Abstract
The posterodorsal medial amygdala (MeApd) and principal nucleus of the bed nucleus of the stria terminalis (pBST) are densely interconnected sites integrating steroid hormone and olfactory information necessary for sociosexual behaviours in many rodents. Our laboratory recently reported sexually dimorphic populations of cells containing tyrosine hydroxylase (TH) located in the MeApd and pBST of prairie voles (Microtus ochrogaster), with males having many more TH-immunoreactive (TH-ir) cells in these sites than do females. We also found that gonadal hormones circulating during adulthood regulated this sex difference, as it was eliminated by castrating adult males or implanting females with testosterone-filled capsules. We here demonstrate that many (25-65%) of TH-ir cells in the MeApd and pBST of both sexes of adult virgin prairie voles also contain immunoreactivity for either the androgen receptor (AR) or oestrogen receptor alpha (ERα). Subcutaneous implants of oestradiol benzoate (EB) mimicked the effects of testosterone (T) and maintained high numbers of TH-ir cells in these sites in castrated males. However, implants of dihydrotestosterone (DHT) did not, and these males had low numbers of TH-ir cells similar to castrated males given empty capsules. A similar effect was found in females, where T or EB greatly increased the number of TH-ir cells in these sites compared to intact or ovariectomized controls, but DHT did not. DHT implants did maintain high seminal vesicle weights in males, though. Thus, many of the TH-ir cells in the prairie vole MeApd and pBST are potentially sensitive to androgens and oestrogens, but maintaining immunocytochemically detectable levels of TH in these cells may depend more on an oestrogen-mediated mechanism in both sexes. These data have implications for understanding how gonadal hormone release across the reproductive cycle modulates species-specific groups of catecholaminergic cells and socially monogamous behaviours in prairie voles.
Keywords: catecholamine, hormones, metabolites, olfaction, voles
The medial amygdala (MeA) is one component of a densely interconnected, steroid hormone-sensitive network of brain sites regulating an array of sociosexual behaviours in rodents (1). Numerous subregions of the MeA receive direct projections from the main olfactory bulbs, the accessory olfactory bulbs, or both (2-6), and this chemosensory input is requisite for appropriate social interactions. In male rats, large lesions of the MeA suppress non-contact erections, alter copulation rate, or severely inhibit copulation in toto (7-10). In female rats, MeA lesions reduce their preference for odors of gonadally intact males over those of castrated males (11) and alleviate the olfactory suppression of maternal behaviours (12). In male and female hamsters, MeA lesions impair social odor recognition, social odor preferences, and/or copulatory behaviours (see 1,13). The MeA projects broadly within and outside the sociosexual behaviour network, but of particular interest are its projections to the bed nucleus of the stria terminalis (BST). The BST of laboratory rats and hamsters receives some of the densest projections emanating from the MeA (14,15), and in some species also receives limited projections directly from the accessory olfactory bulb (3,5,16). Because of their dense connections with each other, similar projections to other areas of the sociosexual network, and olfactory processing abilities, the MeA and medial BST are considered by some to be components of a continuous neural structure termed the “extended olfactory amygdala” (17). Not surprisingly, then, the BST is also implicated in the social behaviours in rodents (18-21).
Prairie voles (Microtus ochrogaster) have become an important rodent model in which to study the neural networks involved in the olfactory and steroid hormone control of social behaviours because their social organization differs from most rodents, and even most mammals. In either wild or laboratory settings prairie voles are highly gregarious, socially monogamous after mating, and biparental towards their offspring (22). Neural sites mediating their unusual social behaviours include many of the sites traditionally involved in the more typical social behaviours of other rodents, including the MeA and BST. Indeed, lesions encompassing the MeA decrease general social contact and paternal behaviours in male prairie voles (23), while exposure to conspecific urinary cues, cohabitation with or without mating, or interactions with pups increase immediate-early gene (IEG) expression in both the MeA and BST (24-28). Although many of the same neural sites are involved in the social behaviour of prairie voles as in other rodents, it has often been suggested that species differences in neurochemistry underlie species differences in social structure and behaviours. In support, the distribution and density of receptors for oxytocin, vasopressin, corticotrophin-releasing hormone, and some gonadal steroids differ between monogamous voles and non-monogamous rodents, and their social behaviours are dramatically altered by manipulating these systems (29).
In addition to neuropeptides and hormones, central dopamine (DA) systems differ between monogamous and non-monogamous voles. Research has focused on the mesolimbocortical system, where prairie voles have higher D2 receptor expression in the medial prefrontal cortex and lower D1 receptor expression in the medial prefrontal cortex and nucleus accumbens than do meadow voles (30,31). Furthermore, mating increases DA release in the nucleus accumbens of both male and female prairie voles, which is necessary for the establishment and maintenance of pairbonds (32), but this release does not occur in non-monogamous voles (32). The existence of species differences in dopamine systems outside the mesolimbocortical system are not often investigated, but could also be expected to contribute to species differences in social behaviour. Our laboratory has described an interesting species difference in the catecholamine innervation of the MeA and BST. We find that prairie voles have hundreds of cells and a dense plexus of fibres containing tyrosine hydroxylase (TH – the rate-limiting enzyme necessary for catecholamine release) centered in the posterodorsal MeA (MeApd) and principal nucleus of the BST (pBST), but relatively few or none of these cells exist in the MeApd and pBST of the non-monogamous species examined (laboratory rats, hamsters, and meadow voles) (33). Because these cells do not contain dopamine-beta-hydroxylase (DBH), they are probably not noradrenergic and instead may be a unique dopaminergic pathway influencing social behaviours in prairie voles.
