Abstract
Cyclophilin-40 (CyP40) is part of the immunophilin family and is found in Hsp90-containing protein complexes. We are interested in identifying proteins that interact with CyP40. CyP40 interacting proteins in HeLa cells were identified using the tandem affinity purification approach. Adenovirus (AdCyP40) expressing human CyP40 protein, fused with a streptavidin and a calmodulin binding peptides at the N-terminus, was generated. Proteins were separated on a SDS-PAGE gel after tandem affinity purification. Ten silver-stained protein bands that were enriched in the AdCyP40-infected lysate and the corresponding regions in the control lysate were excised, digested by trypsin and identified by tandem mass spectrometric analysis. Eleven interacting proteins were identified and four of which (RACK1, Ku70, RPS3, and NF45) were expressed in rabbit reticulocyte lysates, bacteria, and MCF-7 cells. We confirmed that these proteins interact with CyP40. We observed that RACK1 suppressed the cobalt chloride-induced, HRE-dependent luciferase activity in MCF-7 cells but not in MCF-7 stable cells expressing about 5% of the cellular CyP40 content. In addition, RACK1 reduced the HIF-1α protein accumulation after cobalt chloride treatment which was not observed when the CyP40 content was down-regulated. Collectively, we conclude that reduction of the HIF-1α protein by RACK1 is CyP40-mediated.
Keywords: CyP40, RACK1, NF45, RPS3, Ku70, HIF-1α
Introduction
Cyclophilin 40 (CyP40) was first discovered as a cyclosporine A-binding protein [1] and part of the inactivated estrogen receptor complex [2]. It belongs to the immunophilin family which contains two signature motifs – the C-terminal three tetratricopeptide repeats (TPR) and the N-terminal peptidyl prolyl cis/trans isomerase (PPIase) domain, separated by 30 amino acids [3]. TPR repeats are the interaction surfaces which are necessary for binding to Hsp90 [4] whereas PPIase domain catalyzes the prolyl isomerization step which occurs during the protein folding process [5]. There are at least six known human cyclophilins, namely CyPA, CyPB, CyPC, CyPD, hCyP33, and CyP40. CyPB and C contain a localization sequence which targets them into endoplasmic reticulum [6]. CyPD is selectively found in the mitochondrial matrix [7] whereas hCyP33 is localized into the nucleus [8]. Only CyPA and CyP40 appear to be in both cytoplasm and nucleus [9,10]. Expression of CyP40 seems ubiquitous because the CyP40 message is expressed in most human tissues except lung [11]. It has been long discovered that CyP40 is part of the estrogen receptor-Hsp90 complex [12]; however, its significance in steroid receptor complexes has been challenged since researchers failed to locate CyP40 as part of the glucocorticoid receptor complex in L929 and WCL2 cells [13]. CyP40 was suggested to function as a molecular chaperone which helps the client protein to be folded in a somewhat proteolysis-resistant state [14]. Other discovery with regards to possible CyP40 function has been unveiled: for example, knockdown studies have revealed that CyP40 may be essential for the full function of AhR [15] and it may play a role in hepatitis C virus replication in host cells [16]. The DNA binding ability of c-Myb can be inhibited by CyP40 [17], suggesting that Cyp40 may be involved in transcriptional control. Collectively, its cellular role has been suggested but remains quite unclear at present. Hence, it would be informative to identify CyP40-interacting proteins and use them to elucidate the cellular function of CyP40.
Tandem affinity purification (TAP) was originally proposed to identify interacting proteins [18]. Coupled with a proteomic approach, mass spectrometry researchers were able to identify many interacting proteins using this strategy. In principle, two tandem tags are fused to the bait protein so that through sequential affinity purification, interacting proteins can be co-purified with the bait protein. The co-eluted proteins can then be analyzed by denaturing gel electrophoresis to separate the interacting proteins and tandem mass spectrometry to reveal their identity. A tight binding tag to the affinity resin in nanomolar affinity would be ideal for gentle but specific washing during the purification process to allow effective purification of interacting proteins with minimal non-specific protein binding. There are a number of different combinations of affinity tags used for this strategy. For example, a calmodulin binding peptide (CBP) and an IgG binding tag were used to identify interacting proteins of at least 15 TAP fusion proteins [19]; a protein A and a FLAG tags were used to identify the interaction between Hsp70 and SMAD3 [20]; an alternative TAP containing a tandem protein A tag separated from a protein C tag by a TEV protease cleavage site might work better in some occasions [21]. In addition, streptavidin binding peptide (SBP) has been shown to provide nanomolar binding affinity and used to purify recombinant proteins [22,23]. In this paper, we used the InterPlay TAP system from Stratagene (La Jolla, CA) which utilizes a TAP containing SBP and CBP to generate N-terminal TAP fusion of human CyP40. We subsequently generate adenovirus expressing this TAP-CyP40 (Ad-TAP-CyP40). The Ad-TAP-CyP40-infected HeLa cells were used to eventually identify RACK1, Ku70, NF45, and RPS3 as CyP40-interacting proteins.
Material and Methods
Reagents
Anti-CyP40 rabbit polyclonal IgG were purchased from Thermo Scientific (Rockford, IL). Mouse anti-V5 monoclonal IgG (R960) was purchased from Invitrogen (Carlsbad, CA). Rabbit anti-V5 polyclonal IgG (V8137) was purchased from Sigma (St. Louis, MO). MCF-7 and HeLa cells were grown in DMEM supplemented with 10% FBS (Tissue Culture Biologicals, Tulare, CA), 1% glutaMAX-1 (Invitrogen, Carlsbad, CA), 10 U/ml of penicillin (Invitrogen, Carlsbad, CA), and 10 μg/ml of streptomycin (Invitrogen, Carlsbad, CA). Secondary IgG conjugated with IRDye800CW or 680 was purchased from LI-COR Bioscience (Lincoln, NE). Human cDNA clones (XRCC6, GNB2L1, ILF2, and RPS3) were purchased from OriGene (Rockville, MD). CyP40-knockdown stable MCF-7 cells (CID1) were selected under puromycin treatment after transfecting MCF-7 cells with the plasmid containing a shRNA specific to CyP40 (NM_005038, shRNA plasmid #1), according to the manufacturer’s recommended procedure (SuperArray, Frederick, MD).
