Recent methodological developments in the fields of neuroimaging, genetics, and basic neuroscience are reconceptualizing research into mental disorders (1). In particular, findings from functional, structural, and neurochemical imaging studies, along with discoveries from genome-wide association studies have provided novel and promising leads regarding neural circuitry dysfunction in mood disorders. However, translating this accumulating knowledge into practical methods for defining biologically driven disease categories, selecting the most appropriate treatment for a specific patient (i.e., personalized medicine), and developing novel treatments remain major unresolved challenges.
In this context, one of the most promising research breakthroughs in mood disorders was the discovery of the importance of glutamatergic abnormalities in the pathophysiology of these illnesses and the concomitant development of glutamatergic-based treatments (reviewed in [2,3]; Figure 1). In this issue of Biological Psychiatry, Yüksel and Öngür (4) review the evidence from magnetic resonance spectroscopy (MRS) studies of glutamatergic abnormalities in major mood disorders. Their excellent review provides an extensive and detailed framework that integrates MRS findings to date within anatomical, pathophysiological, nosological, and therapeutic considerations.
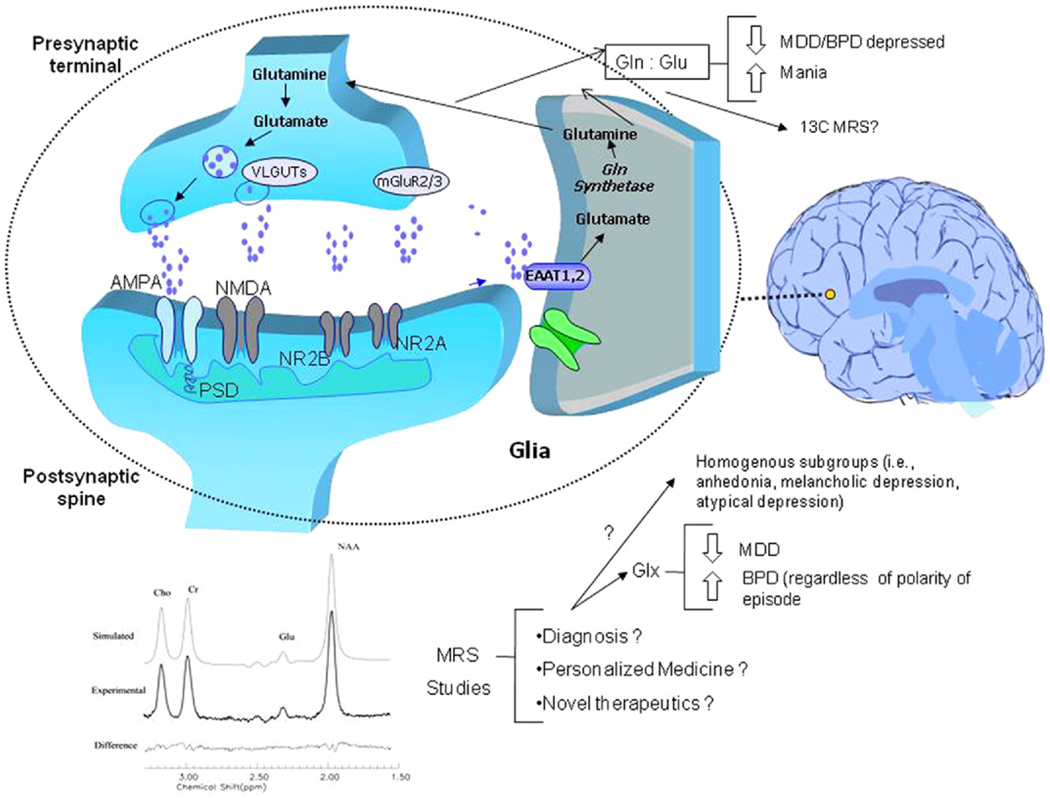
Figure 1.