Not only is there a species difference in TH-immunoreactive (TH-ir) cells in the extended olfactory amygdala, but also a sex difference in prairie voles. Males have hundreds of these cells in both the MeApd and pBST, while females have relatively few TH-ir cells in these sites and most of these cells are weakly immunoreactive. This sex difference is almost completely generated by sex differences in circulating gonadal hormones during adulthood, because we found that castrating adult male prairie voles reduced the number of TH-ir cells in both sites to the low levels found in unmanipulated females, while giving ovariectomized female prairie voles a subcutaneous implant of testosterone raised the number of TH-ir cells in the MeApd and pBST almost to the level found in gonadally intact males (33). It is well understood that testosterone can affect neural function by acting as either an androgen or an oestrogen, depending on the route of its intracellular metabolism and a cell's sensitivity to these metabolites conferred through expression of androgen and oestrogen receptors. Similar to other rodents, the prairie vole MeA and BST express oestrogen receptors and androgen receptors (34,35). Understanding how changes in circulating gonadal hormones in adult prairie voles could affect the number and function of TH-synthesizing cells of the MeApd and pBST, and the social behaviours relying on these cells in prairie voles, requires a better understanding of the sensitivity of these cells to androgens and oestrogens. To accomplish this, we examined immunoreactivity for androgen receptors (ARs) and oestrogen receptor alpha (ERα) within the TH-ir cells of the adult male and female prairie vole extended olfactory amygdala. We then examined whether the ability of exogenous testosterone (T) to maintain the number of TH-ir cells in castrated male prairie voles, and greatly increase the number of these cells in female prairie voles, could be reproduced by either the non-aromatizable androgen, dihydrotestosterone (DHT), or by oestradiol benzoate (EB).
Methods
Experiment 1 - Expression of AR and ERα within TH-ir cells of the MeApd and pBST
Subjects
Male and female prairie voles (Microtus ochrogaster) were born and raised in our colony, from breeding stock that originated from offspring of voles originally captured in 1994 in Urbana, Illinois and brought to Michigan State University in 2002. Animals were maintained on a 14:10-hour light: dark cycle with an ambient temperature maintained at 21°C. At all ages, animals were housed in clear plastic cages (48 × 28 ×16 cm) containing wood chips, wood shavings, and a substantial hay covering. Voles were freely given water and a food mixture containing cracked corn, whole oats, sunflower seeds, and rabbit chow (Tekland rodent diet No. 2031) in a ratio of 1:1:2:2. Pups were weaned from their parents at 20 days of age, placed in mixed-sex sibling groups and housed in these groups until they were 60-95 days old. Voles were then separated into same-sex sibling groups approximately a week before being used in an experiment. Female prairie voles are induced ovulators and subject to incest avoidance (see 22), so were in a chronic state of low ovarian hormone exposure at the time of sacrifice for Experiment 1 and before being ovariectomized and implanted with an empty or hormone-filled capsule in Experiment 2. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) and the Institutional Animal Care and Use Committee at Michigan State University.
Tissue Collection and Immunocytochemistry
Adult virgin males and females (ns = 7-9/sex) from our colony were taken from their home cages and immediately received an overdose of anaesthetic cocktail containing ketamine (62.5 mg/kg), xylazine (7.5 mg/kg), and acepromazine (0.8 mg/kg) and were perfused with 100 mL 0.9% saline followed by 100 mL of a solution of 5% acrolein/4% paraformaldehyde in sodium phosphate buffer (NaPB; pH = 7.6). Brains were removed and post-fixed for 24 hr in 5% acrolein/4% paraformaldehyde. Brains were then submerged in a 20% sucrose/NaPB solution for at least 2 days before cut into 40-μm sections with a freezing microtome. Every other section through the brains was first processed for either ERα- or AR-immunoreactivity and then these sections were all processed for TH-immunoreactivity. The ERα+TH and AR+TH dual-label immunocytochemical procedures were similar to that previously described (36,37). Sections were rinsed in 0.05 M potassium phosphate buffered saline (KPBS; pH = 7.6) prior to and following 10-min incubations in 1% sodium borohydride in KPBS and 0.5% hydrogen peroxide in KPBS. They were then blocked in 20% normal goat serum and 0.3% Triton X-100 in KPBS for 30 min, and then incubated with either a biotinylated rabbit anti-ERα polyclonal antiserum (C1355; 1:15,000; Upstate Biotechnology) or a biotinylated rabbit anti-AR antiserum (N-20, sc-816; 1:10,000; Santa Cruz Biotechnology) in 0.3% Triton-X-KPBS for one hour at room temperature and then for approximately 40 hr (ERα) or 64 hr (AR) at 4°C. Sections were then rinsed in KPBS prior to and following a 60-min incubation in an anti-rabbit secondary antiserum in 0.3% Triton-X-KPBS. They were then rinsed and incubated for 60-min in an avidin-biotin complex (Vectastain Elite, Vector Laboratories), rinsed, and then incubated in a solution containing 3-3′-diaminobenzadine (DAB), which provided a dark brown nuclear label. Sections were then rinsed and blocked again with 20% normal goat serum in 0.3% Triton-X-TBS, and incubated in a rabbit primary antiserum raised against TH (AB152; 1:2000; Chemicon) in 2% normal serum and 0.3% Triton-X-TBS overnight at room temperature as previously described (27,33). Sections were rinsed three times in TBS, incubated in a biotinylated goat anti-rabbit secondary antiserum (1:500; Vector Laboratories, Burlingame, CA) in 0.3% Triton-X and 2% NGS, rinsed three times in TBS, and incubated with avidin-biotin complex (Vectastain Elite, Vector Laboratories) for 60 minutes. After rinsing three times with TBS, TH immunoreactivity was visualized with Vector SG (Vector Laboratories) according to the manufacturer's instructions, which provided a light blue cytoplasmic label. Sections were mounted on microscope slides, dehydrated, and coverslipped. Immunocytochemical control procedures included omission of the primary or secondary antisera, which abolished specific labelling. Single, but separate, immunocytochemical runs were used to visualize AR-TH and ERα-TH.