Generation of adenovirus expressing TAP-CyP40
The full length human CyP40 cDNA was cloned into the EcoRI/Hind III sites of the pNTAP-A plasmid (Stratagene) to generate the pNTAP-A-CyP40 plasmid. The TAP-CyP40 cDNA was amplified from the pNTAP-A-CyP40 plasmid and then cloned into the Not I/Hind III sites of the pShuttle-CMV plasmid (Stratagene) to generate the pShuttle-CMV-TAP-CyP40 plasmid. The Pac I linearized pShuttle-CMV-TAP-CyP40 was used to make adenovirus TAP-CyP40 using the AD293 packaging cells according to the AdEasy protocol (Stratagene). TAP is the tandem tag which contains a SBP (45 amino acids, MDEKTTGWRGGHVVEGLAGELEQLRARLEHHPQGQREPSGGCKLG) and a CBP (26 amino acids, KRRWKKNFIAVSAANRFKKISSSGAL).
Tandem affinity purification
Stratagene InterPlay Mammalian TAP system (Stratagene, La Jolla, CA) was used to isolate the TAP-CyP40 interacting proteins. Five 150-mm dishes of HeLa cells (about 90% confluent) were infected with 75 μl/dish of the high titer Ad-TAP-CyP40 while another five dishes were not infected (as the negative control). After 72 h, infected and non-infected HeLa cells were harvested after being washed with PBS and cells were centrifuged at 1500g for 10 min at 4 °C to obtain the pellet. The pellets were resuspended in lysis buffer (Stratagene, La Jolla, CA) containing protease inhibitors (1 mM PMSF, 2 μg/ml leupeptin, and 1X Roche’s complete protease inhibitor cocktail). The lysates underwent four cycles of freeze/thaw and were centrifuged at 16,000g for 10 min at 4 °C. The resulting supernatants were the loading samples for TAP purification, according to the manufacturer’s protocol (Stratagene, La Jolla, CA).
Silver staining
The cell lysates and eluates from the streptavidin and calmodulin binding steps were resolved on a 4–15% acrylamide gel and then fixed overnight in 50% methanol and 5% acetic acid. The fixed gel was washed once in 50% methanol for 20 min and water twice for 15 min. The washed gel was sensitized for 3 min in 1.27 mM sodium thiosulphate, followed by washing in water twice of 1 min each before being stained with 0.15% silver nitrate for 30 min. The stained gel was washed twice in water of 2 min each. After that, developer solution (0.04% 6 formalin and 2% sodium carbonate) was added to the gel. Once the bands appeared, the developer was discarded and the gel was incubated in a stop solution (5% acetic acid) for at least 5 min. The gel was then stored in 1% acetic acid. Protein bands were excised from the gel and stored in 1% acetic acid, ready for in-gel tryptic digestion.
In-gel Tryptic Digestion
In-gel digestion of individual protein bands was carried out utilizing a procedure as previously described [24]. Typically, 100 ng of porcine trypsin (side chain-protected; Promega, Madison, WI) was used to digest each gel band at 37 °C for 4 h. Peptides were extracted from gel pieces three times with 50 μl of 50% acetonitrile containing 2% acetic acid, and the extracts were combined and concentrated into about a 5 μl volume.
On-line Capillary LC-MS and LC-MS-MS Analysis
A 1 μl aliquot of the digest was separated by a 75 μm × 15 cm reverse-phase capillary column at a flow rate of 300 nl/min. A linear gradient from 2 to 35% acetonitrile in 30 min in 0.1% formic acid was utilized for peptide separation. Solvents were delivered by a Thermo Finnigan Surveyor pump (Thermo Scientific, Waltham, MA) with a precolumn flow split. The eluant was connected directly to a nanoelectrospray ionization source of an LTQ Orbitrap XL mass spectrometer (Thermo Scientific, Waltham, MA). LC-MS data were acquired in an information-dependent acquisition mode, cycling between a MS scan (m/z 310–2,000) acquired in the orbitrap, followed by low-energy CID analysis on 6 most intense multiply charged precursors acquired in the linear ion trap. Activation time was 30 msec; automatic gain control targets were set at 200,000 for MS scans and 30,000 for MS/MS scans. The centroided peak lists of the CID spectra were searched against the National Center for Biotechnology Information (NCBI) protein database using Batch-Tag, a program in the in-house version of the University of California San Francisco Protein Prospector package. A precursor mass tolerance of 15 ppm and a fragment mass tolerance of 0.6Da were used for protein database search. Protein hits were reported with a Protein Prospector protein score ≥22, protein discriminant score ≥0.0, and a peptide expectation value ≤0.01 [25]. This set of protein identification parameters threshold did not return any substantial false positive protein hit when searched against a random-concatenated database [26].
Generation of the rabbit reticulocyte lysate (RRL) expressed RACK1, Ku70, RPS3, and NF45
The GNB2L1 cDNA was amplified and cloned into NheI and XbaI sites while ILF2, RPS3, and XRCC6 cDNAs were cloned into NheI and XhoI sites of the pcDNA6/V5-His A plasmid (Invitrogen, Carlsbad, CA). The primers used to amplify the full length cDNAs were listed as follows: for GNB2L1, forward primer, 5′-CTAGCTAGCGCCATGACTGAGCAGATGACCCTTCGTGG-3′, reverse primer, 5′-GCTCTAGAGCGTGTGCCAATGGTCACCTGCCAC-3′; for ILF2, forward primer, 5′-CTAGCTAGCGCCATGAGGGGTGACAGAGGCCGTGGTCG-3′, reverse primer, 5′-CCGCTCGAGCTCCTGAGTTTCCATGCTTTCTTCTTCCTCTCCTTG-3′; for RPS3, forward primer, 5′-CTAGCTAGCAAGATGGCAGTGCAAATATCCAAGAAGAGG-3′, reverse primer, 5′-CCGCTCGAGTGCTGTGGGGACTGGCTGGGGCATGGC-3′; for XRCC6, forward primer, 5′-CTAGCTAGCAACATGTCAGGGTGGGAGTCATATTAC-3′, reverse primer, 5′-CCGCTCGAGGTCCTGGAAGTGCTTGGTGAGGGC-3′. The resulting pcDNA6/V5-His-GNB2L1, pcDNA6/V5-His-ILF2, pcDNA6/V5-His-RPS3, and pcDNA6/V5-His-XRCC6 plasmids were used to generate V5/6His fusions of RACK1, NF45, RPS3, and Ku70, respectively, using the RRL system from Promega (Madison, WI).
