Abstract
Effects of a muscarinic receptor agonist oxotremorine-M (oxo-M) on bladder afferent nerve (BAN) activity were studied in an in vitro bladder–pelvic nerve preparation. Distension of the bladder induced rhythmic bladder contractions that were accompanied by multiunit afferent firing. Intravesical administration of 25 and 50 µM oxo-M significantly increased afferent firing from 41±2 spikes/s to 51±4 spikes/s and 60.5±5 spikes/s, respectively, but did not change the maximum amplitude of spontaneous bladder contractions. The afferent nerve firing induced by isotonic distension of the bladder (10–40 cmH2O) was increased 22–100% by intravesical administration of 50 µM oxo-M. Electrical stimulation on the surface of the bladder elicited action potentials (AP) in BAN. Oxo-M significantly decreased the voltage threshold by 40% (p < 0.05) and increased by 157% (p < 0.05) the area of the AP evoked at a submaximal stimulus intensity. These effects were blocked by intravesical injection of 5 µM atropine methyl nitrate (AMN). Intravesical administration of 5 µM AMN alone did not alter BAN firing or the amplitude of bladder contractions. The facilitatory effects induced by oxo-M on BAN activity were also suppressed (p < 0.05) by intravesical administration of 2′,3′-0-trinitrophenyl-ATP (TNP-ATP) (30 µM). In preparations pretreated with capsaicin (125 mg/kg, s.c.) the facilitatory effects of 50 µM oxo-M on BAN activity were absent. These results suggest that activation of muscarinic receptors facilitates mechano-sensitive, capsaicin-sensitive BAN activity in part by mechanisms involving purinergic receptors located near the luminal surface of the bladder and ATP release which presumably occurs in the urothelium.
Keywords: Urothelium, Oxotremorine-M, Bladder afferent nerve, Capsaicin, Purinergic receptor
1. Introduction
The functions of the urinary bladder to store and periodically eliminate urine are regulated by neural circuits in the brain and spinal cord that receive inputs from Aδ and C-fiber afferent receptors at various sites in the bladder wall (de Groat and Yoshimura, 2009; de Groat et al., 1993; Fowler et al., 2008; Häbler et al., 1990; Nishiguchi et al., 2005; Sengupta and Gebhart, 1994). Afferent nerve endings located near the urothelium and in the smooth muscle (Birder et al., 2008, 2009; de Groat, 2006; Gabella and Davis, 1998; Milsom et al., 2001; Chancellor and de Groat, 1999) monitor bladder volume and intramural tension as well as the chemical environment (Birder et al., 2009; de Groat and Yoshimura, 2010; Häbler et al., 1990; Kanai et al., 2007). The sensation of bladder filling in humans is triggered by afferent activity traveling in the pelvic nerves (Birder et al., 2009; de Groat et al., 1993; Namasivayam et al., 1999). Sensory input is the prerequisite for conscious bladder control, and changes in sensory mechanisms may give rise to disturbances in bladder function (Birder et al., 2009; Cruz et al., 1997; de Groat and Yoshimura, 2009, 2010; de Groat, 1997).
Overactive bladder which is characterized by frequency, urgency and incontinence is a common disabling condition that affects health related quality of life (Milsom et al., 2001; Stewart et al., 2003). Antimuscarinic drugs are the first choice for treatment of this condition (Abrams et al., 2006; Andersson et al., 2009; Birder et al., 2009; de Groat and Yoshimura, 2001; Kumar et al., 2005). It is commonly believed that the primary site of action of these drugs is the post-junctional receptors located in the bladder smooth muscle. Blockade of these receptors suppresses bladder contractions induced by acetylcholine released from parasympathetic efferent nerves (Abrams et al., 2006; Finney et al., 2006). However recent evidence has indicated that muscarinic receptors expressed in urothelial cells (Kullmann et al., 2008b,a) or bladder afferent nerves may also be important modulators of bladder sensory pathways (de Groat, 2006) and therefore targets for antimuscarinic drugs (Abrams et al., 2006; Kim et al., 2005). The ability of antimuscarinic agents to reduce urgency sensations during bladder filling when parasympathetic nerves are quiescent (Andersson, 2004) and presumably when the post-junctional muscarinic receptors in the muscle are also inactive supports the idea that these agents act on sensory pathways (Andersson, 2004; Birder et al., 2009; de Groat, 2006).
Intravesical administration of muscarinic agonists, oxotremorine- M (oxo-M) or carbachol in urethane anesthetized rats (de Groat, 2006; Kim et al., 2005; Kullmann et al., 2008a) or awake spinal cord injured cats (de Groat, 2006) modulates reflex bladder activity. In rats oxo-M elicits concentration-dependent purinergic excitatory and nitric oxidergic inhibitory effects on voiding frequency presumably mediated in part by ATP and NO release from the urothelium (Kullmann et al., 2008a). The urothelium which is the epithelial lining of the urinary tract is thought to play an important role in the regulation of bladder activity and exhibits neuron-like properties that contribute to sensory function including the expression of various receptors and the ability to release transmitters that can influence the excitability of nearby afferent nerves (Abrams et al., 2006; Birder and de Groat, 2007; Birder et al., 2008, 2009; de Groat, 2004; Hanna-Mitchell et al., 2007; Sun and Chai, 2004). The ability of muscarinic receptor agonists to release ATP from urothelial cells provides further evidence for a role of cholinergic mechanisms in bladder sensory pathways (Hanna-Mitchell et al., 2007; Kullmann et al., 2008b).