Tissue and Data Analyses
Slides were coded and analyzed by one person (BLC) using a Nikon E400 microscope at 200× magnification with the aid of a reticle placed in one of the ocular lenses. The number of cells containing detectable TH immunoreactivity was counted by eye. The pBST was analyzed bilaterally from two consecutive sections in the series through the middle and caudal pBST, roughly corresponding to plates 20-22 of Swanson's atlas of the rat brain (38), which is where the most TH-ir cells in the pBST are found. The three sections through the MeApd with the most TH-ir cells were examined, beginning with the section roughly corresponding to plate 28 of Swanson's rat atlas (38) and ending approximately at the level corresponding to atlas plate 30. There were few or no TH-ir cells rostral or caudal to the chosen sections in either sex and the rostrocaudal levels containing the maximum number of TH-ir cells was similar between the sexes. As with our previous analyses of these cell groups (27,33), the quantification included any TH-ir cells found close to, but possibly outside, the traditional boundaries of the pBST or MeApd described in other rodents.
The number of cells singly labelled with TH-ir, and double labelled with TH-ir and either AR-ir or ERα-ir, were totaled for each subject and this total used for statistical analyses. Cells were considered double labelled when dark brown labelling for AR or ERα in the nucleus was surrounded by blue cytosolic labelling for TH. Unpaired t-tests were used to compare the sexes in the number of TH-ir cells, and the percentage of TH-ir cells that were double-labelled with either AR or ERα. The relevant sections of the MeApd of one male were lost during immunocytochemical processing for TH- and ERα-ir, and the same was true for one female during immunocytochemical processing for TH- and AR-ir, resulting in accordingly reduced sample sizes for those analyses. Statistical significance was indicated by p < 0.05.
Experiment 2 - Androgenic and oestrogenic effects on the number of TH-ir cells in the MeApd and pBST
Gonadectomy and Hormone Treatment
Adult virgin male and females voles from our colony were anesthetized with an intraperitoneal injection of anesthetic cocktail (see above) and males received a sham surgery in which testes were visualized but not removed, or were castrated via a midline incision to the scrotum. Females received a sham surgery where ovaries were visualized but not removed or were ovariectomized via a ventral midline incision. Both male and female sham animals received a subcutaneous implant of an empty silastic capsule (Sham; 2.5-cm-long, 0.62 mm inner diameter, 0.95 mm outer diameter; VWR, West Chester, PA) in the nape of their neck. The remaining male and female subject were implanted with a silastic capsule of the same diameter containing nothing (GDX, 2.5-cm-long), crystalline T (2.5-cm-long), EB (0.5-cm-long), or DHT (1.0-cm-long). Capsules of these sizes containing T and EB were chosen because they provide hormone levels that maintain masculine and feminine neurobehavioural characteristics in prairie voles (37). The length of the DHT capsule was based on its ability to maintain precopulatory behaviour and seminal vesicle weight in castrated laboratory mice (39), which are similar in weight to prairie voles. Sample sizes were 8-11 animals per sex in each hormone treatment group.
Tissue Collection and Immunocytochemistry
Voles were overdosed with anesthetic cocktail three weeks after surgery and perfused with saline followed by 4% paraformaldehyde in NaPB (pH = 7.6). Seminal vesicles were removed from a group of randomly selected males and weighed immediately before perfusion to verify hormone presence or absence. Brains were removed after perfusion, post-fixed overnight in 4% paraformaldehyde in NaPB, then submerged in a 20% sucrose/NaPB solution before sectioning into 40-μm sections with a freezing microtome. Single-label ICC for TH was performed on every other section through the brain as previously described (33). Briefly, sections were rinsed three times for 5 minutes each in Trisma-buffered saline (TBS; pH 7.6), incubated in 0.1% sodium borohydride for 15 min, rinsed three times in TBS, incubated in 0.3% Triton X-100 and 1% hydrogen peroxide in TBS for 10 min, rinsed three times in TBS, blocked with 20% normal goat serum in 0.3% Triton X in TBS for 15 min, and then incubated with a rabbit anti-tyrosine hydroxylase polyclonal primary antiserum (AB152; 1:2,000; Chemicon, Temecula, CA) in 0.3% Triton X and 2% NGS in TBS at room temperature for approximately 18 hr. From that point, TH-immunochemical procedures were identical to those described in Experiment 1. Two immunocytochemical runs were used, with a similar number of male and female subjects from each treatment group included in each run.
Tissue and Data Analyses
Data were analyzed using one-way ANOVAs for each sex for each of the two brain sites analyzed. A one-way ANOVA was also used to analyze seminal vesicle weights in males. ANOVAs were followed by Fisher's LSD post-hoc tests to compare individual groups. Statistical significance was indicated by p < 0.05.
Results
Experiment 1 - Expression of AR and ERα within TH-ir cells of the MeApd and pBST
TH/AR
As expected (33), males had more TH-ir cells in the MeApd (t12 = 3.25, p = 0.007) and pBST (t13 = 6.18, p = 0.0001) than did females (Table 1). Consistent with their higher number of all TH-ir cells, males also had more TH-ir cells that contained AR-ir than did females in both the MeApd (t12 = 3.18, p = 0.008) and pBST (t13 = 4.16, p = 0.001; Table 1, Figure 1). Although the raw number of dual-labelled cells greatly differed between the sexes, the percentage of TH-ir cells that contained AR-ir did not differ between the sexes in either the MeApd (∼65%; t12 = 0.08, p = 0.939) or pBST (∼50-60%; t13 = 0.89, p = 0.387; Table 1).
Table 1.