The tripartite glutamatergic system and magnetic resonance spectroscopy studies in mood disorders. The glutamate (Glu)-glutamine (Gln) cycle plays a key role in the regulation of presynaptic and postsynaptic ionic and metabotropic glutamate receptors that have been implicated in the pathophysiology of mood disorders. In the brain, glutamate can either be synthesized de novo from glucose via the Krebs cycle and the transamination of α-oxoglutatrate or it can be recycled through the Glu/Gln cycle (see above). Glutamate is transported into synaptic vesicles by vesicular Glu transporters. Once released, Glu binds to and activates ionotropic and metabotropic receptors found throughout the central nervous system that have wide-ranging effects on neural excitability. There are three excitatory ionotropic receptors identified by their pharmacological properties as AMPA, kainate, and NMDA receptors. Metabotropic glutamate receptors couple to different signal transduction systems and ligand sensitivities. Glutamate is cleared from the extracellular space via high-affinity excitatory amino acid transporters in neighboring glial cells, which convert Glu into Gln via the action of glutamine synthetase. Glutamine is then transported back into the glutamatergic neuron where it is hydrolyzed by glutaminase back into Glu. AMPA, α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid; BPD, bipolar disorder; 13C MRS, carbon-13 magnetic resonance spectroscopy; EAAT, excitatory amino acid transporter; Glx, glutamine plus glutamate; MDD, major depressive disorder; mGluR2/3, metabotropic glutamatergic receptor 2 and 3; NMDA, N-methyl-D-aspartate; NR2A, NMDA receptor sybtype 2A; NR2B, NMDA receptor subtype 2B; PSD, postsynaptic density; VLGUT, vesicular Glu transporters.
Notably, Yüksel and Öngür (4) also present evidence highlighting the considerable heterogeneity across MRS studies with regard to subject selection, field strength, MRS sequences, and anatomical placement of the voxel of interest. Despite these methodological differences, the studies performed to date show a consistent pattern of a decreased composite peak formed by glutamate, glutamine, and gamma-aminobutyric acid (Glx) in major depressive disorder (MDD) (unipolar depression) and increased Glx in bipolar disorder (BPD), regardless of mood polarity. If those findings continue to be confirmed in future studies, glutamate-related MRS measures may extend our understanding of the pathophysiology of both MDD and BPD and may prove to be valuable new tools for improving diagnostic accuracy and defining patient cohorts.
The review also highlights evidence regarding the possibility of using glutamate-related measures to more clearly identify biologically homogenous subgroups of patients; for instance, glutamatergic abnormalities appear to be more pronounced in patients with melancholic depression (5) or with a high degree of anhedonia (6) compared with patients with atypical depression or little anhedonia. Indeed, the need to “enrich” study samples (e.g., with clinical and family history criteria) is not a novel concept in neuroimaging studies of mood disorders (7) and is key to defining biological differences between patients and healthy control subjects. This is critical for disentangling the diagnostic complexity of MDD into more homogenous subgroups that might share not only clinical features, but also biological determinants, such as genetic vulnerability or common patterns of glutamatergic abnormalities. In this context, glutamatergic abnormalities consistently identified by MRS studies might ultimately serve as key biomarkers—loosely defined as biochemical features or facets that can be used to measure both the progress of a disease or treatment responsiveness.
Another important methodological point highlighted by Yüksel and Öngür (4) is that most recent MRS studies conducted at higher field strengths (mostly using J-resolved magnetic resonance spectroscopy) have been able to resolve the overlapping resonances of glutamate and glutamine; as a result, these provide valuable information that may be more directly linked to brain anatomy and physiology. Because proton MRS studies measure the whole glutamate and glutamine content in the voxel of interest, they cannot provide direct information about the glutamate-glutamine cycle between neurons and astroglial cells. However, because glutamate measures reflect mainly the glutamate intracellular pool present in glutamatergic neurons and glutamine measures reflect the glial intracellular content, those measures, taken together, may provide an indirect window into the overall status of the glutamatergic system. Methodologically, this represents a significant step forward compared with measuring overall Glx tissue content.
As Yüksel and Öngür (4) note, only a few studies have used these newer MRS sequences to study patients with mood disorders; thus, the reliability of such measurements is still not comparable with Glx measures obtained using more conventional MRS sequences (e.g., point-resolved spectroscopy, stimulated echo acquisition mode). For now, such studies therefore need to be considered hypothesis generating and preliminary. Despite these caveats, the studies indicate that patients with MDD and BPD experiencing a major depressive episode appear to have reduced glutamine relative to glutamate; in contrast, the opposite pattern—increased glutamine relative to glutamate—appears to characterize the manic phase in individuals with BPD. Yüksel and Öngür (4) hypothesize that decreased glutamine might indicate lower glutamate conversion by glial cells, thereby reflecting an overall reduction in glutamatergic neurotransmitter flux during depression, while higher glutamate-to-glutamine conversion might occur during mania. As the authors recognize, future carbon-13 MRS studies might provide specific information about glutamate-glutamine flux between neurons and glial cells.