In this study we used an in vitro whole bladder–pelvic nerve preparation to examine the effect of the muscarinic receptor agonist, oxo-M, on bladder afferent nerve activity. Preliminary data have been presented in an abstract (Yu and de Groat, abstract 36th Annual Meeting of the Society for Neuroscience).
2. Results
Multiunit afferent activity in the pelvic nerve was induced by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min. Two components of afferent activity were identified: (1) phasic firing which occurred during bladder contractions and (2) tonic firing which occurred between bladder contractions (Fig. 1). Firing reached a peak 5–7 min after the start of infusion and slowly declined after the infusion was stopped at 8 min. The average maximum intravesical pressure during bladder contractions was 17.7±1.5 cmH2O and the average peak firing rate was 41±2.4 spikes/s (n = 16) measured for a period of 10 min after the end of infusion. After emptying the bladder, the rhythmic bladder contractions and afferent firing returned to control level. Repeated bladder distensions elicited consistent responses including the peak amplitude of bladder contractions (bladder pressure 17±1.2 cmH2O during the first distension and 18±0.8 cmH2O during the second distention n = 12) and afferent firing (36.4±1.9 spikes/s during the first distension and 39.3±2.3 spikes/s during the second tension n = 12).
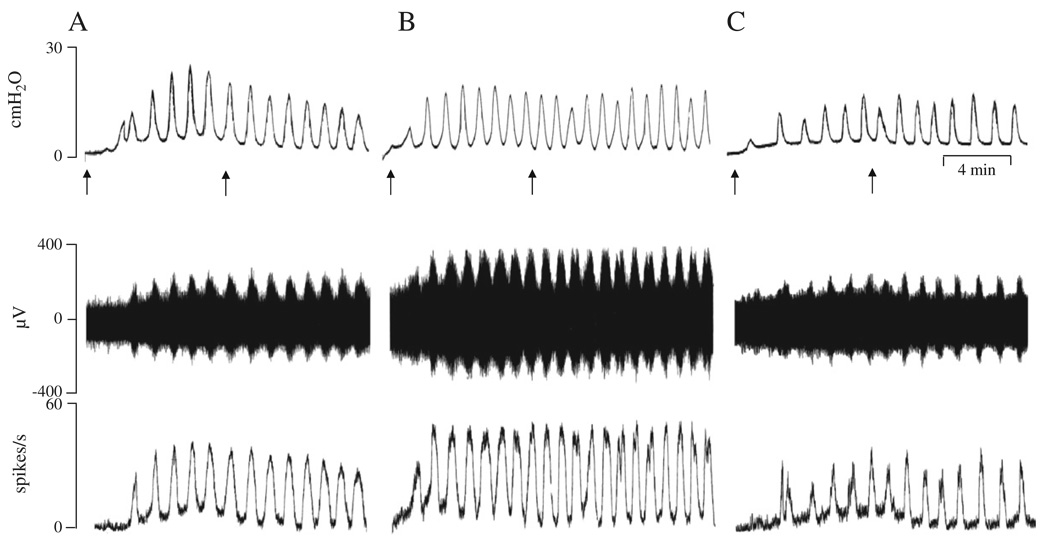
Fig. 1.
Enhancement of pelvic afferent nerve firing during bladder filling and after intravesical administration of oxo-M. The top traces represent bladder contractile activity measured as intravesical pressure, the middle traces represent pelvic afferent nerve activity and the bottom traces represent ratemeter recording of afferent firing (spikes/s). All records were obtained in the same preparation with 30–60 min periods between recordings. A: Control recording during bladder distension induced by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min. Arrows indicate the start and end of infusion. B: Recording during intravesical infusion of 50 µM oxo-M. This preparation had also received an intravesical infusion of 25 µM oxo-M 40 min prior to this recording. C: Intravesical infusion of 50 µM oxo-M in combination with 5 µM AMN after pretreatment with 0.3 ml AMN (5 µM) for 10 min. Horizontal calibration represents 4 min.