Number (Mean ± SEM) of cells in the MeApd and pBST of male and female prairie voles immunoreactive for TH or both TH and AR, and the percentage of TH-immunoreactive cells also containing AR-ir. Significant sex difference within each site indicated by different superscript letters, p < 0.05.
| # TH-ir cells | # Dual-labelled cells | % TH-ir cells also AR-ir | |
|---|---|---|---|
| MeApd | |||
| Males (n = 7) | 149 ± 17a | 97 ± 15a | 64 ± 5 |
| Females (n = 7) | 43 ± 28b | 27 ± 16b | 65 ± 13 |
| pBST | |||
| Males (n = 7) | 92 ± 13a | 62 ± 14a | 62 ± 7 |
| Females (n = 8) | 13 ± 4b | 8 ± 2b | 49 ± 12 |
Figure 1.
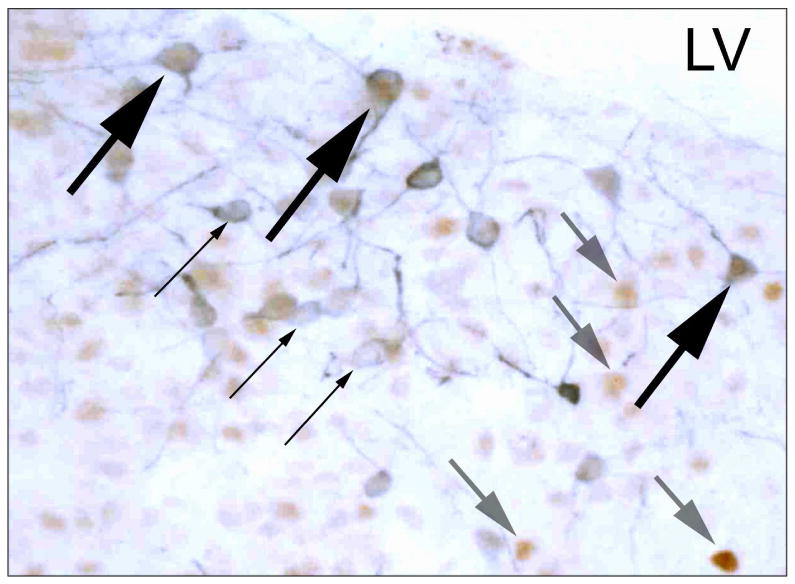
Photomicrograph of the pBST of a male prairie vole showing representative cells immunoreactive for TH alone (small black arrows, blue cytoplasmic label), immunoreactive for AR alone (medium gray arrows, brown nuclear label), and immunoreactive for both TH and AR (large black arrows, blue cytoplasmic plus brown nuclear labels). LV = lateral ventricle.
TH/ERα
Similar to above, males had more TH-ir cells in the MeApd (t13 = 3.91, p = 0.0018) and pBST (t14 = 5.36, p = 0.0001) than did females (Table 2). Males also had more TH-ir cells that contained ERα-ir than did females in both the MeApd (t13 = 4.76, p = 0.0004) and pBST (t14 = 5.88, p = 0.0001; Table 2, Figure 2). The sexes did not differ in the percentage of TH-ir cells that contained ERα-ir in the MeApd (∼30-40%; t13 = 1.20, p = 0.250), but they did differ in the pBST, with males having a significantly greater percentage of TH-ir cells that were also ERα-ir than did females (∼60 vs. 25%; t14 = 4.53, p = 0.0005; Table 2).
Table 2.
Number (Mean ± SEM) of cells in the MeApd and pBST of male and female prairie voles immunoreactive for TH or both TH and ERα, and the percentage of TH-ir cells also containing ERα-ir. Significant sex difference within each site indicated by different superscript letters, p < 0.05.
| # TH-ir cells | # Dual-labelled cells | % TH-ir cells also ERα-ir | |
|---|---|---|---|
| MeApd | |||
| Males (n = 8) | 193 ± 25a | 76 ± 11a | 39 ± 2 |
| Females (n =7) | 48 ± 27b | 13 ± 7b | 30 ± 8 |
| pBST | |||
| Males (n = 9) | 92 ± 13a | 55 ± 7a | 60 ± 2a |
| Females (n = 7) | 11 ± 5b | 4 ± 2b | 23 ± 9b |
Figure 2.
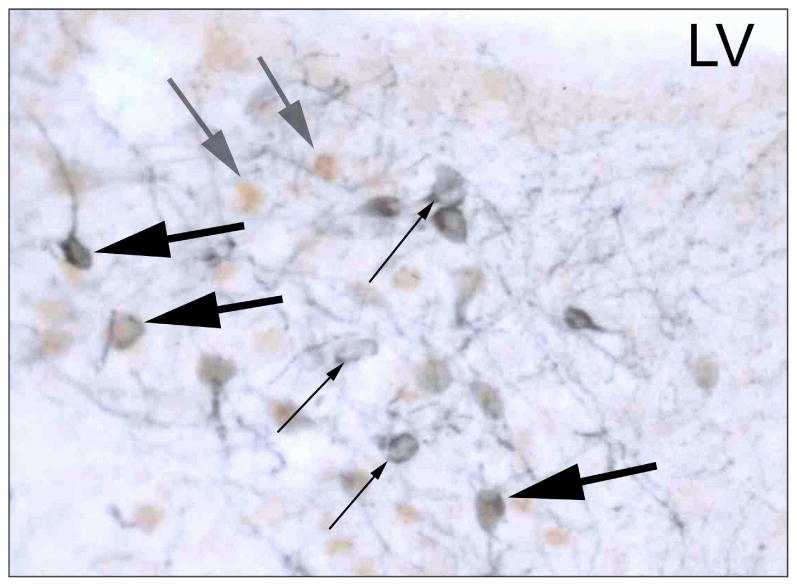
Photomicrograph of cells in the pBST of a male prairie vole showing representative cells immunoreactive for TH alone (small black arrows, blue cytoplasmic label), immunoreactive for ERα alone (medium gray arrows, brown nuclear label), and immunoreactive for both TH and ERα (large black arrows, blue cytoplasmic plus brown nuclear labels). LV = lateral ventricle.