Generation of the bacterially expressed RACK1, Ku70, RPS3, and NF45
GNB2L1, ILF2, RPS3, and XRCC6 cDNAs with a V5 tag at the 3′ end were amplified and cloned into SphI and SalI sites of the Qiagen pQE80 plasmid (Valencia, CA). The same reverse primer (5′-ACGCGTCGACCTACGTAGAATCGAGACCGAGGA-3′) was used with the sequence-specific forward primers to amplify the V5 fusion cDNAs. Sequences of the forward primers are as follows: GNB2L1, 5′-ACATGCATGCACTGAGCAGATGACCCTTCG-3′; ILF2, 5′-ACATGCATGCAGGGGTGACAGAGGCCGTGG-3′; RPS3, 5′-ACATGCATGCGCAGTGCAAATATCCAAGAA-3′; and XRCC6, 5′-ACATGCATGCTCAGGGTGGGAGTCATATTA-3′. The resulting pQE80-His-GNB2L1-V5, pQE80-His-ILF2-V5, pQE80-His-RPS3-V5, and pQE80-His-XRCC6-V5 plasmids were used to generate bacterially expressed V5 and His-tagged RACK1, NF45, RPS3, and Ku70 proteins, respectively. These proteins were expressed in JM109 and affinity purified using the TALON resin (BD Biosciences Clontech, Mountain View, CA) as described previously [27].
Co-immunoprecipitation assay
Using RRL expressed V5 fusions: 40 μl of RRL containing either RACK1-V5, NF45-V5, RPS3-V5, Ku70-V5, D1-V5 (negative control, D1 is a protein not known to interact with CyP40) or uncharged RRL (negative control) was incubated with 2 μg of TALON purified, bacterially expressed 6His-CyP40 for 10 min at room temperature. Four μl of the reaction mixture was set aside to determine the level of protein expression in RRL while 2 μl was set aside as the % input (referring to the amount of CyP40 used). The remaining mixture was incubated with 1 μl of the mouse anti-V5 antibody (Invitrogen, Carlsbad, CA) for 30 min at room temperature. The mixture was then added to 10 μl of pre-equilibrated Dynabead Protein G along with 400 μl of HEDG buffer (25 mM HEPES, pH 7.4, 1 mM DTT, 1 mM EDTA and 10% glycerol). The resulting mixture was rotated in a tumbling fashion at 60 rpm for 1 h at 4 °C. The Dynabead Protein G complex was washed four times with 1 ml of HEDG containing 0.5% Tween-20 and then the precipitated proteins were eluted with 20 μl of the SDS-PAGE sample buffer (50 mM Tris, pH 6.8, 2% SDS, 0.025% bromophenol blue, 10% glycerol, and 5% β-mercaptoethanol). Samples were analyzed by chemiluminescent Western using the rabbit anti-CyP40 IgG (PA3-022, Thermo Fisher Scientific, Rockford, IL). Using bacterially expressed V5 fusions: 16.5 μl of TALON purified, bacterially expressed 6His-RACK1-V5, 6His-NF45-V5, 6His-RPS3-V5, 6His-Ku70-V5 or buffer (25 mM HEPES, pH 7.4, 10% glycerol, 0.3 M KCl, 0.5 M imidazole) was incubated with 10 μg of TALON purified, bacterially expressed 6His-CyP40, 1.15 mg of Sf9 lysate, 1 μl of the mouse anti-V5 antibody (Invitrogen, Carlsbad, CA) and HEDG buffer (to a final volume of 1 ml) for 30 min at room temperature. The mixture was added to a new tube containing 10 μl of pre-equilibrated Dynabead Protein G, followed by a tumbling incubation at 60 rpm for 1 h at 4 °C. The Dynabead Protein G complex was washed four times with 1 ml of HEDG buffer containing 0.5% Tween-20. The precipitated proteins were eluted with 25 μl of SDS-PAGE sample buffer and analyzed by chemiluminescent Western using the rabbit anti-CyP40 IgG (PA3-022, Thermo Fisher Scientific, Rockford, IL). Using electroporated V5 fusions: MCF-7 cells (20–25 passages) were used for electroporation. Cells (5×106 cells/ml) were washed twice in DMEM containing 2.5% FBS before electroporation. The pcDNA-V5/His cloned plasmid (40 μg) was added to cells (400 μl) in a 4 mm cuvette. After 5 min at room temperature, electroporation was performed using a BTX ECM 830 electroporator (Holliston MA) with the following setting: LV mode at 99 msec/500V, 145 volts for 70 msec. After 5 min at room temperature, the electroporated cells were seeded onto a 10 cm plate containing 8 ml of DMEM with 10% FBS, 1% glutaMAX-1, 10 U/ml of penicillin, and 10 μg/ml of streptomycin and then harvested after 72 h. The cell pellets were lysed in 100 μl of the lysis buffer (HEDG containing 0.4 M KCl). After three cycles of freeze/thaw to rupture the plasma membrane, the lysate was incubated on ice for 30 min, followed by dilution with the HEDG buffer to 0.1 M final KCl concentration. The diluted lysates were centrifuged at 16,000g for 10 min at 4 °C and the supernatants were used for co-immunoprecipitation experiments as follows: samples (500 μg) were incubated at 30 °C for 10 min, followed by a 30-min, room-temperature incubation with 1 μl of the mouse anti-V5 IgG (Invitrogen, Carlsbad, CA). The resulting samples were added to a new tube containing the pre-equilibrated Dynabeads protein G (5 μl) and 0.5 ml of the IP buffer (HEDG containing 0.1% Tween-20) and then the mixtures were incubated in a tumbling fashion for 1 h at 4 °C. The Dynabeads protein G complexes were washed with the IP buffer four times with a 5-min incubation at 4 °C between each wash. The precipitated proteins were eluted with 20 μl of SDS-PAGE sample buffer and analyzed by quantitative Western analysis.