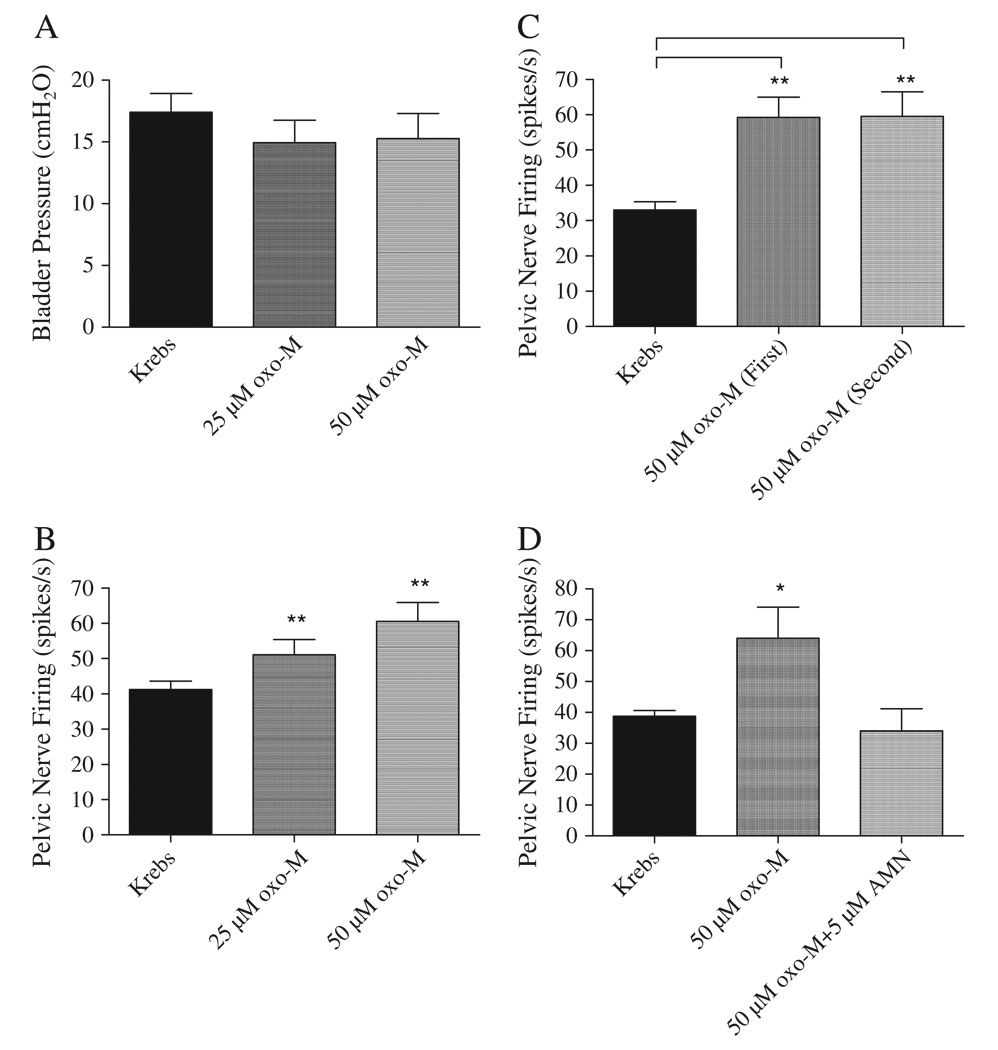
Intravesical administration of oxo-M at an infusion rate of 0.04 ml/min for 8 min in concentrations (25 and 50 µM) that enhance voiding frequency in vivo (Kullmann et al., 2008a) did not significantly change the peak bladder pressure (Figs. 1 and 2A) or the frequency of bladder contractions. The two concentrations of oxo-M were administered sequentially in the same preparations at a 30–60 min interval during which the bladder was flushed once with Krebs solution. The maximum bladder pressures were 14.7±1.8 cmH2O and 15.6±1.9 cmH2O after infusion of 25 µM (n = 16) and 50 µM oxo-M (n=16), respectively. However both tonic and phasic afferent firing were increased by oxo-M (Figs. 1 and 2B). Phasic firing which was the focus of this study increased 24% to 51±4 spikes/s in response to application of 25 µM oxo-M (n = 16, p < 0.05) and 48% to 60.5±5.4 spikes/s at the concentration of 50 µM oxo-M (n = 16, p < 0.05). The facilitatory effect of oxo-M on afferent activity occurred within 10 min after starting infusion and persisted for at least 30 min. After washout with Krebs solution the pelvic afferent nerve activity returned to the control level within 30 to 60 min following the high concentration of oxo-M. In four of these experiments when oxo-M (50 µM) was administered a second time at a 60 min interval after flushing the bladder with Krebs solution it elicited a similar enhancement of afferent firing (Fig. 2C). In five additional experiments oxo-M (50 µM) was administered before and after treatment with AMN, a nonselective muscarinic receptor antagonist (Fig. 2D) in a concentration (5 µM) that blocked the facilitatory effect of the agonist in vivo (Kullmann et al., 2008a). AMN blocked the facilitatory effect of oxo-M (Fig. 2D). Intravesical infusion of AMN (5 µM, n = 4) alone did not significantly alter bladder activity or pelvic afferent firing.
Fig. 2.