Experiment 2 - Androgenic and oestrogenic effects on the number of TH-ir cells in the MeApd and pBST
Hormone manipulations significantly affected the number of TH-ir cells in the MeApd and pBST of both sexes. Castrating males significantly reduced the number of TH-ir cells in the MeApd (F(4,41) = 4.45, p = 0.004; Figures 3) and pBST (F(4,41) = 6.15, p = 0.0006; Figures 3, 4), while a high number of cells was maintained in castrated animals given capsules filled with T, as reported previously (33). Similar to the effects of T-filled capsules, EB also maintained a high number of TH-ir cells in both sites in castrated males at levels similar to that found in gonadally intact males. Notably, DHT did not produce this effect, and these males had as few TH-ir cells as that found in castrates that received an empty capsule. Seminal vesicle weights significantly differed among the groups of males (F(4,36) = 3.69, p = 0.013; Table 3). Castrated males had the lightest seminal vesicles, but intact males or castrated males receiving either T or DHT had the highest. Castrated males that received EB had intermediate seminal vesicle weights that did not significantly differ from any other group.
Figure 3.
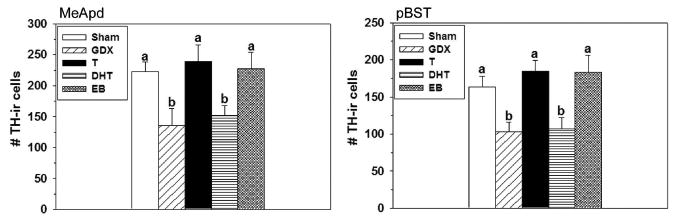
Number (Mean ± SEM) of TH-ir cells in the MeApd (left) and pBST (right) of male prairie voles that were gonadally intact (Sham; n = 10), castrated and implanted with an empty capsule (GDX; n = 10), or castrated and implanted with a capsule containing T (n = 8), DHT (n = 9), or EB (n = 9). Significant differences among groups indicated by different letters above bars, p < 0.05.
Figure 4.
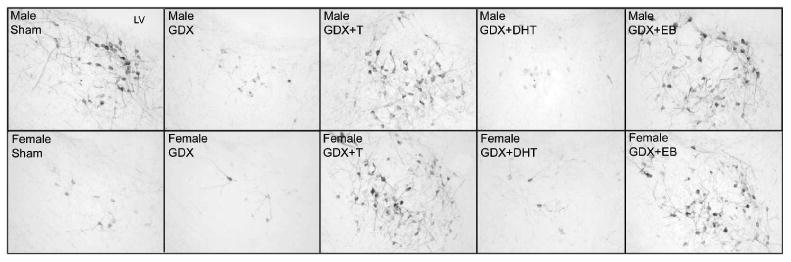
Photomicrographs of TH-ir cells in the pBST of representative male (top) and female (bottom) prairie voles that were gonadally intact (Sham) or gonadectomized (GDX) and implanted with an empty capsule or one filled with T, DHT, or EB. LV = lateral ventricle.
Table 3.
Seminal vesicle weights (Mean ± SEM) of male prairie voles that were gonadally intact (Sham) or castrated and implanted with an empty capsule (GDX) or one filled with T, DHT, or EB. Significant differences between groups indicated by different superscript letters, p < 0.05.
| Sham | GDX | T | DHT | EB | |
|---|---|---|---|---|---|
| Seminal Vesicles (g) | 6.4 ± 0.4a | 3.4 ± 0.3b | 6.2 ± 0.6a | 7.1 ± 0.8a | 5.5 ± 1.1ab |
In females, ovariectomy had no significant effect on the already low number of TH-ir cells in the MeApd (F(4,45) = 6.31, p = 0.0004; Figure 5) or pBST (F(4,45) = 11.63, p = 0001; Figures 4, 5), while capsules filled with T had the expected effect of notably increasing the number of TH-ir cells in ovariectomized females in both sites (33). Similar to males, EB-treated females had a high number of TH-ir cells in the MeApd and pBST, reaching levels similar to what was found in females receiving T. DHT did not produce this effect, and the number of TH-ir cells in DHT-treated females was similar to that found in both the sham-operated females and ovariectomized females that did not receive hormone.
Figure 5.
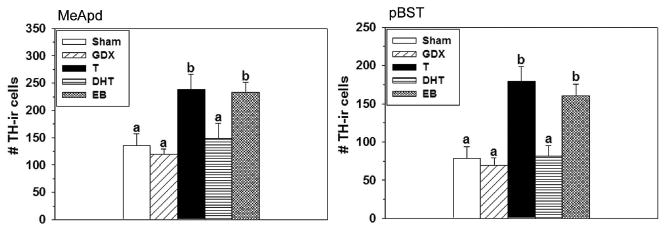
Number (Mean ± SEM) of TH-ir cells in the MeApd (left) and pBST (right) of female prairie voles that were gonadally intact (Sham; n = 10), ovariectomized and implanted with an empty capsule (GDX; n = 9), or ovariectomized and implanted with a capsule containing T (n = 9), DHT (n = 11), or EB (n = 11). Significant differences among groups indicated by different letters above bars, p < 0.05.