Luciferase assay
MCF-7 or CID1 cells were seeded onto a 24-well plate (1.2 × 105 cells/well) in media (DMEM containing 10% of charcoal/dextran stripped FBS) one day before the transfection experiment. To each well, a transfection mix (40 μl) containing 0.8 μg of plasmids (0.15 μg of the pGL3-EPO reporter plasmid, 0.05 μg of the pCH110 β-galactosidase expressing plasmid, and 0.6 μg of a combination of pcDNA plasmids of pcDNA/V5, pcDNA/V5-CΔ553 or pcDNA/V5-GNB2L1) and 1.6 μl of Fugene HD in Opti-MEM was added. After 24 h at 37 °C, fresh media containing either 100 μM cobalt chloride or water was exchanged and the cells were allowed to incubate at 37 °C for another 18 h before harvest for Galacton-Plus Dual-Light luciferase assay according to the manufacturer’s recommended protocol (Applied Biosystems, Bedford, MA).
Chemiluminescent Western analysis
Samples were resolved by 10% SDS-PAGE and then transferred onto a nitrocellulose membrane using a Bio-Rad mini trans-Blot cell (2 h at 4 °C at 300 mA). After transfer, the membranes were blocked briefly with 5% non-fat dry milk (Bio-Rad, Hercules, CA) in TBST buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 0.05% Tween-20) and then incubated overnight with either the rabbit anti-CyP40 IgG (1:5,000) or the rabbit anti-V5 IgG (1:2,500) at 4 °C, followed by the goat anti-rabbit IgG-HRP (1:10,000, Sigma, St. Louis, MO) or anti-rabbit IgG-AP (1:20,000, Sigma, St. Louis, MO) for 1 h at room temperature. The SuperSignal West Pico chemiluminescent or Lumi-Phos substrate (Pierce, Rockford, IL) was used to visualize the results.
Quantitative Western analysis
After transfer, the nitrocellulose membrane was blocked for 1 h at room temperature with the Odyssey blocking buffer (LI-COR, Lincoln, NE) and then incubated overnight with the mouse anti-V5 IgG (1:5,000, Invitrogen, Carlsbad, CA) at 4 °C. The membrane was washed with TBST containing 0.1% Tween-20 three times, followed by incubation with the donkey anti-mouse IgG conjugated with IRDye680CW (1:10,000, LI-COR, Lincoln, NE) for 1 h at room temperature. After washing with TBST containing 0.1% Tween-20 three times, the membrane was analyzed using a LI-COR Odyssey imaging system to visualize the results.
Statistical analysis
We performed unpaired two-tailed t-test to determine the statistical significance using the GraphPad Prism 5 software.
Results and discussion
In an effort to identify CyP40-interacting proteins, we generated adenovirus expressing TAP-CyP40 and found that we could express the TAP-tagged CyP40 at a similar or higher level as the endogenous CyP40 protein in a variety of mammalian cells without causing significant cell death (Fig. 1). We anticipated that the yield of CyP40-interacting proteins would be low since TAP-CyP40 must compete with the endogenous CyP40 for binding with interacting proteins. In addition, proteins which only interact with CyP40 upon stimulus would not be identified by our method; for example, it has been reported that S100 proteins interact with CyP40 only in the presence of calcium [28].
Fig. 1. Western data showing dose-dependent expression of TAP-CyP40 in mammalian cell lines.
Different amount of adenovirus expressing TAP-CyP40 was added to cells in a 6-well plate: COS-1 (0, 1, 2, 5, 10, 20 μl), MCF-7 (0, 2, 5, 10, 20, 50 μl), and HeLa (0, 2, 5, 10, 20, 50 μl). Arrows indicate TAP-CyP40 (upper) and cellular CyP40 (lower). MCF-7 and COS-1 Western analyses were performed using Lumi-Phos whereas HeLa Western analysis was performed using LI-COR the IRDye-conjugated secondary antibody.
Eluants from both streptavidin purification (Fig. 2, lanes 4, 5) and TAP purification (Fig. 2, lanes 6, 7) were separated by SDS-PAGE. Protein components in major silver-stain bands were analyzed by tryptic in-gel digestion and identified by LC-MS and LC-MS/MS analyses. The calmodulin affinity eluant contained TAP-CyP40, Hsp90, and Hsp70 (Fig. 2, lane 7). This result is consistent with the existing literature that CyP40 is a member of the Hsp90 chaperone complex, validating that our purification procedure is effective in isolating CyP40-interacting proteins. However, low silver-stain signal suggested an extensive sample loss in TAP purification. It has been reported that there is a putative calmodulin binding site present at the C-terminal end of the human CyP40 (amino acids 353-369) which is also found in FKBP59 [12]. This sequence is completely different from the engineered CBP sequence of our TAP-CyP40. We expect that most of the endogenous CyP40 should be excluded after the streptavidin affinity purification; therefore, it is unlikely, but cannot be ruled out, that this putative calmodulin binding sequence would interfere with the co-elution of interacting proteins at the latter calmodulin affinity purification. The eluant from just the first affinity purification in this case should still be adequate to identify CyP40-interacting proteins since the SBP tag has a strong binding affinity to the streptavidin resin (KD = 2.5 nM [22]) and a mild TEV protease elution condition prevents co-elution of the non-specific protein binders. To fully rule out the non-specific binding to the resin, we intentionally analyzed the same regions from the lysate that did not contain TAP-CyP40 as negative controls (Fig. 2, lane 4). Among the eleven identified interacting proteins present in the TAP-CyP40 expressing lysate but absent in the control lysate, we focused on four novel interacting proteins – NF45, Ku70, RACK1, and RPS3 for further characterization in hopes to unveil the cellular function of CyP40 (Fig. 2). We excluded the cytoskeletal proteins (actin and tubulins) at this point since many proteins are known to interact with these proteins and it would be difficult to discover the CyP40 function by these interactions. During our screen, we were unsure why we obtained a variety of hits for phaseolins since they are non-human proteins found in beans but not HeLa cells. We had ruled out the possibility of contaminations during our mass spectrometric work up; however, since we were interested in human CyP40-interacting proteins, we did not further pursue phaseolins.
Fig. 2. TAP-CyP40 purification.