Summaries of the effects of intravesical infusion of Krebs solution and oxo-M on the average amplitude of phasic bladder contractions (A) and pelvic afferent nerve firing (B, C, D). A: oxo-M (n = 16) did not change peak contraction pressure, but enhanced pelvic afferent nerve firing (25 µM, p < 0.05, n = 16; 50 µM, p < 0.05, n = 16) (B). C: The oxo-M enhancement of afferent nerve firing could be repeated when oxo-M was administered at 40 min intervals (n = 4) and was blocked by AMN (5 µM) (n = 5) (D). * represents P < 0.05, ** represents P < 0.01. Data in A and B are from the same experiments. Data in C and D are from different experiments.
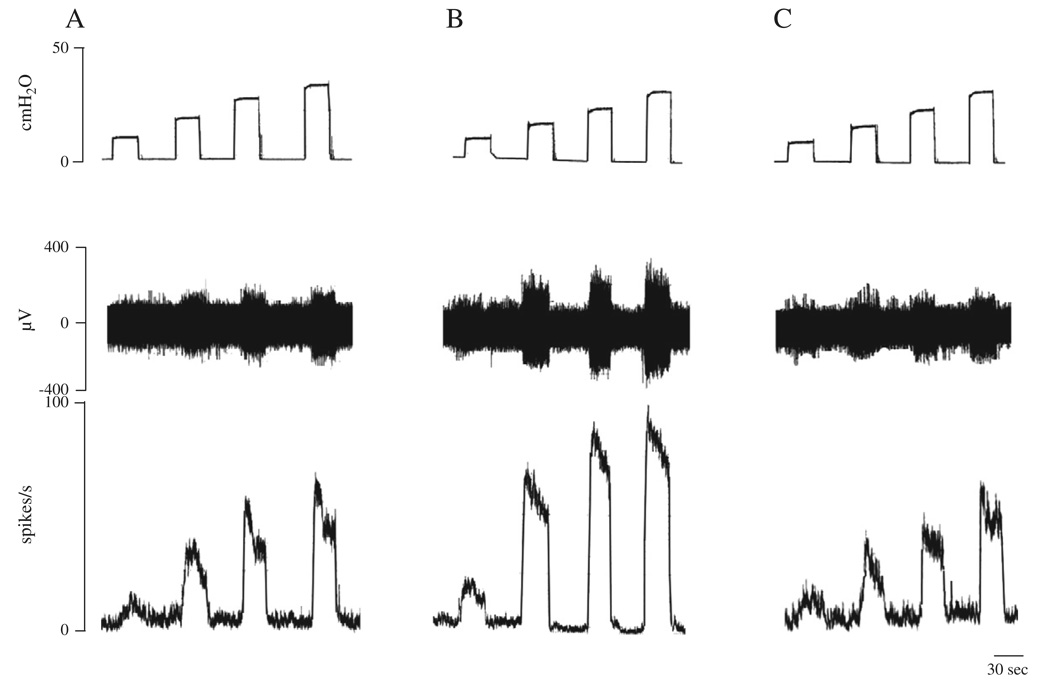
In four experiments afferent nerve activity was also elicited by isotonic distension of the bladder with Krebs solution at 10, 20, 30 and 40 cmH2O pressures for 30 s at 30 s intervals to evaluate the pressure–response relationship of afferent nerve activity (Table 1 and Fig. 3). The afferent nerve firing reached a maximum within seconds after isotonic distention of the bladder and then declined indicating some adaptation at a constant intravesical pressure (Fig. 3). The enhancement of afferent nerve firing was similar during a second series of isotonic bladder distensions applied after a 30–60 min recovery period (Table 2). After intravesical administration of 50 µM oxo-M the afferent nerve firing induced by isotonic distension of the bladder was significantly increased at 10, 20 and 30 but not at 40 cmH2O (Table 1 and Fig. 3B). Consistent responses to intravesical administration of 50 µM oxo-M could be obtained during the second series of isotonic distensions of the bladder at 10–40 cmH2O pressures after a 30–60 min recovery period (n = 4, p > 0.05) (Table 2). These facilitatory effects of oxo-M on the bladder afferent nerve firing were completely blocked by intravesical administration of 5 µM AMN in combination with 50 µM oxo-M (Fig. 3C). However intravesical application of 5 µM AMN alone (n = 4) in the absence of oxo-M did not significantly alter afferent firing induced by isotonic distension of the bladder at 10–40 cmH2O pressures.
Table 1.
Effects of oxotremorine-M (oxo-M, 50 µM) and atropine methyl nitrate (AMN, 5 µM) on pelvic afferent firing induced by isotonic distention of the bladder in untreated, capsaicin vehicle and capsaicin pretreated preparations.
| IVP (cmH2O) |
Untreated (n = 4) |
Capsaicin vehicle pretreated (n = 3) |
Capsaicin pretreated (n = 4) |
|||||
|---|---|---|---|---|---|---|---|---|
| Krebs | oxo-M | oxo-M+ AMN | Krebs | oxo-M | oxo-M+ AMN | Krebs | oxo-M | |
| 10 | 22±5 | 46±10* | 32±6 | 12±3 | 26±2* | 18±1 | 15±4 | 21±7 |
| 20 | 35±6 | 56±3* | 42±6 | 30±7 | 59±8* | 47±4 | 28±8 | 35±9 |
| 30 | 59±10 | 86±7* | 62±10 | 50±10 | 88±5 | 65±8 | 35±9 | 43±10 |
| 40 | 73±7 | 93±6 | 51±5 | 51±12 | 95±3 | 63±9 | 41±8 | 49±11 |
oxo-M, oxotremorine-M (50 µM); AMN, atropine methyl nitrate (5 µM) and IVP, intravesical pressure. The numbers in brackets represent the number of experiments, * indicates significant difference from measurements in Krebs solution p < 0.05. Values are means±SE.
