SA00 demonstrate average amino acid identities of 71% (versus 94% between OV-SA00 and OV-IA82), consistent with the classification of BPSV and ORFV as two PPV species. BPSV and ORFV share 44, 58, and 27 genes with 81 to 100%, 61 to 80%, and 29 to 60% amino acid identity, respectively. About half of the most similar ORFs (81 to 100% amino acid identity) are associated with transcription, transcription regulation, or RNA processing (Table 1).
BPSV and ORFV contain 15 and 16 ORFs, respectively, which share no significant homology to known proteins and are primarily located at the right end of the genome. Fourteen of these ORFs (ORFs 001, 005, 012, 013, 024, 073, 113, 115, 116, 119 to 121, 124, and 125) are present in both BPSV and ORFV, with amino acid identities ranging from 29 to 64%, two (ORFs 002 and 118) are present only in ORFV, and one (ORF 133) is unique to BPSV. Consistent with a possible host range function, homologues of six of these (ORFs 012, 024, 118, 119, 121, and 125) are transcribed at early times in cells infected with ORFV strain orf-11 (Table 1).
Of the 26 most distantly related ORFs between BPSV and ORFV (amino acid identity < 60%), 10 are unique to PPVs (ORFs 005, 012, 013, 113, 115, 116, 119 to 121, and 124), 3 are characterized ORFV NZ2 strain genes with putative (ORFs 020 and 117) or known (ORF 006/132) roles in pathogenesis, 10 show homology with VACV genes of known structure or function (ORFs 061, 080, 088, 103, 109, 110, 112, 126, 128, and 129), and 3 show homology with VACV virus ORFs of unknown function (ORFs 009, 016, and 122).
Highly variable PPV proteins include homologues of the VACV proteins H5R, A4L, A12L, A26L, A33R, A34R, M1L, and B4R. BPSV and ORFV 061, orthologues of VACV H5R gene, are only 51% identical. H5R encodes a late transcription factor (VLTF-4) which is synthesized before and after DNA synthesis, is phosphorylated by viral kinases, and is hypothesized to have multiple roles in the viral replicative cycle (5, 36). Notably, in closely related capripoxviruses, sheeppox virus and goatpox virus (genomes which share 96% nucleotide identity), VLTF-4 homologues are among the least conserved genes. It is tempting to speculate that PPV ORF 061 may play a role in host range during virus infection.
BPSV and ORFV 080 encode homologues of VACV A4L, a gene with significant variability in other poxvirus genera. A4L encodes an immunodominant late protein associated with the virion core and necessary for viral morphogenesis (84). OV-SA00 and OV-IA82 080 encode products that are 84 and 80 amino acids longer than the BPSV 080 product, respectively, due in part to the lack of four Cys-(Pro-Ala)3 motifs separated by additional Pro/Ala-rich sequences in BPSV 080. Similar Pro/Ala-rich repeats are present in the molluscum contagiosum virus (MOCV) orthologue MC107L but not in A4L. Tandem repeat motifs in A4L-like proteins are thought to be involved in protein-protein interactions and antigenicity (7).
BPSV and ORFV 088, orthologues of VACV A12L virion core protein, share only 56% amino acid identity, an unusual degree of intragenus variability for A12L orthologues (e.g., >90% amino acid identity within orthopoxvirus [OPV], leporipoxvirus, and capripoxvirus). Notably, BPSV and ORFV 088 encode proteins of 223 and 260 amino acid, respectively, while non-PPV ChPV A12L orthologues are 156 to 195 amino acids. The difference in length is partially due to a positively charged 20-residue insertion immediately downstream of the predicted VNA/GG cleavage site (position 170 in ORFV 088) and might suggest specific PPV core structure requirements (83).
PPV 102 and 103 are variable ORFs, with PPV 102 being more conserved between species than 103 (67 to 76% versus 57 to 58% amino acid identity, respectively). PPV 102 and 103 share similarity to each other (32 to 37% amino acid identity) and to homologues of OPV genes involved in formation of virus-filled A-type inclusions (ATIs) (46, 80). Both 102 and 103 are most similar to homologues of OPV P4c, an intracellular mature virion (IMV)-specific protein which helps direct IMV into ATIs. The carboxy termini of PPV 102 and OPV P4c proteins share sequences found in the VACV A27L fusion protein. PPV 103 has weak similarity to OPV ATI proteins, which constitute the crystalline matrix of OPV ATIs. Given the variable nature of these genes in different ChPV genera, PPV homologues may affect genera-specific and species-specific mechanisms of retaining or disseminating IMVs.