Silver staining of 4–15% SDS-PAGE gel. Lane 1, New England Biolabs prestained molecular weight marker (P7708S); lane 2, control HeLa lysate; lane 3, HeLa lysate expressing TAP-CyP40; lane 4, streptavidin eluant from control lysate; lane 5, streptavidin eluant from TAP-CyP40 lysate; lane 6, calmodulin eluant from control lysate; lane 7, calmodulin eluant from TAP-CyP40 lysate. Arrows on lane 4 indicate regions that were analyzed as negative controls. Prestained marker was shown to provide a rough estimate of the relative molecular weight. The reported molecular weights of RPS3, RACK1, NF45 and ku70 are approximately 27, 35, 43 and 70 kDa, respectively.
Since we identified the CyP40-interacting proteins from the first affinity purification, we want to unambiguously show that these proteins interact with CyP40. Thus we expressed the NF45, Ku70, RACK1, and RPS3 proteins in a number of expression systems (E. coli, rabbit reticulocyte lysate, and mammalian cells) and proved that they indeed interact with CyP40. We purchased the human full-length cDNAs of ILF2 (coded for the NF45 protein), GNB2L1 (coded for the RACK1 protein), XRCC6 (coded for the Ku70 protein), and RPS3 from OriGene (Rockville, MD) and used them as PCR templates to amplify the cDNAs for subsequent cloning into the pcDNA/V5-His and pQE80 plasmids. The cloned pcDNA/V5-His plasmids express a tandem V5/6His fusion at the C-termini of these proteins in the rabbit reticulocyte lysate and MCF-7 cells whereas the cloned pQE80 plasmids were designed to express an N-terminal 6His and a C-terminal V5 of these proteins in E. coli. For E. coli expression of these proteins, we further purified them using the TALON affinity resin. All expressions were validated by Western blot analysis using the anti-V5 antibody. In E. coli, the abundance of expression was approximated using the anti-V5 antibody in the following order: RPS3 > RACK1 > NF45 ~ Ku70. All of these bacterially expressed V5 fusions interacted with the bacterially expressed human CyP40 [29] and CyP40 was co-precipitated in the following order: RPS3 ~ Ku70 ~ NF45 > RACK1 (Fig. 3). The sample lacking any V5 fusion did not co-precipitate CyP40 (Fig. 3, lanes 5, 11), showing that CyP40 was selectively co-precipitated by the V5 fusions. Among these CyP40-V5 fusion interactions, it appeared that RACK1 interacted with CyP40 the weakest since the amount of expression was among the highest and the amount of CyP40 co-precipitated was the least among all. In the rabbit reticulocyte lysate, RPS3 expressed the best whereas NF45 the least (Fig. 4A). RPS3 and Ku70 were duplets, possibly due to post-translational modification. The rabbit reticulocyte lysate expressed V5 fusions of RACK1, NF45, RPS3, and Ku70 interacted with bacterially expressed human CyP40 and CyP40 was co-precipitated in the following order: RACK1 ~ NF45 ~ Ku70 > RPS3 (Fig. 4B). Coupled with the level of expression observed in Fig. 4A, NF45 appeared to interact with CyP40 the best. These interactions should be specific between the CyP40-interacting proteins and CyP40 since the rabbit reticulocyte lysate alone and containing the V5 fusion of D1, which is an aryl hydrocarbon receptor deletion construct which is not known to interact with CyP40, did not co-precipitate CyP40 (Fig. 4B, lanes 12, 13). Next, we addressed whether these CyP40-interacting proteins would interact with the cellular CyP40 in MCF-7 cells. We introduced the plasmid expressing the V5-fusion of Ku70, NF45, RACK1 or RPS3 into MCF-7 cells by electroporation. After that, we harvested the lysates and performed co-immunoprecipitation experiments using the mouse anti-V5 monoclonal IgG to co-precipitate the cellular CyP40. After electroporation, we found that expression of Ku70 was noticeably weaker than the other three V5-fusions in MCF-7 cells (Fig. 5, upper panel, lanes 1–4). Co-immunoprecipitation results of the same samples showed that NF45, RACK1, and RPS3 co-precipitated the MCF-7 CyP40 but not Ku70 when 500 μg of lysates were used for the experiment (Fig. 5, lower panel, lanes 1–10). When we used a lysate from another electroporation that contained more Ku70 for a co-immunoprecipitation experiment, we were able to observe the interaction between Ku70 and CyP40 (Fig. 5, upper panel, lanes 5–6 and lower panel, lanes 11–14), suggesting that the low yield of the Ku70 expression after electroporation was the reason for failing to observe the Ku70::CyP40 interaction in the prior experiment. In this co-immunoprecipitation experiment, the transfected RACK1 interacted with the cellular CyP40 best in MCF-7 cells. Collectively, data from our interacting studies using Ku70, NF45, RACK1, and RPS3 expressed in bacteria, rabbit reticulocyte lysate, and mammalian cells validated that these proteins interact with CyP40. The binding affinities of these four proteins with CyP40 appear to be different depending on the methodologies used to study the interaction. For example, using bacterially expressed human CyP40 and V5-fusion proteins of RACK1, NF45, RPS3 and Ku70 suggested that RACK1 interacted with CyP40 the weakest but data from interaction study in MCF-7 cells suggested that RACK1 interacted with CyP40 the best among those four proteins. The discrepancies could be due to differences in post-translational modification of CyP40 and V5-fusion proteins used in the study, depending on how the proteins were expressed (bacteria vs. rabbit reticulocyte lysate vs. mammalian cells).
Fig. 3. Western data showing that the bacterially expressed RACK1, NF45, RPS3, and Ku70 interacted with bacterially expressed human CyP40.
TALON-purified 6His/V5 fusions of RACK1, NF45, RPS3, and Ku70 were used to immunoprecipitate TALON-purified, bacterially expressed human CyP40 using the mouse anti-V5 monoclonal IgG. Rabbit anti-CyP40 polyclonal was used to detect CyP40 (arrow) using chemiluminescent HRP Western. Lanes 1–6 were 2.5% input of V5 fusion proteins (1–4) or CyP40 (6) used for the experiment whereas lanes 7–10 show the co-precipitated CyP40. Lanes 5 and 11 contained rabbit reticulocyte lysate (RRL) and are the negative control without any V5 fusion protein added.