Fig. 3.
Enhancement of pelvic afferent nerve activity by isotonic bladder distension with Krebs solution and with oxo-M (50 µM). A: Afferent nerve activity was induced by distention of bladder with Krebs solution at 10, 20, 30 and 40 cmH2O for 30 s at 30 s intervals. B: Enhancement of afferent firing during distension with 50 µM oxo-M. C: Enhancement of afferent firing did not occur during distension with 5 µM AMN and 50 µM oxo-M. The top traces in A, B and C show intravesical pressure during isotonic distention of the bladder. Middle traces show the afferent nerve activity and the bottom traces represent ratemeter recording (spikes/s) of afferent nerve activity. All data are from the same preparation.
Table 2.
Effects of isotonic distention of the bladder with Krebs solution or with oxotremorine-M on pelvic afferent firing were reproducible during repeated applications.
| IVP (cmH2O) |
Krebs | oxo-M (first test) |
After washout |
oxo-M (second test) |
|---|---|---|---|---|
| 10 | 23.8±6 | 47.8±13* | 22.5±5.2 | 50±12* |
| 20 | 45.5±12.4 | 61±12* | 50.5±7 | 70±9* |
| 30 | 60±6.7 | 81.8±9.2* | 61.3±6.4 | 78.8±7* |
| 40 | 69±5.1 | 84±9.4 | 66±6.3 | 80±10 |
oxo-M, oxotremorine-M (50 µM) and IVP, intravesical pressure. Data are the averages from four experiments. Asterisks indicate significant difference from measurements in Krebs solution, *p < 0.05. Values are mean±SE.