PPV 109 and 110 are orthologues of VACV A33R and A34R, respectively, genes encoding envelope type II glycoproteins expressed in intracellular enveloped virions and in EEV (44, 66). Mutations in these genes affect EEV formation (A33R and A34R), cell-to-cell spread of virus (A33R), and infectivity and virulence (A34R) (reviewed in reference 74). PPV 109 and 110, although distantly related to the VACV orthologues, are predicted to contain amino-terminal transmembrane regions and external Cys residues suggesting a similar protein topology and structure. Notably, OV-SA00 109 and 110 are as distantly related to OV-IA82 orthologues (56 and 49% amino acid identity) as they are to BPSV proteins (49 to 50% amino acid identity) (Table 1), with amino acids differences being largely concentrated in the predicted external domain. An explanation for this intraspecies variability is not immediately obvious. Alignment of available ORFV 109 sequences grouped OV-IA82, NZ2, and Orf-11 A33R homologues in a single cluster with 80 to 97% amino acid identity, excluding OV-SA00 A33R, which showed 51 to 55% amino acid identity relative to other ORFV sequences. A similar situation is observed when available ORF 110 sequences are compared. OV-SA00 is the only strain originally isolated from goats, whereas OV-IA82, NZ2, and ORF-11 strains were isolated from sheep. Thus, there appears to be a correlation between the species from which the virus was isolated and clustering of ORFV 109 and 110 amino acid sequences. This raises the possibility that differences in the external domain of both A33R and A34R are associated with host-specific requirements during virus infection by EEV. Differences in disease course have been shown following experimental infection with ORFV isolated from sheep or goats (87).
Variable PPV 126, 128, and 129 correspond to the Ank-1, Ank-2, and Ank-3 genes previously described for the BPSV B177 and ORFV D1701 strains (67). These ORFs are 58, 57, and 50% identical between BPSV and ORFV, respectively, and encode ankyrin repeat-containing proteins (ARPs). Cellular ARPs engage other proteins to form regulatory complexes which are involved in the control of processes such as cell cycle and cell differentiation (71). Many poxviruses encode ARPs, several of which have been linked to host range functions, apoptosis inhibition, and virulence (34, 56). Notably, BPSV contains two additional ARPs in the left terminal genomic region (ORFs 003 and 004) which are not present in ORFV (Table 1).
PPV genes involved in pathogenesis.
ORFV encode several proteins with known or putative roles in pathogenesis: 020, an orthologue of vaccinia E3L that functions in interferon (IFN) resistance; 127, a viral homologue of IL-10; 117, a secreted granulocyte-macrophage colony-stimulating factor (GM-CSF) inhibitor (GIF); 112, a putative chemokine-binding protein; and 132, a viral homologue of VEGF. These genes are present in BPSV with predicted amino acid identities ranging from 37 to 77%.
E3L encodes a double-stranded-RNA (dsRNA)-binding protein kinase inhibitor with orthologues in all ChPV genera except Avipoxvirus and Molluscipoxvirus. The E3L gene product provides IFN resistance to VACV-infected cells and broad host range to virus infection in tissue culture and is a virulence determinant (4, 9). The ORFV NZ2 strain E3L homologue is also involved in IFN resistance (31) and is 93% and 53% identical to its OV-SA00 and BPSV counterparts, respectively. ORFV and BPSV 020 are most similar at the carboxy-terminal half of the protein, which is predicted to bind dsRNA through a conserved binding motif (10). The amino-terminal half of the protein is less conserved (45% amino acid identity) and includes two deletions of six and four amino acids in ORFV. In VACV, the amino-terminal half of E3L is dispensable for replication in cell culture and is not required for IFN resistance. However, this region is required for full virulence in a mouse intranasal inoculation model (9). It is thus possible that differences between ORFV and BPSV in the amino-terminal half of 020 are significant for host range and pathogenesis in their respective hosts.
OV-SA00 and OV-IA82 127 are orthologues (95% amino acid identity) of the previously described ORFV NZ2 and NZ7 strain IL-10 genes (23). BV-AR02 127 is divergent from ORFV homologues (77% amino acid identity) with most amino acid differences concentrated in the amino terminal third of the protein (27% amino acid identity in the first 50 amino acids). Nevertheless, a putative signal peptide is present in the amino terminus of all three IL-10 homologues presented here. The carboxy two-thirds of PPV IL-10 are highly conserved with cellular IL-10, with all ORFV interleukin 10 (IL-10) proteins sequenced to date being most similar to ovine IL-10 (23; this work). Notably, and in agreement with previous results, BV-AR02 127 shares in this conserved region six residues identical to bovine but not to ovine and ORFV IL-10, including a His residue at position 75 predicted to interact with the IL-10 receptor chain 1 (67). These features may reflect specific adaptation to the natural host.