Another promising application of proton MRS measures related to the glutamatergic system is the use of multimodal imaging studies that combine MRS with other imaging techniques (e.g., functional magnetic resonance imaging, positron emission tomography, magnetoencephalography). Indeed, multimodal imaging may eventually have important implications for integrating information obtained across multiple sources to expand our understanding of both normal and pathological brain physiology.
Thus, MRS studies of the glutamatergic system in mood disorders create novel avenues for understanding the pathophysiology of these devastating illnesses, refining their diagnosis, potentially improving our ability to provide individualized treatment, and developing new and improved therapeutics. Nevertheless, and as noted above, several challenges—both clinical and methodological—still need to be addressed before this knowledge can be translated into practice. First, most MRS studies to date have had small sample sizes; for instance, only 3 out of 12 studies of individuals with MDD had more than 20 patients, and only 5 out of 11 studies of individuals with BPD had more than 20 patients. A related issue concerns clinically enriched samples (e.g., patient subgroups selected because of their melancholic features or high rates of anhedonia); in general, such results were based on secondary analyses and therefore underpowered.
Next, despite accumulating evidence regarding the importance of the glutamatergic system in the pathophysiology and treatment of mood disorders, whether glutamate-related MRS measures can provide clinically relevant information remains unknown. In this context, clinically relevant information would include novel biological measures that could guide treatment selection and provide information regarding longitudinal prognosis for any given patient. In fact, very few longitudinal studies have investigated whether glutamate-related MRS measures are affected by antidepressant treatment or whether pretreatment glutamate-related measures differentiate treatment responders from nonresponders. Such studies will be key to understanding the extent to which glutamatergic measures can contribute to clinical decision making.
Magnetic resonance spectroscopy studies of glutamatergic measures in mood disorders may also assist in drug development. Novel glutamatergic agents to treat mood disorders being studied include N-methyl-D-aspartate and N-methyl-D-aspartate receptor subtype 2B antagonists, riluzole, and metabotropic glutamate receptor modulators (2). Nevertheless, several challenges remain before such work can be done successfully. In addition to the considerable heterogeneity that exists in diagnosing mood disorders, difficulties remain in assessing and developing new technologies, minimizing confounding factors such as the use of other medications, and controlling for course of illness with a comparator treatment.
Finally, one methodological issue regarding MRS studies conducted to assess glutamate and glutamine levels instead of Glx is that the voxel of interest chosen was relatively large (i.e., 8–27 cc); this was done to obtain enough signal-to-noise ratio to resolve individual glutamate and glutamine peaks. This method, however, yields low spatial resolution, which may limit our ability to detect meaningful differences in glutamate and glutamine concentrations in the voxel investigated. Furthermore, because no reliable MRS imaging sequences yet exist for quantifying glutamate-related metabolites, most of the studies have used single voxel spectroscopy. Therefore, potential abnormalities that fall outside the a priori anatomical region of interest may not have been detected. It is possible, however, that future studies conducted at higher field strength may be able to quantify glutamate levels in smaller voxels of interest. Magnetic resonance spectroscopy imaging sequences to quantify glutamate in extended regions of the brain are presently under development.
Taken together, the evidence presented in this review (4) provides a very valuable and critical overview of this topic and highlights the most important controversies in the interpretation of MRS measures. Glutamate-related measures obtained via MRS clearly appear to be a promising—but still preliminary—technology for elucidating the role of glutamatergic abnormalities in mood disorders. Nevertheless, this review also clearly draws attention to the tremendous need to adopt a consistent scientific framework for evaluating biomarkers. Any such evaluation must occur in a systematic manner to ensure that biomarkers of interest are reliable, reproducible, adequately sensitive, adequately specific, and associated with the clinical outcome of interest (8); this work must, obviously, take place before proceeding to the clinic.
Despite these limitations, it is clear that glutamate-related measures obtained via MRS hold considerable promise in our quest to elucidate the role of glutamatergic abnormalities in mood disorders. Ultimately, such measures could be used to refine diagnosis, develop novel and improved therapeutics, and create more personalized and successful treatment regimens for individual patients with mood disorders.
Acknowledgments
Funding for this work was supported by the Division of Intramural Research Programs of the National Institute of Mental Health, National Institutes of Health, and Department of Health and Human Services.
Footnotes
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression. Dr. Zarate has assigned his patent rights on ketamine to the US government. Dr. Salvadore reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 2.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA., Jr Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009;15:1595–1611. doi: 10.2174/138161209788168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 6.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine of the National Academies. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]