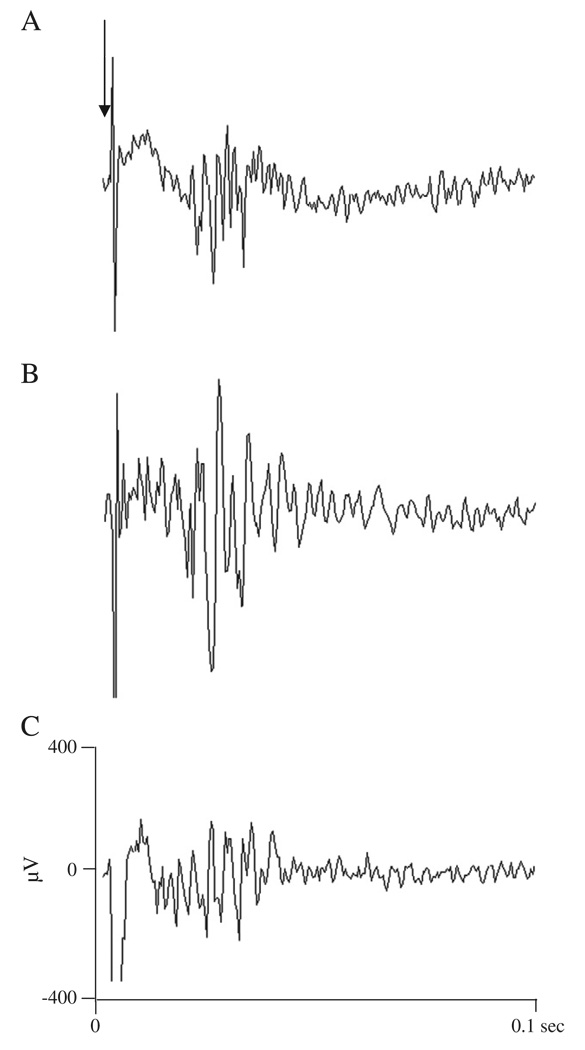
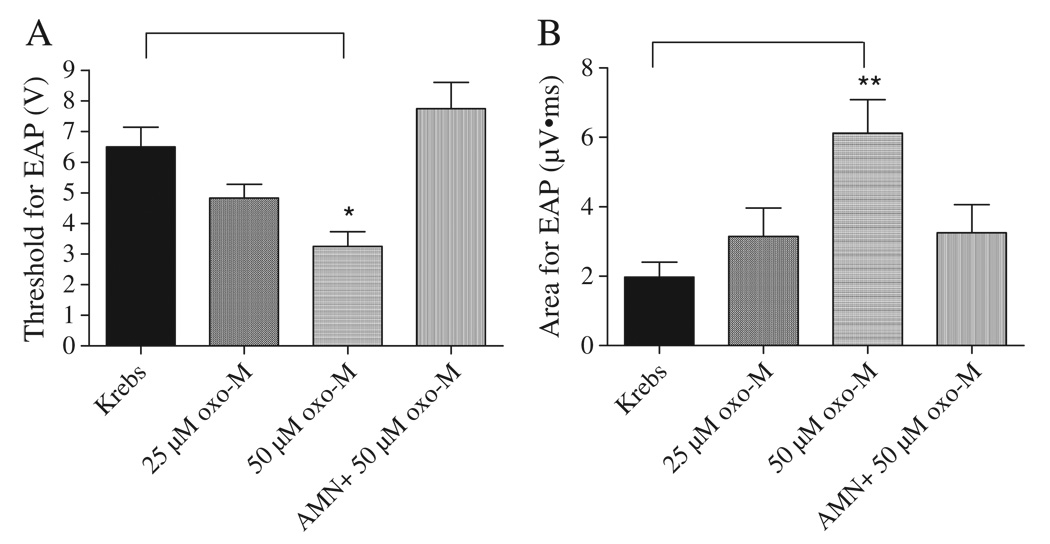
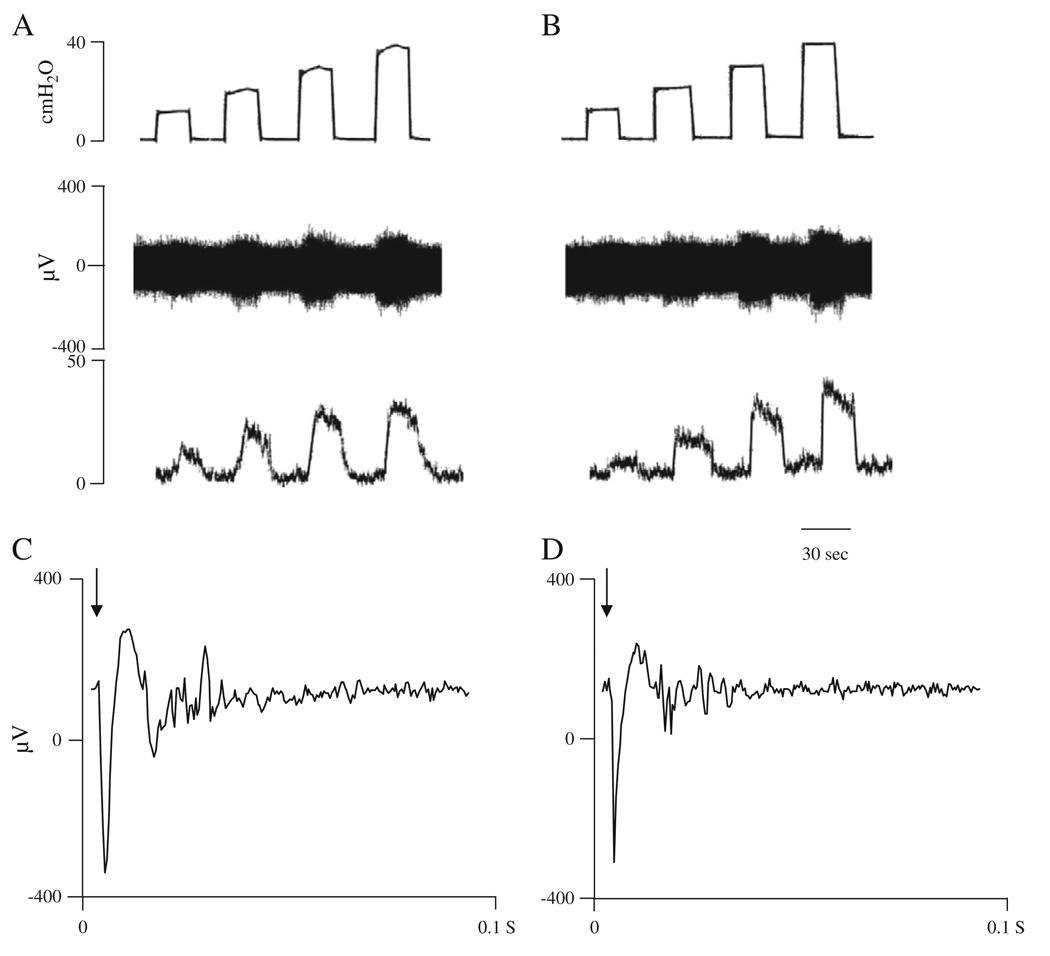
In four experiments after filling the bladder with Krebs solution or oxo-M at the rate of 0.04 ml/min for 8 min, electrical stimulation with bipolar electrodes was applied to the serosal surface of the bladder close to the neck and evoked compound action potentials were recorded on the pelvic nerve (Fig. 4). Action potentials were characterized by short and long latency components corresponding to axonal conduction velocities ranging from 0.3 to 10 m/s at 27 °C. The largest amplitude action potentials in the recording occurred at short latencies. Lower amplitude action potentials occurred at slower conduction velocities ranging from 0.3 to 1.0 m/s. After intravesical administration of 50 µM oxo-M the threshold for evoked compound action potentials decreased approximately 50% from 6.5±0.6 to 3.3±0.5 V (n = 4, p < 0.05) (Fig. 5A). In addition the area of evoked action potentials including the short and long latency potentials induced at a submaximal stimulus intensity (80 V, 0.15 ms duration) increased about 3 fold after administration of 50 µM oxo-M from 2.0±0.4 µV·ms to 6.1±2.0 µV·ms (n = 4, p < 0.05) (Fig. 5B). The effects of oxo-M were blocked by 5 µM AMN administered intravesically in combination with 50 µM oxo-M (n = 4) (Figs. 4C and 5).