PPVs 117 are orthologues of ORFV GIF, a protein which binds and inhibits GM-CSF and IL-2 in vitro and may function as an immunomodulatory factor in vivo (15). OV-SA00, OV-IA82, and NZ2 strain GIF homologues are very similar to each other (94% amino acid identity), containing at least six potentially structurally significant Cys residues and an amino-terminal signal peptide. While BV-AR02 GIF shares these structural features, it is only 38 to 40% identical to ORFV GIF, an unexpected divergence considering the similarity between ovine and bovine GM-CSF and IL-2 (83 and 96% amino acid identity, respectively). Notably, PPV 117 shares 22 to 25% amino acid identity with PPV 112, a gene also predicted to encode a signal peptide and expressed at early times postinfection in ORFV strain Orf-11 (Table 1). Both PPV 117 and 112 share limited sequence similarity and/or Cys patterns with VACV C23L and myxoma virus MT-1 chemokine binding proteins and VACV A41L virulence factor. Taken together, these data suggest that PPV 117 and 112 may be members of a divergent family of poxviral chemokine- and cytokine-binding virulence factors.
ORFV 132 and BPSV 006 encode homologues of mammalian VEGFs, angiogenic factors that bind receptor tyrosine kinases to affect embryonic development and tumor neovascularization (11, 53, 62, 85). ORFV, PCPV, and BPSV encode the only known viral VEGFs (vVEGFs), all of which contain a characteristic cystine knot motif, a potential signal sequence, potential N- and O-linked glycosylation sites, a carboxy-terminal Thr/Pro-rich motif unique to vVEGFs, and an Asp residue (position 85 in BPSV VEGF) associated with specific VEGF receptor binding (38, 79; this paper). All vVEGFs are flanked by similar putative transcriptional control elements (38, 79; this paper) suggesting that, as is the case for ORFV (38), these genes are expressed at early times postinfection. ORFV VEGF is known to play a role in ORFV pathogenesis associated with vascularization and epidermal lesion proliferation (70).
OV-SA00 and OV-IA82 VEGF are 38% identical to each other and most similar to NZ7 and NZ2-like VEGFs (90 and 80% amino acid identity, respectively). Previous sequence analysis of ORFV isolates from diverse geographic origins segregated VEGFs into two divergent groups, a more conserved NZ7-like group and a more variable NZ-2 group (52). The data presented here for U.S. ORFV isolates further supports the notion that VEGF type is independent of the geographic origin (52).
BV-AR02 006 represents a novel variant of PPV VEGF, previously not found in BPSV strain B177 by DNA hybridization (67). BV-AR02 006 is located in a BPSV-specific, left terminal genomic region contrasting the right terminal location of ORFV and PCPV VEGFs. BV-AR02 VEGF is 35 to 50% identical to other vVEGFs and contains a unique charged pentapeptide located at positions 34 to 39. This suggests that, as for PCPV, BPSV VEGF is distinct from ORFV VEGF prototypes. Sequence divergence revealed here may explain the lack of hybridization when BPSV B177 strain DNA was probed with ORFV VEGF probe (67). Alternatively, terminal genomic variability observed for BPSV isolates (26) may have resulted in loss of the gene from the B177 strain. The presence of vVEGF in BPSV suggests its importance in PPV pathogenesis; however, the divergent nature of BPSV VEGF may imply functions or binding specificities distinct from other vVEGFs.
Comparison of PPV with other poxvirus genera.
BPSV and ORFV contain 16 and 17 ORFs, respectively, which have no significant homology to genes from other poxvirus genera, and with the exception of VEGF, to other known proteins (Table 1). Although six of them are transcribed in ORFV strain Orf-11-infected cell cultures, their functions are not known. BPSV and ORFV contain a total of 113 and 111 genes, respectively, with homology to genes from other poxvirus genera. These include homologues of 88 of the 90 genes conserved within all other ChPVs, with 7 of the 11 most similar (≥60% amino acid identity; Table 1) involved in transcription, indicating that PPVs utilize basic ChPV replicative mechanisms (81). PPVs are unique within the ChPV subfamily in that they lack homologues of VACV F15L, a gene of unknown function, and VACV D9R, a gene encoding a putative nucleoside triphosphate pyrophosphohydrolase containing a mutT motif similar to that in VACV D10R, a protein affecting viral transcription (55).