Discussion
The prairie vole is an invaluable rodent in which to study the neurobiology of sociality because they are highly gregarious, pairbond after mating, and are biparental (22). An abundance of research demonstrates that species differences in neurochemistry underlie some species differences in social behaviors (29). Our lab has shown that the prairie vole MeApd and pBST are remarkable because unlike any other mammal studied to date, these sites contain hundreds of cells densely immunoreactive for TH. TH-synthesizing cells do exist in the extended amygdala of adult Syrian hamsters, but these cells are not in the same MeA and BST locations as the TH-ir cells in prairie voles, and the TH content in the hamster MeA and BST is so low under basal conditions that it is immunocytochemically undetectable in all but a few cells (40,41). Other studies have described cells synthesizing immunocytochemically detectable levels of TH in the central amygdala and lateral BST of laboratory rats, but most of these cells only transiently produce TH and are undetectable by the time rats are 50 days old (42). The existence of a large number of cells in the MeApd and pBST of adult prairie voles containing high levels of TH indicates that these cells could be unique participants in extended amygdalar function in this species, including integration of steroid hormone and olfactory signals necessary for the display of monogamous behaviours such as pairbonding and biparental care.
We found that a substantial number of TH-ir cells in the MeApd and pBST of both sexes contained AR or ERα, potentially rendering these cells sensitive to androgens and oestrogens. We do not know if the same TH-ir cells express both AR and ERα, but such co-localization is common in cells of unknown chemical phenotype in the male rat and hamster MeApd and pBST (43,44) and could permit synergistic effects of these steroids on TH cell activity. Expectedly, we found that not all TH-ir cells were immunoreactive for AR or ERα, and in both sites the expression of each hormone receptor occurred in up to ∼65% of TH-ir cells. This is similar to that found in hamsters treated with colchicine to increase TH in the MeA and BST to detectable levels, where 75-80% of TH-ir cells contain AR (41). We also found a significant sex difference in the percentage of TH-ir cells in the pBST also labelled with ERα, which was lower in females compared with males (23% vs. 60% of TH-ir cells). This male bias is not due to an overall sex difference in ERα-ir in the pBST, because we find no sex difference in prairie voles from our colony in the number of pBST or MeApd cells (of any chemical phenotype) containing ERα-ir (37), and others who find of a sex difference in ERα in these sites find it in favor of female prairie voles (34). In any case, it may be prudent to consider the relevance of this finding negligible, because females have so few TH-ir cells in the pBST in the first place (average of 11 TH-ir cells) that even one or two additional dual-labelled cells in each female could have eliminated this apparent sex difference in the percentage that contain ERα.
In the second experiment, we again found that the number of TH-ir cells in the MeApd and pBST of adult prairie voles is tremendously affected by circulating gonadal hormones (33). In males, castration greatly reduced the number of TH-ir cells in these sites, while capsules filled with T maintained a high number of these cells. Females treated with T had an increased number of these cells that approached levels found in gonadally intact males. These effects of T are unique to the MeApd and pBST, because we do not find them in numerous other TH-containing cells groups in the prairie vole forebrain (33,45). Another unique aspect of these potent hormone effects in male prairie voles is related to the fact that castrated male hamsters treated with colchicine show fewer TH-ir cells in the anterior MeA (but not the posterior MeA or BST) compared to intact controls, but this castration effect cannot completely be reversed by T-filled implants (41). An important new finding from the present study was that EB produced effects almost identical to those of T in both sexes of voles. This suggests that many of the TH-ir cells in the MeApd and pBST probably contain aromatase, which is found in abundance in these brain sites in other rodents (46,47). Treating T- or EB-implanted voles with an aromatase inhibitor or ER receptor antagonist would help clarify the mechanisms underlying how these hormones each stimulate high TH immunoreactivity in these cells. Even so, the similar effects of T and EB strongly suggests that ER activity is required for the integrity of these species-specific populations of catecholaminergic cells, ones we believe are involved in prairie voles' unique monogamous behaviours (see 27, 33 and discussion below). However, work from the Cushing laboratory leads to the conclusion that high ERα expression in the MeA and BST is inconsistent with monogamous behaviours in voles and other rodents (34,36) and they have elegantly demonstrated that increasing ERα expression in the MeA reduces male prairie voles' alloparental behaviour and preferences for a familiar female (48). It is challenging to reconcile how ERα could both promote and inhibit monogamous behaviours, unless it depends on the locus of ERα expression and activity. A broad increase in ERα activity in many types of cells in the MeApd and pBST may suppress monogamous behaviours, but more restricted ERα activity within TH-ir cells may promote these social behaviours in prairie voles. It would be difficult to imagine circulating gonadal hormones targeting just the TH/ERα-ir cells in these sites, as opposed to all ERα-containing cells, so it may be that ERα activity specifically in TH-ir cells helps counter the monogamy-suppressing effects of more generalized ERα expression and activity in the prairie vole MeApd and pBST.
In contrast to EB, DHT did not reproduce the effects of T on the number of TH-ir cells in the MeApd or pBST. Given that Experiment 1 revealed more TH-ir cells containing AR immunoreactivity than ERα immunoreactivity (∼60% vs. ∼40%, collapsed across sex and site), this lack of an effect is surprising. This is probably not due to insufficient hormone provided by the capsules because males' seminal vesicle weights were maintained at control levels by the DHT-filled implants. Instead of having no function at all, it is more conceivable that AR activity does influence TH-associated and other processes of these cells, but not whether they contain immunocytochemically detectable levels of TH. The inability of DHT to reproduce the effects of T on TH immunoreactivity in the adult brain has been previously observed (e.g., 49,50), but analyzing TH mRNA may have revealed a more subtle effect of DHT at the level of transcription.