Fig. 4.
Effect of intravesical administration of oxo-M on evoked action potentials in the pelvic afferent nerves. A: Submaximal electrical stimulation (80 V, 0.15 ms) of the nerves on the surface of the bladder at the arrow evoked a discharge on the pelvic nerve during infusion of Krebs solution. B: 50 µM oxo-M transvesical infusion increased the evoked discharge. C: After infusion of 5 µM AMN in combination with 50 µM oxo-M the evoked discharge decreased. Evoked action potentials represent the computer average of 5 individual responses.
Fig. 5.
Effect of oxo-M administered by intravesical infusion on electrically evoked pelvic afferent nerve activity (n = 4). A: Threshold for evoked AP was decreased by oxo-M (50 µM) and this effect was blocked by AMN (5 µM). B: Area of evoked AP was increased by oxo-M (50 µM) and this effect was blocked by AMN (5 µM). * represents P < 0.05, ** represents P < 0.01.
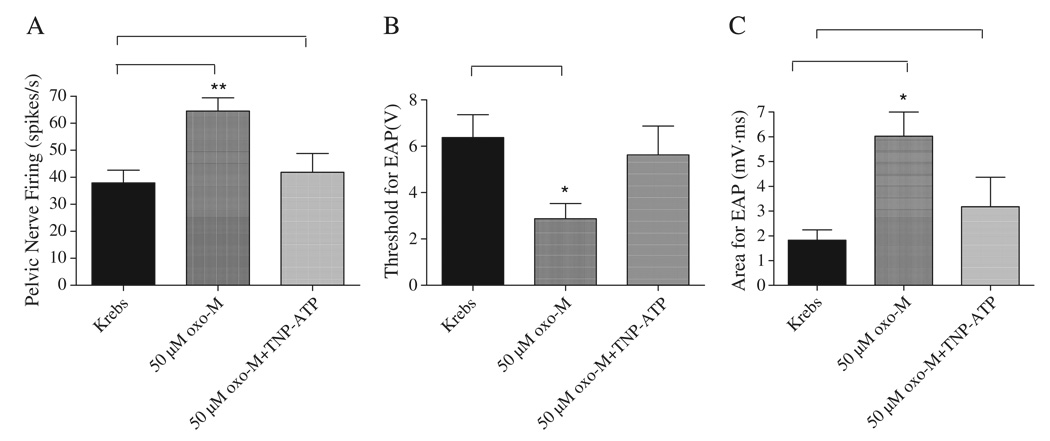
To evaluate the possible contribution of purinergic mechanisms to the facilitatory effect of oxo-M on afferent nerve activity, a purinergic receptor antagonist, 2′,3′-0-trinitrophenyl- ATP (30 µM, n = 8) was administered intravesically for 10 min followed by isotonic bladder distension with a combination of 50 µM oxo-M and TNP-ATP at 20 cmH2O pressure for 30 s (Fig. 6). We have shown previously that this concentration of TNP-ATP suppresses the afferent excitatory effect of α,β-methylene-adenosine 5′-triphosphate administered intravesically (Yu and de Groat, 2008). The peak afferent nerve firing at 20 cmH2O pressure was increased from 37.9±4.7 spikes/s when distending with Krebs solution to 64.5±5 spikes/s after distention with 50 µM oxo-M (n = 8, p < 0.05). After pretreatment with 30 µM TNP-ATP for 10 min intravesical administration of TNP-ATP (30 µM) in combination with 50 µM oxo-M at 20 cmH2O pressure the peak afferent firing was significantly decreased to 41.9±6.9 spikes/s (n = 8, p < 0.05) (Fig. 6A). The afferent firing induced by isotonic distension of the bladder with Krebs solution at 20 cmH2O was not significantly changed by 30 µM TNP-ATP (36.5±2.5 spikes/s in Krebs solution and 34±4.9 spikes/s in 30 µM TNP-ATP, n = 6, p > 0.05).
Fig. 6.