PPVs, although distinct, share a number of features with MOCV, the sole member of the molluscipoxvirus genus. PPV and MOCV are the only characterized poxviruses with genomes rich in G+C (64%), while others are rich in A+T. Homology searches revealed that 46 of 104 PPV proteins were most similar to MOCV orthologues, while 26 proteins were more similar to OPV orthologues (Table 1 and data not shown).
PPV 014, 015, and 029 are putative orthologues of MOCV MC026L, 027L, and 043L, respectively, based on amino acid identity and similar genomic location (72, 73). These ORFs of unknown function have no counterparts outside PPV and MOCV and are 61 to 68% identical between ORFV and BPSV. PPV 029 is transcribed at early times postinfection in Orf-11-infected cells (Table 1). PPV 014 and MC026L contain a RING-H2 motif which is present in proteins from diverse organisms. RING-H2 proteins are subunits of heteromeric ubiquitin ligases (E3s) which affect multiple cellular processes including cell cycle regulation and immune response (37).
PPVs and MOCV both lack genes present or conserved in other poxviruses. These comprise homologues of most poxviral genes likely involved in nucleotide metabolism, including homologues of OPV ribonucleotide reductase, thymidine kinase, guanylate kinase, thymidylate kinase, and a putative ribonucleotide reductase cofactor, VACV glutaredoxin O2L (3). PPVs, MOCV, and Melanoplus sanguinipes entomopoxvirus are the only poxviruses known to lack a thymidine kinase gene. In contrast, PPV 007, a gene not essential for growth of ORFV in vitro, is a dUTPase gene missing in MOCV (22, 43, 72, 73). Notably, PPVs and MOCV are the only ChPVs lacking homologues of VACV B1R, a Ser/Thr protein kinase similar to cellular VRK1 homologues and giving a temperature-sensitive DNA-negative phenotype (61). PPVs and MOCV lack homologues of VACV A50R DNA ligase, a gene also absent in yatapoxviruses (81). Also absent in PPV and MOCV are serine protease inhibitor and kelch-like gene families present in other ChPVs. These gene families are known to affect host responses, including inflammation, apoptosis, complement activation, and coagulation (39), and are associated with virulence (78). The lack of ChPV-like genes in both PPVs and MOCV may reflect adaptation for specific tissue tropism, which is notable considering that PPVs and MOCV appear to replicate in cycling cells (17, 45).
Features similar in both PPV and MOCV—nucleotide composition, amino acid similarity, and gene content—suggest that they are distinct from other known mammalian poxvirus genera. Phylogenetic analysis of protein sequences also supports the idea that, although divergent, PPVs and MOCV are distinct from other known mammalian ChPVs (Fig. 1).
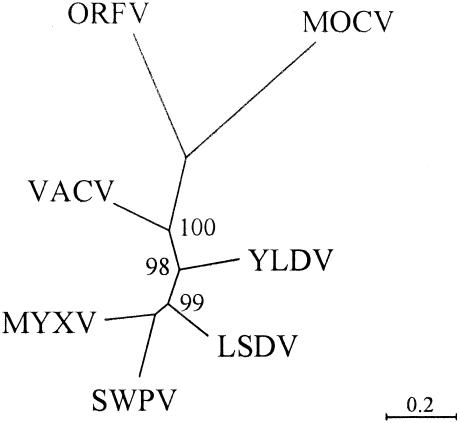
FIG. 1.
Phylogenetic analysis of PPV proteins. PPV025 DNA polymerase and homologous sequences were aligned using ClustalW. Unrooted trees were generated using the neighbor-joining algorithm with Poisson correction for multiple substitutions. Bootstrap values greater than 95% after 1,000 replicates are indicated at appropriate nodes. Homologous protein sequences from the following viruses and accession numbers were compared: ORFV, AY386264; MOCV, MCU60315; VACV, M35027; yaba-like disease virus (YLDV), YDI293568; lumpy skin disease virus (LSDV), AF325528; myxoma virus (MYXV), AF170726; and swinepox virus (SWPV), AF410153. Similar results were obtained for 16 additional conserved PPV proteins, with 15 maintaining 100% bootstrap support for separation of PPV and MOCV from other mammalian ChPVs. Similar results were also obtained for these 17 proteins using the maximum likelihood algorithm with Dayhoff correction for multiple substitutions and for whole genomic nucleotide alignment utilizing amino acid translation as implemented by using Dialign (54).