The present studies do not address the source of hormone-induced increases in TH-ir cells, nor the fate of the cells that apparently “disappear” after hormone deprivation. The increase could result from steroid hormone induced neurogenesis, which occurs in the female prairie vole MeA after cohabitation and mating (51). These events are associated with increased ovarian function, but this as a prerequisite for increased TH-ir cells is tempered by the finding that neurogenesis does not occur in the MeA of unmated female prairie voles treated with exogenous oestradiol (35). Instead of being additional cells, the newly appearing TH-ir cells in our gonadectomized females and males treated with hormones may always exist and have the capacity to produce TH, but remain relatively dormant until being stimulated by testosterone, oestradiol, or other factors to upregulate TH mRNA transcription and/or protein translation sufficient enough to detect this enzyme imunocytochemically. Indeed, the AR and ERα can each act on the promoter region of the rat TH gene to modulate its activity (52,53). On the other hand, low levels of TH-ir cells in unmanipulated females and the apparent loss of TH-ir cells after adult castration in males is probably due to TH mRNA or protein downregulation. In castrated males, it may also result from increased expression of apoptotic genes that eventually contribute to death of these cells when animals are deprived of gonadal hormones (54,55). Evaluating these possibilities would be useful and will require an analysis of total cell numbers, as well as neurogenesis and apoptotic cell profiles, in the MeApd and pBST of voles in different gonadal hormone conditions.
Our data demonstrating hormone-mediated plasticity in the extended olfactory amygdala of adult prairie voles are reminiscent of studies on other rodent species. Castrating adult male rats or mice significantly reduces MeApd volume and soma size (56), and a similar effect is found on posterior MeA soma size and dendritic branching in male hamsters (57). Castrating male rats also reduces the volume of their pBST (58). There are species differences in how hormones can maintain the integrity of these sites post-castration, with the reduction in MeApd soma size in castrated rats prevented by EB, DHT, or their combination, but neither hormone prevents the effects of castration on MeApd volume (56) and only oestradiol prevents the regressive effects of castration on the hamster posterior MeA (56,57). Consistent with our data from female prairie voles, T and EB each have proliferative effects on MeApd volume and soma size in female rats and mice (56,59). Hormone-mediated plasticity of particular neurochemicals in the adult MeA or BST has also been observed in other rodents, including the number of cells synthesizing arginine-vasopressin, cholecystokinin, enkephalins, somatostatin, and substance P. In all cases, and similar to what is suggested by our findings, the neurochemical profiles of these cells seem to be maintained by an ER-associated mechanism (60).
At this point, we can only conjecture about the functional significance of gonadal hormone-induced plasticity of TH-ir cells in the prairie vole extended olfactory amygdala. The location of these cells implicates them in steroid hormone and olfactory control of social behaviours. In support of this, lesions encompassing the male prairie vole MeA reduce their social contact with females or pups (23). We recently found that IEG expression in TH-ir cells of the male prairie vole MeApd and pBST is greatly increased by cohabitation and mating with a female, but not by interactions with pups (27), suggesting relevance to mating and the consequent formation or maintenance of an olfactory memory necessary for pairbonding. These TH-ir cells in male prairie voles may also be involved in more general detection and processing of the social environment, as we also found that any social contact with conspecifics maintains basal IEG expression within these cells (27) and others find that the number of these TH-ir cells drops when adult males and females are socially isolated (61). This isolation-induced drop in the number of TH-ir cells is similar to our castration effects, but socially isolating adult male prairie voles for four weeks does not significantly affect circulating T or oestradiol (nor corticosterone) (62), so the isolation effect is probably controlled by non-gonadal factors.
In sum, many of the TH-ir cells in the prairie vole extended olfactory amygdala contain receptors rendering them sensitive to either androgens or oestrogens, and the functional significance of this sensitivity is reflected by tremendous plasticity of these cells to experimental manipulations in gonadal hormones during adulthood. This is consistent with the hormone-mediated plasticity of other systems within these brain sites in adult rats, mice, and hamsters. Widespread hormone-induced plasticity in the extended olfactory amygdala of animals with such disparate social organizations suggests it is a ubiquitous target for gonadal hormone effects on many type of social and non-social behaviours in rodents. In the context of prairie vole reproduction, this plasticity could be related to how natural changes in circulating gonadal hormones at different periods of the reproductive cycle promote flexibility in the display of monogamous behaviours. We have data indicating that the number of TH-ir cells in the pBST and MeApd increase after female prairie voles cohabitate with an unfamiliar male for long enough to be induced into estrus, or after females mate and later gain extensive parental experience with multiple litters of pups (63). This increase could reflect changes in how the pBST and MeApd process gonadal hormone and olfactory cues necessary for pairbonding and parenting behaviours.
Acknowledgments
This work was supported by NSF grant 0515070 (JSL). We appreciate the advice of Dr. Bruce Cushing on the ERα immunocytochemistry and Dr. Cynthia Jordan on the AR immunocytochemistry.