Fig. 4. Western data showing that the rabbit reticulocyte lysate (RRL) expressed RACK1, NF45, RPS3, and Ku70 interacted with bacterially expressed CyP40.

pcDNA/V5-His plasmid containing the cDNA of GNB2L1, ILF2, RPS3, XRCC6, D1 (an aryl hydrocarbon receptor deletion construct aa 83-295) or empty plasmid was used to express protein in RRL. A. Lanes 1–6 show expression of V5 fusion proteins in RRL in 11% of the amount of RRL used for experiment (% input). Mouse anti-V5 monoclonal IgG (Invitrogen) was used to detect V5 fusions. IRDye700-conjugated secondary antibody was used with a LI-COR Odyssey system to capture the signals. B. Lanes 1–6 show the amount of CyP40 present in 5% of the amount of RRL used for the experiment (% input) and lane 7 shows the CyP40 intensity present in 2.5% of the amount of RRL used for the experiment (% input). Lanes 8–13 show the amount of CyP40 co-precipitated with V5 fusions using anti-mouse V5-monoclonal. Rabbit anti-CyP40 polyclonal was used to detect CyP40 using chemiluminescent HRP western. This experiment was repeated once with similar results.
Fig. 5. Western data showing that the transfected RACK1, NF45, RPS3, and Ku70 interacted with CyP40 in MCF-7 cells.
The upper panels show the expression of V5 fusions after electroporation. 500 μg of MCF-7 lysate was loaded per lane. Lanes 1 and 6 are the same lysate and lanes 5 and 6 are lysates from two separate electroporations. The lower panels show co-immunoprecipitation results. Mouse anti-V5 antibody was used to co-immunoprecipitate CyP40 in MCF-7 cells and rabbit anti-CyP40 antibody was used to visualize CyP40 (arrow). CP, co-precipitated CyP40; I, 2% input (referring to the amount of CyP40 used in the experiment).
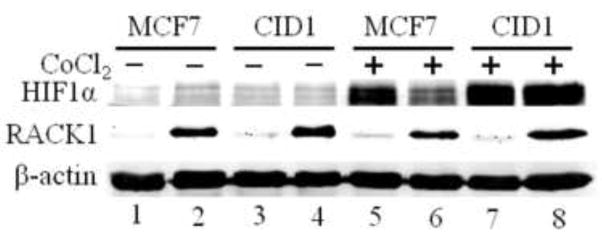
RACK1 binds HIF-1α and subjects it to 26S proteasomal degradation in a pVHL-independent process [30]. Suppression of Hsp90 binding to HIF-1α allows RACK1 to bind HIF-1α, causing the HIF-1α protein degradation. The phosphorylation status of RACK1 governs its homodimerization and calcineurin inhibits RACK1 dimerization by dephosphorylation and subsequently inhibits the RACK1 effect on HIF-1α [31]. Our proteomic screen uncovered the interaction between RACK1 and CyP40. We then addressed whether the RACK1 effect on HIF-1α is CyP40-dependent. Our transfection results showed that RACK1 suppressed more than 40% of the cobalt chloride-induced, HRE-driven luciferase activity in MCF-7 cells (Fig. 6A). This suppression was similar to the suppression caused by CΔ553 – an aryl hydrocarbon receptor deletion construct which has been shown to dimerize with Arnt and suppress the HIF-1α signaling pathway [32]. Interestingly, when we performed a similar transfection experiment using the CyP40-knockdown stable MCF-7 cells which express about 5% of the CYP40 content, we observed that RACK1 was no longer able to suppress the luciferase activity, suggesting that this RACK1 inhibitory effect of the HIF-1α function is CyP40-mediated (Fig. 6B and C). Next, we addressed whether RACK1 would affect the HIF-1α protein content in these cells by quantitative Western analysis. After treating MCF-7 cells with 120 μM cobalt chloride for 18 h in a 6-well plate, high salt extracts were obtained and analyzed for the HIF-1α protein content. We observed that the amount of the HIF-1α protein was increased and this increase was suppressed in the presence of RACK1 (Fig. 7A, lanes 5 and 6), consistent with the literature that RACK1 promotes the Hsp90-dependent proteasomal degradation of HIF-1α [30]. However, RACK1 did not suppress the HIF-1α protein accumulation in CID1 cells (Fig. 7A, lanes 7 and 8). In an effort to quantify the amount of the HIF-1α protein, we performed a similar experiment in replicates (n = 5) by adding the SDS-PAGE sample buffer directly to cells in a 24-well plate, followed by Western analysis. We observed that the HIF-1α protein was accumulated to about 10-fold in the presence of cobalt chloride in MCF-7 cells and RACK1 suppressed this content to about 55% (Fig. 7B). However, the cobalt chloride-induced HIF-1α protein content was not altered significantly in the presence of RACK1 in CID1 cells. Taken together, RACK1 down-regulates the accumulation of the HIF-1α protein by cobalt chloride in MCF-7 cells and this effect is CyP40-mediated. Interestingly, the amount of cobalt chloride-induced accumulation of the HIF-1α protein was more dramatic in CID1 than in MCF-7 cells, suggesting that the endogenous CyP40 might interfere with the protein accumulation process.
Fig. 6. Effect of RACK1 on HIF-1α in MCF-7 and CID cells.


(A) Luciferase data showing the effect of RACK1 on cobalt chloride-induced, HRE-driven luciferase activity in MCF-7 cells. Cells were transfected with empty plasmid (left), plasmid expressing CΔ553 (middle), or plasmid expressing RACK1 (right) ± 100 μM cobalt chloride. (B) Quantitative Western data showing the CyP40 content in the CyP40-knockdown stable MCF-7 (CID1) cells using a LI-COR Odyssey imaging system. 10 μg of the whole cell lysate was loaded. Arrows indicate CyP40 (upper) and GAPDH (lower). 95% knockdown of the CyP40 protein content was observed in CID1 cells, as compared to the wild-type MCF-7 cells. (C) Luciferase data similar to (A) except that CID1 cells were used.
Fig. 7. Effect of RACK1 on HIF-1α protein accumulation in MCF-7 and CID cells.