Conclusions.
PPV resembles other poxviruses in genome organization and gene content, sharing specific genomic features only with MOCV. Genome sequences of a BPSV strain and two ORFV strains described here provide a comparative view of PPV genomics and basic knowledge of viral functions associated with virus replication and manipulation of cellular responses. Significant differences occur between BPSV and ORFV genomes, and these may account for differences in host range. An improved understanding of PPV biology will permit the engineering of novel vaccine viruses and expression vectors with enhanced efficacy and greater versatility.
ADDENDUM
Since the completion of the analysis presented here, an additional ORFV genomic sequence has been deposited in the patent database (accession no. AX754989).
Acknowledgments
We thank T. McKenna for providing the PPV BV-AR02 strain and A. Zsak and A. Lakowitz for providing excellent technical assistance.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73:533-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, B. Y., and B. Moss. 1992. Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascorbate reductase activities. Proc. Natl. Acad. Sci. USA 89:7060-7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. [DOI] [PubMed] [Google Scholar]
- 5.Beaud, G., R. Beaud, and D. P. Leader. 1995. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase. J. Virol. 69:603-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becher, P., M. König, G. Müller, U. Siebert, and H.-J. Thiel. 2002. Characterization of sealpox virus, a separate member of the parapoxviruses. Arch. Virol. 147:1133-1140. [DOI] [PubMed] [Google Scholar]
- 7.Boulanger, D., P. Green, T. Smith, C. P. Czerny, and M. A. Skinner. 1998. The 131-amino-acid repeat region of the essential 39-kilodalton core protein of fowlpox virus FP9, equivalent to vaccinia virus A4L protein, is nonessential and highly immunogenic. J. Virol. 72:170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman, K. F., R. T. Barbery, L. J. Swango, P. R. Schnurremberger. 1981. Cutaneous form of bovine papular stomatitis in man. JAMA 246:2813-2818. [PubMed] [Google Scholar]
- 9.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology 194:537-547. [DOI] [PubMed] [Google Scholar]
- 11.Clauss, M. 2000. Molecular biology of the VEGF and the VEGF receptor family. Semin. Thromb. Hemostasis 26:561-569. [DOI] [PubMed] [Google Scholar]
- 12.Cottone, R., M. Büttner, B. Bauer, M. Henkel, E. Hettich, and H.-J. Rziha. 1998. Analysis of genomic rearrangement and subsequent gene deletion of the attenuated Orf virus strain D1701. Virus Res. 56:53-67. [DOI] [PubMed] [Google Scholar]
- 13.Darbyshire, J. H. 1961. A fatal, ulcerative mucosal condition of sheep associated with the virus of contagious pustular dermatitis. Br. Vet. J. 117:97-105. [Google Scholar]
- 14.Dashtseren, T., B. V. Solovyev, F. Vareja, and A. Khokhoo. 1984. Camel contagious ecthyma (pustular dermatitis). Acta Virol. 28:122-127. [PubMed] [Google Scholar]
- 15.Deane, D., C. J. McInnes, A. Percival, A. Wood, J. Thomson, A. Lear, J. Gilray, S. Fleming, A. Mercer, and D. Haig. 2000. Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2. J. Virol. 74:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein, W. L., M. A. Conant, and H. Krasnobrod. 1966. Molluscum contagiosum: normal and virus infected epidermal cell kinetics. J. Investig. Dermatol. 46:91-103. [DOI] [PubMed] [Google Scholar]
- 18.Ewing, B., and P. Green. 1998. Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res. 8:186-194. [PubMed] [Google Scholar]
- 19.Ewing, B., L. Hillier, M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 8:175-185. [DOI] [PubMed] [Google Scholar]
- 20.Fleming, S. B., K. M. Fraser, A. A. Mercer, and A. J. Robinson. 1991. Vaccinia virus-like early transcriptional control sequences flank an early gene in orf virus. Gene 97:207-212. [DOI] [PubMed] [Google Scholar]