References
- 1.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 2.Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–34. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Bañón I, Crespo C, Insausti R, Martinez- Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–80. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- 4.Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Bañon I, de la Rosa-Prieto C, Saiz-Sanchez D, Martinez-Marcos A. Projections of olfactory bulbs to the olfactory and vomeronasal cortices. Neuroreport. 2008;19:1541–4. doi: 10.1097/WNR.0b013e32831126ee. [DOI] [PubMed] [Google Scholar]
- 5.Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- 6.Winans SS, Scalia F. Amygdaloid nucleus: new afferent input from the vomeronasal organ. Science. 1970;170:330–2. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge FH, Oldenburger WP, Louwerse AL, Van de Poll NE. Changes in male copulatory behavior after sexual exciting stimuli: effects of medial amygdala lesions. Physiol Behav. 1992;52:327–32. doi: 10.1016/0031-9384(92)90279-b. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51:939–43. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- 9.Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1998;91:215–22. [PubMed] [Google Scholar]
- 10.McGregor A, Herbert J. Differential effects of excitotoxic basolateral and corticomedial lesions of the amygdala on the behavioural and endocrine responses to either sexual or aggression-promoting stimuli in the male rat. Brain Res. 1992;574:9–20. doi: 10.1016/0006-8993(92)90793-9. [DOI] [PubMed] [Google Scholar]
- 11.Kondo Y, Sakuma Y. The medial amygdala controls the coital access of female rats: a possible involvement of emotional responsiveness. Jpn J Physiol. 2005;55:345–53. doi: 10.2170/jjphysiol.RP001105. [DOI] [PubMed] [Google Scholar]
- 12.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–43. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 13.Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2009;200:260–7. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 15.Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- 16.von Campenhausen H, Mori K. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur J Neurosci. 2000;12:33–46. doi: 10.1046/j.1460-9568.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 17.Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–53. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–95. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- 20.Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–14. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–13. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JT, Wang Z. Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster) Physiol Behav. 2003;80(1):95–101. doi: 10.1016/s0031-9384(03)00226-9. [DOI] [PubMed] [Google Scholar]
- 25.Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–11. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- 26.Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–8. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- 27.Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–32. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 29.Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:401–10. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 31.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500:103–15. doi: 10.1002/cne.21148. [DOI] [PubMed] [Google Scholar]
- 34.Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le WW, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016:247–54. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–79. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cushing BS, Wynne-Edwards KE. Estrogen receptor-alpha distribution in male rodents is associated with social organization. J Comp Neurol. 2006;494:595–605. doi: 10.1002/cne.20826. [DOI] [PubMed] [Google Scholar]
- 37.Northcutt KV, Lonstein JS. Sex differences and effects of neonatal aromatase inhibition on masculine and feminine copulatory potentials in prairie voles. Horm Behav. 2008;54:160–9. doi: 10.1016/j.yhbeh.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson LW. Brain maps: structure of the rat brain. 2nd. Amsterdam: Elsevier; 1998. [Google Scholar]
- 39.Bean NJ, Nyby J, Kerchner M, Dahinden Z. Hormonal regulation of chemosignal-stimulated precopulatory behaviors in male housemice (Mus musculus) Horm Behav. 1986;20:390–404. doi: 10.1016/0018-506x(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 40.Asmus SE, Kincaid AE, Newman SW. A species-specific population of tyrosine hydroxylase-immunoreactive neurons in the medial amygdaloid nucleus of the Syrian hamster. Brain Res. 1992;575:199–207. doi: 10.1016/0006-8993(92)90080-s. [DOI] [PubMed] [Google Scholar]
- 41.Asmus SE, Newman SW. Tyrosine hydroxylase neurons in the male hamster chemosensory pathway contain androgen receptors and are influenced by gonadal hormones. J Comp Neurol. 1993;331:445–57. doi: 10.1002/cne.903310402. [DOI] [PubMed] [Google Scholar]
- 42.Verney C, Gaspar P, Febvret A, Berger B. Transient tyrosine hydroxylase-like immunoreactive neurons contain somatostatin and substance P in the developing amygdala and bed nucleus of the stria terminalis of the rat. Brain Res. 1988;470:45–58. doi: 10.1016/0165-3806(88)90200-3. [DOI] [PubMed] [Google Scholar]
- 43.Gréco B, Edwards DA, Michael RP, Clancy AN. Androgen receptors and estrogen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinology. 1998;67:18–28. doi: 10.1159/000054294. [DOI] [PubMed] [Google Scholar]
- 44.Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–97. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- 45.Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66:197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- 46.Foidart A, Harada N, Balthazart J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res. 1995;280:561–74. doi: 10.1007/BF00318360. [DOI] [PubMed] [Google Scholar]
- 47.Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 48.Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci. 2008;28:10399–403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brawer J, Bertley J, Beaudet A. Testosterone inhibition of tyrosine hydroxylase expression in the hypothalamic arcuate nucleus. Neurosci Lett. 1986;67:313–8. doi: 10.1016/0304-3940(86)90328-9. [DOI] [PubMed] [Google Scholar]
- 50.Dias BG, Ataya RS, Rushworth D, Zhao J, Crews D. Effect of incubation temperature and androgens on dopaminergic activity in the leopard gecko, Eublepharis macularius. Dev Neurobiol. 2007;67:630–6. doi: 10.1002/dneu.20382. [DOI] [PubMed] [Google Scholar]
- 51.Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–28. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 52.Jeong H, Kim MS, Kwon J, Kim KS, Seol W. Regulation of the transcriptional activity of the tyrosine hydroxylase gene by androgen receptor. Neurosci Lett. 2006;396:57–61. doi: 10.1016/j.neulet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors alpha and beta and interactions with cyclic AMP. J Neurochem. 2005;93:1502–14. doi: 10.1111/j.1471-4159.2005.03142.x. [DOI] [PubMed] [Google Scholar]
- 54.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 55.Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–38. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231:498–509. doi: 10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- 58.Malsbury CW, McKay K. Neurotrophic effects of testosterone on the medial nucleus of the amygdala in adult male rats. J Neuroendocrinol. 1994;6:57–69. doi: 10.1111/j.1365-2826.1994.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 59.Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008;1190:115–21. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier; New York: 2002. pp. 137–191. [Google Scholar]
- 61.Perry A, Carter CS, Cushing B. The effects of social isolation on reproductive behavior in the prairie vole. Society for Behavioral Neuroendocrinology Abstracts. 2009;P2.06 [Google Scholar]
- 62.Klein SL, Hairston JE, Devries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm Behav. 1997;32:30–9. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- 63.Cavanaugh BL, Lonstein JS. Effects of inter- or intra-sexual contact and ovarian hormones on tyrosine hydroxylase immunoreactivity in the female prairie vole extended olfactory amygdala. Society for Neuroscience. 2008;866.6:NN2. [Google Scholar]