(A) An image of quantitative Western data showing the expression of the HIF-1α protein ± 100 μM cobalt chloride ± RACK1. Each sample was from one well of a 6-well plate transfected with 4 μg of pcDNA-V5 or pcDNA-V5-GNB2L1 and 10 μl of Fugene HD. After 24 h, cells were treated with 120 μM cobalt chloride or water for 18 h. Cells were harvested into HEDG containing 0.4 M KCl (60 μl/well). After 30 min on ice, the high salt extract was obtained from the 16,000g supernatant for Western. 35 μg of protein was loaded per lane. Rabbit anti-HIF-1α monoclonal IgG (2015-1, Epitomics) was used to detect HIF-1α, rabbit anti-V5 polyclonal IgG (V8137, Sigma) for RACK1, and mouse anti-β-actin monoclonal IgG (AM4302, Applied Biosystems) for β-actin. The HIF-1α protein contents were normalized by β-actin. (B) A graph of quantitative Western data showing the expression of the HIF-1α protein ± 100 μM cobalt chloride ± RACK1. Each sample was from one well of a 24-well plate transfected with 0.8 μg of pcDNA/V5 or pcDNA/V5-GNB2L1. 30 μl of SDS-PAGE sample buffer was added to well and then used directly for Western. The error bars represent ± SD of five experiments (n = 5). All luciferase activities were normalized by the internal standard of β-galactosidase activities. *p ≤ 0.05 and †p > 0.05 (not significant).
Expression of IL-2 is a hallmark of T-cell activation upon antigen recognition [33]. The CsA-CyPA complex binds calcineurin, which is a serine/threonine phosphatase, and inhibits its function, suppressing the signaling of nuclear factor of activated T cells (NFAT), which upregulates IL-2 gene expression in T cells [34; 35]. Activation of IL-2 also occurs in T cells via binding of the NF45-NF90 heterodimer to the antigen receptor response element (ARRE), leading to the IL-2 gene transcription [36]. Interestingly, NF45 is the gene product of ILF2; we showed that CyP40 interacts with NF45, suggesting that CyP40 might play a role in the ARRE-mediated IL-2 gene expression. This is very interesting since the immunosuppressive effect of cyclosporine A is primarily attributed to its binding to CyPA, which in turn inhibits the calcineurin function in NFAT-mediated T-cell activation [37]. Our discovery of the CyP40-NF45 interaction revealed that CyP40 may be important in NF45-mediated T-cell activation. In addition, Ku70, which is involved in double-strand DNA break repair during the nonhomologous end joining process (for review, see [38]), appears to interact with NF45 and may take part in gene regulation [39]. Our co-immunoprecipitation results showed that CyP40 interacts with Ku70 in the absence of NF45, suggesting that CyP40 may be involved in forming the NF45/Ku70 containing complex during gene regulation.
Although RPS3 is a part of the 40S ribosome for general protein synthesis, it possesses other functions ranging from DNA repair [40] to apoptosis [41] to transcriptional control [42]. RPS3 can be found to be part of the NFκB complex and directs gene-specific binding of NFκB to its target gene promoters [43]. Activity of RPS3 may be modulated by the Hsp90-dependent proteasomal degradation of the RPS3 protein [44]. Interestingly, CyP40 interacts with both RPS3 and Hsp90. It is tempting to speculate that CyP40 may be involved in regulating the RPS3 function.
Table 1.
Identification of CyP40-interacting proteins by mass spectrometry
| Database Accession # | Protein | #of Unique Peptides | Sequence Coverage (%) |
|---|---|---|---|
| P07900 | Hsp90, alpha | 31 | 42 |
| P08238 | Hsp90, beta | 29 | 45 |
| P11142 | Hsp7C | 15 | 23 |
| P08107 | Hsp71 | 11 | 20 |
| P12956 | Ku70 | 6 | 11 |
| P68363 | Tubulin, alpha | 15 | 40 |
| P68371 | Tubulin, beta | 13 | 31 |
| P60709 | Actin | 15 | 61 |
| Q12905 | NF45 | 4 | 18 |
| P63244 | RACK1 | 4 | 16 |
| P23396 | RPS3 | 8 | 37 |
Acknowledgments
This work is supported by the National Institutes of Health grant R01 ES014050 (WKC) and a research development grant from COLSA at UNH and the NH Agricultural Experiment Station (FC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kieffer LJ, Thalhammer T, Handschumacher RE. Isolation and characterization of a 40-kDa cyclophilin-related protein. J Biol Chem. 1992;267:5503–7. [PubMed] [Google Scholar]
- 2.Ratajczak T, Hlaing J, Brockway MJ, Hahnel R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem. 1990;35:543–53. doi: 10.1016/0022-4731(90)90197-z. [DOI] [PubMed] [Google Scholar]
- 3.Mok D, Allan RK, Carrello A, Wangoo K, Walkinshaw MD, Ratajczak T. The chaperone function of cyclophilin 40 maps to a cleft between the prolyl isomerase and tetratricopeptide repeat domains. FEBS Lett. 2006;580:2761–8. doi: 10.1016/j.febslet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Ratajczak T, Carrello A. Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J Biol Chem. 1996;271:2961–5. doi: 10.1074/jbc.271.6.2961. [DOI] [PubMed] [Google Scholar]
- 5.Lang K, Schmid FX, Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987;329:268–70. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- 6.Bergsma DJ, Eder C, Gross M, Kersten H, Sylvester D, Appelbaum E, Cusimano D, Livi GP, McLaughlin MM, Kasyan K, Porter TG, Silverman C, Dunnington D, Hand A, Prichett WP, Bossard MJ, Brandt M, Levy MA. The cyclophilin multigene family of peptidyl-prolyl isomerases, Characterization of three separate human isoforms. J Biol Chem. 1991;266:23204–14. [PubMed] [Google Scholar]
- 7.Johnson N, Khan A, Virji S, Ward JM, Crompton M. Import and processing of heart mitochondrial cyclophilin D. Eur J Biochem. 1999;263:353–9. doi: 10.1046/j.1432-1327.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 8.Mi H, Kops O, Zimmermann E, Jaschke A, Tropschug M. A nuclear RNA-binding cyclophilin in human T cells. FEBS Lett. 1996;398:201–5. doi: 10.1016/s0014-5793(96)01248-3. [DOI] [PubMed] [Google Scholar]
- 9.Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones. 2001;6:59–70. doi: 10.1379/1466-1268(2001)006<0059:hciahs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Q, Li M, Yang H, Chai H, Fisher W, Chen C. Roles of cyclophilins in cancers and other organ systems. World J Surg. 2005;29:276–80. doi: 10.1007/s00268-004-7812-7. [DOI] [PubMed] [Google Scholar]