- 21.Fleming, S. B., J. Blok, K. M. Fraser, A. A. Mercer, and A. J. Robinson. 1993. Conservation of gene structure and arrangement between vaccinia virus and Orf virus. Virology 195:175-184. [DOI] [PubMed] [Google Scholar]
- 22.Fleming, S. B., D. J. Lyttle, J. T. Sullivan, A. A. Mercer, and A. J. Robinson. 1995. Genomic analysis of a transposition-deletion variant of Orf virus reveals a 3.3 kbp region of non-essential DNA. J. Gen. Virol. 76:2969-2978. [DOI] [PubMed] [Google Scholar]
- 23.Fleming, S. B., C. A. McCaughan, A. E. Andrews, A. D. Nash, and A. A. Mercer. 1997. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser, K. M., D. F. Hill, A. A. Mercer, and A. J. Robinson. 1990. Sequence analysis of the inverted terminal repetition in the genome of the parapoxvirus, orf virus. Virology 176:379-389. [DOI] [PubMed] [Google Scholar]
- 25.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biol. Sci. 12:543-554. [DOI] [PubMed] [Google Scholar]
- 26.Gassmann, U., R. Wyler, and R. Wittek. 1985. Analysis of parapoxvirus genomes. Arch. Virol. 83:17-31. [DOI] [PubMed] [Google Scholar]
- 27.Glover, R. E. 1928. Contagious pustular dermatitis of the sheep. J. Comp. Pathol. 41:318-340. [Google Scholar]
- 28.Gordon, D., C. Abajian, and P. Green. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:192-202. [DOI] [PubMed] [Google Scholar]
- 29.Guo, J., Z. Zhang, J. F. Edwards, R. W. Ermel, C. Taylor, Jr., and A. de la Concha-Bermejillo. 2003. Characterization of a North American orf virus isolated from a goat with persistent, proliferative dermatitis. Virus Res. 93:169-179. [DOI] [PubMed] [Google Scholar]
- 30.Haig, D. M., and A. A. Mercer. 1998. Ovine diseases. Orf. Vet. Res. 29:311-326. [PubMed] [Google Scholar]
- 31.Haig, D. M., C. J. McInnes, J. Thomson, A. Wood, K. Bunyan, and A. Mercer. 1998. The Orf virus gene OV20.0L gene product is involved in interferon resistance and inhibits an interferon-inducible, double-stranded RNA-dependent kinase. Immunology 93:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haig, D. M., and C. J. McInnes. 2002. Immunity and counter-immunity during infection with the parapoxvirus orf virus. Virus Res. 88:3-16. [DOI] [PubMed] [Google Scholar]
- 33.Huang, X., and A. Madan. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9:868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ink, B. S., C. S. Gilbert, and G. I. Evan. 1995. Delay of vaccinia virus-induced apoptosis in nonpermissive Chinese hamster ovary cells by the cowpox virus CHOhr and adenovirus E1B 19K genes. J. Virol. 69:661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoshima, Y., K. Murakami, T. Yokoyama, and H. Sentsui. 2001. Genetic heterogeneity among parapoxviruses isolated from sheep, cattle and Japanese serows (Capricornis crispus). J. Gen. Virol. 82:1215-1220. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs, G. R., and B. Moss. 1996. The vaccinia virus H5R gene encodes late gene transcription factor 4: purification, cloning, and overexpression. J. Virol. 70:6796-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leverson, J. D., C. A. P. Joazeiro, A. M. Page, H. Huang, P. Hieter, and T. Hunter. 2000. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell. 11:2315-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyttle, D. J., K. M. Fraser, S. B. Fleming, A. A. Mercer, and A. J. Robinson. 1994. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J. Virol. 68:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mark, R., L. Buller, and G. J. Palumbo. 1991. Poxvirus pathogenesis. Microbiol. Rev. 55:80-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayr, A., and M. Büttner. 1990. Bovine papular stomatitis virus, p. 23-28. In Z. Dinter and B. Morein (ed.), Virus infections of ruminants. Elsevier, Amsterdam, The Netherlands.
- 41.Mazur, C., and R. D. Machado. 1989. Detection of contagious pustular dermatitis virus of goats in a severe outbreak. Vet. Rec. 125:419-420. [DOI] [PubMed] [Google Scholar]
- 42.McInnes, C. J., A. R. Wood, and A. A. Mercer. 1998. Orf virus encodes a homolog of the vaccinia virus interferon-resistance gene E3L. Virus Genes 17:107-115. [DOI] [PubMed] [Google Scholar]
- 43.McInnes, C. J., A. R. Wood, P. F. Nettleton, and J. Gilray. 2001. Genomic comparison of an avirulent strain of orf virus with that of a virulent wild type isolate reveals that the orf virus G2L gene is non-essential for replication. Virus Genes 22:141-150. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh, A. A., and G. L. Smith. 1996. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J. Virol. 70:272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKeever, D. J., P. E. McEwan, D. M. Jenkinson, G. Hutchinson, and H. W. Reid. 1988. Studies of the pathogenesis of orf virus infection in sheep. J. Comp. Pathol. 99:317-328. [DOI] [PubMed] [Google Scholar]
- 46.McKelvey, T. A., S. C. Andrews, S. E. Miller, C. A. Ray, and D. J. Pickup. 2002. Identification of the orthopoxvirus p4c gene, which encodes a structural protein that directs intracellular mature virus particles into A-type inclusions. J. Virol. 76:11216-11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meechan, J. G., and R. I. MacLeod. 1992. Human labial orf: a case report. Br. Dent. J. 173:343-344. [DOI] [PubMed] [Google Scholar]
- 48.Mercer, A. A., K. Fraser, G. Barns, and A. J. Robinson. 1987. The structure and cloning of orf virus DNA. Virology 157:1-12. [DOI] [PubMed] [Google Scholar]
- 49.Mercer, A. A., D. J. Lyttle, E. M. Whelan, S. B. Fleming, and J. T. Sullivan. 1995. The establishment of a genetic map of orf virus reveals a pattern of genomic organization that is highly conserved among divergent poxviruses. Virology 212:698-704. [DOI] [PubMed] [Google Scholar]
- 50.Mercer, A. A., K. M. Fraser, and J. J. Esposito. 1996. Gene homology between orf virus and smallpox variola virus. Virus Genes 13:175-178. [DOI] [PubMed] [Google Scholar]
- 51.Mercer, A. A., and D. M. Haig. 1999. Parapoxviruses, p. 1140-1146. In A. Granoff and R. G. Webster (ed.), The encyclopedia of virology, 2nd ed. Academic Press, New York, N.Y.
- 52.Mercer, A. A., L. M. Wise, A. Scagliarini, C. J. McInnes, M. Buttner, H. J. Rziha, C. A. McCaughan, S. B. Flemming, N. Ueda, and P. F. Nettleton. 2002. Vascular endothelial growth factors encoded by Orf virus show surprising sequence variation but have a conserved, functionally relevant structure. J. Gen. Virol. 83:2845-2855. [DOI] [PubMed] [Google Scholar]
- 53.Meyer, M., M. Clauss, A. Lepple-Wienhues, J. Waltenberger, H. G. Augustin, M. Ziche, C. Lanz, M. Buttner, H. Rziha, and C. Dehio. 1999. A novel vascular endothelial factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinase. EMBO J. 18:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgenstern, B., K. Frech, A. Dress, and T. Werner. 1998. DIALIGN: finding local similariries by multiple sequence alignment. Bioinformatics 14:290-294. [DOI] [PubMed] [Google Scholar]
- 55.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monathy, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, Pa.
- 56.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of the poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nettleton, P. F., R. Munro, I. Pow, J. Gilray, E. W. Gray, and H. W. Reid. 1995. Isolation of a parapoxvirus from a grey seal (Halichoerus grypus). Vet. Rec. 137:562-654. [DOI] [PubMed] [Google Scholar]
- 58.Newsom, I. E., and F. Cross. 1934. Sore mouth in sheep transmissible to man. J. Am. Vet. Med. Assoc. 84:799-802. [Google Scholar]
- 59.Ogino, H., D. Nakabayashi, M. Nabeya, K. Hoshi, and T. Okazawa. 1996. Contagious papular dermatitis of Japanese serows in Niigata Prefecture. J. Jpn. Vet. Med. Assoc. 49:615-618. [Google Scholar]
- 60.Okada, H., K. Matsukawa, and Y. Chihaya. 1986. Experimental transmission of contagious pustular dermatitis from a Japanese serow, Capricornis crispus, to a calf and goats. J. Jpn. Vet. Med. Assoc. 39:578-581. [Google Scholar]
- 61.Rempell, R. E., and P. Traktman. 1992. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J. Virol. 66:4413-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 63.Robinson, A. J., G. Ellis, and T. Balassu. 1982. The genome of orf virus: restriction endonuclease analysis of viral DNA isolated from lesions of orf in sheep. Arch. Virol. 71:43-55. [DOI] [PubMed] [Google Scholar]