- 11.Kieffer LJ, Seng TW, Li W, Osterman DG, Handschumacher RE, Bayney RM. Cyclophilin-40, a protein with homology to the P59 component of the steroid receptor complex, Cloning of the cDNA and further characterization. J Biol Chem. 1993;268:12303–10. [PubMed] [Google Scholar]
- 12.Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, House AK. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–92. [PubMed] [Google Scholar]
- 13.Banerjee A, Periyasamy S, Wolf IM, Hinds TD, Jr, Yong W, Shou W, Sanchez ER. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–80. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin CyP40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–20. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 15.Luu TC, Bhattacharya P, Chan WK. Cyclophilin-40 has a cellular role in the aryl hydrocarbon receptor signaling. FEBS Lett. 2008;582:3167–73. doi: 10.1016/j.febslet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto K, Watashi K, Inoue D, Hijikata M, Shimotohno K. Identification of cellular and viral factors related to anti-hepatitis C virus activity of cyclophilin inhibitor. Cancer Sci. 2009;100:1943–50. doi: 10.1111/j.1349-7006.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leverson JD, Ness SA. Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol Cell. 1998;1:203–11. doi: 10.1016/s1097-2765(00)80021-0. [DOI] [PubMed] [Google Scholar]
- 18.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–2. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 19.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–29. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 20.Knuesel M, Wan Y, Xiao Z, Holinger E, Lowe N, Wang W, Liu X. Identification of novel protein-protein interactions using a versatile mammalian tandem affinity purification expression system. Mol Cell Proteomics. 2003;2:1225–33. doi: 10.1074/mcp.T300007-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Schimanski B, Nguyen TN, Gunzl A. Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot Cell. 2005;4:1942–50. doi: 10.1128/EC.4.11.1942-1950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keefe AD, Wilson DS, Seelig B, Szostak JW. One-step purification of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. Protein Expr Purif. 2001;23:440–6. doi: 10.1006/prep.2001.1515. [DOI] [PubMed] [Google Scholar]
- 23.Lamla T, Erdmann VA. The Nano-tag, a streptavidin-binding peptide for the purification and detection of recombinant proteins. Protein Expr Purif. 2004;33:39–47. doi: 10.1016/j.pep.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Lee JH, Chu F, Burlingame AL, Gunzl A, Wang CC. Identification of a novel chromosomal passenger complex and its unique localization during cytokinesis in Trypanosoma brucei. PLoS One. 2008;3:e2354. doi: 10.1371/journal.pone.0002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalkley RJ, Baker PR, Hansen KC, Medzihradszky KF, Allen NP, Rexach M, Burlingame AL. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: I. How much of the data is theoretically interpretable by search engines? Mol Cell Proteomics. 2005;4:1189–93. doi: 10.1074/mcp.D500001-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–14. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 27.Shetty PV, Bhagwat BY, Chan WK. p23 enhances the formation of the aryl hydrocarbon receptor-DNA complex. Biochem Pharmacol. 2003;65:941–8. doi: 10.1016/s0006-2952(02)01650-7. [DOI] [PubMed] [Google Scholar]
- 28.Shimamoto S, Kubota Y, Tokumitsu H, Kobayashi R. S100 proteins regulate the interaction of Hsp90 with Cyclophilin 40 and FKBP52 through their tetratricopeptide repeats. FEBS Lett. 2010;584:1119–25. doi: 10.1016/j.febslet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 29.Shetty PV, Wang X, Chan WK. CyP40, but not Hsp70, in rabbit reticulocyte lysate causes the aryl hydrocarbon receptor-DNA complex formation. Arch Biochem Biophys. 2004;429:42–49. doi: 10.1016/j.abb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–17. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YV, Hubbi ME, Pan F, McDonald KR, Mansharamani M, Cole RN, Liu JO, Semenza GL. Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem. 2007;282:37064–73. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen KA, Luu TC, Chan WK. A truncated Ah receptor blocks the hypoxia and estrogen receptor signaling pathways: a viable approach for breast cancer treatment. Mol Pharm. 2006;3:695–703. doi: 10.1021/mp0600438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–61. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 35.O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–4. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 36.Kao PN, Chen L, Brock G, Ng J, Kenny J, Smith AJ, Corthesy B. Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J Biol Chem. 1994;269:20691–9. [PubMed] [Google Scholar]
- 37.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SH, Kim CH. DNA-dependent protein kinase complex: a multifunctional protein in DNA repair and damage checkpoint. Mol Cells. 2002;13:159–66. [PubMed] [Google Scholar]
- 39.Aoki Y, Zhao G, Qiu D, Shi L, Kao PN. CsA-sensitive purine-box transcriptional regulator in bronchial epithelial cells contains NF45, NF90, and Ku. Am J Physiol. 1998;275:L1164–72. doi: 10.1152/ajplung.1998.275.6.L1164. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Chubatsu LS, Admon A, Stahl J, Fellous R, Linn S. Implication of mammalian ribosomal protein S3 in the processing of DNA damage. J Biol Chem. 1995;270:13620–9. doi: 10.1074/jbc.270.23.13620. [DOI] [PubMed] [Google Scholar]
- 41.Jang CY, Lee JY, Kim J. RpS3, a DNA repair endonuclease and ribosomal protein, is involved in apoptosis. FEBS Lett. 2004;560:81–5. doi: 10.1016/S0014-5793(04)00074-2. [DOI] [PubMed] [Google Scholar]
- 42.Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, Deutsch WA, Lenardo MJ. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–39. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TS, Jang CY, Kim HD, Lee JY, Ahn BY, Kim J. Interaction of Hsp90 with ribosomal proteins protects from ubiquitination and proteasome-dependent degradation. Mol Biol Cell. 2006;17:824–33. doi: 10.1091/mbc.E05-08-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]