- 64.Robinson, A. J., G. Barns, K. Fraser, E. Carpenter, and A. A. Mercer. 1987. Conservation and variation in orf virus genomes. Virology 157:13-23. [DOI] [PubMed] [Google Scholar]
- 65.Robinson, A. J., and A. A. Mercer. 1995. Parapoxvirus of red deer: evidence for its inclusion as a new member in the genus parapoxvirus. Virology 208:812-815. [DOI] [PubMed] [Google Scholar]
- 66.Roper, R. L., L. G. Payne, and B. Moss. 1996. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 70:3753-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rziha, H.-J., B. Bauer, K.-H Adam, M. Röttgen, R. Cottone, M. Henkel, C. Dehio, and M. Büttner. 2003. Relatedness and heterogeneity at the near-terminal end of the genome of a parapoxvirus bovis 1 strain (B177) compared with parapoxvirus ovis (Orf virus). J. Gen. Virol. 84:1111-1116. [DOI] [PubMed] [Google Scholar]
- 68.Sainsbury, A. W., and J. Gurnell. 1995. An investigation into the health and welfare of red squirrels, Sciurus vulgaris, involved in reintroduction studies. Vet. Rec. 137:367-370. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez, R. L., A. Hebert, H. Lucia, and J. Swedo. 1985. Orf. A case report with histologic, electron microscopic, and immunoperoxidase studies. Arch. Pathol. Lab. Med. 109:166-170. [PubMed] [Google Scholar]
- 70.Savory, L. J., S. A. Stacker, S. B. Fleming, B. E. Niven, and A. A. Mercer. 2000. Viral vascular endothelial growth factor plays a critical role in orf virus infection. J. Virol. 74:10699-10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sedgwick, S. G., and S. J. Smerdon. 1999. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem. Sci. 24:311-316. [DOI] [PubMed] [Google Scholar]
- 72.Senkevich, T. G., J. J. Bugert, J. R. Sisler, E. V. Koonin, G. Darai, and B. Moss. 1996. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science. 273:813-816. [DOI] [PubMed] [Google Scholar]
- 73.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 74.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 75.Strimmer, K., and A. von Haeseler. 1996. Quartet Puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 76.Sullivan, J. T., K. M. Fraser, S. B. Fleming, A. J. Robinson, and A. A. Mercer. 1995. Sequence and transcriptional analysis of an Orf virus gene encoding ankyrin-like repeat sequences. Virus Genes 9:277-282. [DOI] [PubMed] [Google Scholar]
- 77.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, J.-H. Sur, N. T. Sandybaev, U. Z. Kerembekova, V. L. Zaitsev, G. F. Kutish, and D. L. Rock. 2002. The genomes of sheeppox and goatpox viruses. J. Virol. 76:6054-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ueda, N., L. M. Wise, S. A. Stacker, S. B. Fleming, and A. A. Mercer. 2003. Pseudocowpox virus encodes a homolog of vascular endothelial growth factor. Virology 305:298-309. [DOI] [PubMed] [Google Scholar]
- 80.Ulaeto, D., D. Grosenbach, and D. E. Hruby. 1996. The vaccinia virus 4c and A-type inclusion proteins are specific markers for the intracellular mature virus particle. J. Virol. 70:3372-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 77:7590-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wesley, R. D., and A. E. Tuthill. 1984. Genome relatedness among African swine fever virus field isolates by restriction endonuclease analysis. Prev. Vet. Med. 2:53-62. [Google Scholar]
- 83.Whitehead, S. S., and D. E. Hruby. 1994. Differential utilization of a conserved motif for the proteolytic maturation of vaccinia virus proteins. Virology 200:154-161. [DOI] [PubMed] [Google Scholar]
- 84.Williams, O., E. J. Wolffe, A. S. Weisberg, and M. Merchlinsky. 1999. Vaccinia virus WR gene A5L is required for morphogenesis of mature virions. J. Virol. 73:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wise, L., T. Veikhola, A. Mercer, L. Savory, S. Fleming, C. Caesar, A. Vitali, T. Makinen, K. Alitalo, and S. Stacker. 1999. Vascular endothelial growth factor (VEGF)-like protein from orf virus strain NZ2 binds to VEGFR-2 and neurophilin-1. Proc. Natl. Acad. Sci. USA 96:3071-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wittek, R., C. C. Kuenzle, and R. Wyler. 1979. High G+C content in paramyxovirus DNA. J. Gen. Virol. 43:231-234. [DOI] [PubMed] [Google Scholar]
- 87.Zamri-Saad, M., I. Roshiah, and K. Al-Ajeeli. 1994. Experimental cross-infection of sheep and goats with different isolates of contagious ecthyma virus. Aust. Vet. J. 71:218-220. [DOI] [PubMed] [Google Scholar]