Effect of TNP-ATP (30 µM), a purinergic receptor antagonist, administered by intravesical infusion, on afferent nerve activity evoked by isotonic distention of the bladder at 20 cmH2O for 30 s with Krebs solution or 50 µM oxo-M (A) and on electrically evoked AP (B, C). Oxo-M increased pelvic nerve firing (A) (p < 0.05, n = 8), decreased the threshold (B) (p < 0.05, n = 4) and increased the area of evoked AP (C) at a submaximal stimulus intensity (80 V, 0.15 ms pulse duration) (n = 4, p < 0.05). The facilitatory effects were reversed by TNP-ATP (30 µM). * represents P < 0.05, ** represents P < 0.01.
In four of these experiments the interactions between TNP-ATP and oxo-M on electrically evoked action potentials were also examined. Individual responses were averaged with a computer. The threshold for electrically evoked action potentials was 6.4±1.0 V in Krebs solution and decreased to 2.9±0.7 V (0.15 ms duration) after intravesical administration of 50 µM oxo-M (n = 4, p < 0.05) (Fig. 6B). After pretreatment with TNP-ATP (30 µM) which alone did not change either the threshold or the area of evoked action potentials (n = 4, p > 0.05) the intravesical administration of TNP-ATP (30 µM) in combination with 50 µM oxo-M did not significantly change the threshold for electrically evoked action potentials (5.6±1.2 V, n = 4, p > 0.05). The area of evoked action potentials at submaximal stimulation intensity (80 V, 0.15 ms) was 1.8±0.4 µV·ms in Krebs solution and increased to 6.0±1.0 µV·ms after intravesical administration of 50 µM oxo-M (n = 4, p < 0.05) (Fig. 6C). However after pretreatment with 30 µM TNP-ATP and the intravesical administration of 30 µM TNP-ATP in combination with 50 µM oxo-M the increase in the area of evoked action potentials was significantly less (3.2±1.2 µV·ms) (p < 0.05) than in the presence of oxo-M alone.
To identify the types of bladder afferent nerves affected by the muscarinic receptor agonist, capsaicin (125 mg/kg s.c.), an afferent neurotoxin was administered 4 days prior experiments (n = 5) to desensitize C-afferent nerve fibers (Cheng et al., 1993). In these preparations intravesical infusion of oxo-M did not enhance the afferent firing induced by isotonic distension of the bladder at different pressures (Table 1 and Fig. 7B) or alter the threshold or the area of action potentials evoked at submaximal stimulation intensity (80 V, 0.15 ms duration) (Fig. 7D, n = 5). On the other hand, pretreatment with capsaicin vehicle did not alter the oxo-M (50 µM) facilitation of afferent nerve activity induced by isotonic distension of the bladder at the pressures of 10–40 cmH2O (Table 1, n = 3). Capsaicin pretreatment also blocked the oxo-M enhancement of afferent firing induced by slow filling of the bladder for 8 min. In these experiments the maximum afferent firing (31±2 spikes/s) and peak bladder contractions (15±3 cmH2O) after oxo-M administration were not significantly different than the firing (30±3 spikes/s) and contractions (14.8±1 cmH2O) induced by the infusion of Krebs solution (n = 4, p>0.05).
Fig. 7.
Effects of oxo-M (50 µM) administered by intravesical infusion on afferent nerve activity in capsaicin pretreated preparations (125 mg/kg, s.c., 4 days prior to experiments).The top traces in A and B show isotonic distention of the bladder with Krebs solution (A) or 50 µM oxo-M (B) at 10, 20, 30 and 40 cmH2O for 30 s. Middle traces show the afferent activity and the bottom traces show ratemeter recording (spikes/s) of afferent nerve firing. C and D: Action potentials (average of 5 evoked responses) evoked by a submaximal stimulus intensity (80 V, 0.15 ms pulse duration) after filling the bladder with Krebs solution (C) or 50 µM oxo-M (D). Arrows indicate the stimulus artifact. Note that oxo-M did not alter afferent nerve firing in capsaicin pretreated experiments (n = 4).
To evaluate the efficacy of capsaicin pretreatment capsaicin was injected into the bath solution at the end of several experiments. In control experiments the afferent nerve excitatory effect of capsaicin (10 µM) was evident as a marked increase in bladder pressure (115%) and increased afferent firing (62%) (n = 2). These effects were not detected in capsaicin pretreated preparations (n = 2).
3. Discussion
The present study examined the influence of muscarinic receptors on the activity of mechano-sensitive bladder afferent nerves in an in vitro whole bladder–afferent nerve preparation. Intravesical administration of oxo-M, a muscarinic receptor agonist, increased the peak firing of pelvic afferent nerves elicited by distension and/or contraction of the bladder, decreased the threshold and increased the area of electrically evoked action potentials. The effects of oxo-M were suppressed by AMN or by TNP-ATP, a P2X receptor antagonist. These results indicate that activation of muscarinic receptors near the luminal surface of the bladder can enhance the mechano-sensitivity of afferent nerves by indirect mechanisms involving ATP release.
It is likely that oxo-M and AMN administered intravesically act on muscarinic receptors close to the luminal surface because these hydrophilic molecules contain positively charged quaternary nitrogen groups that should limit their ability to penetrate the urothelial barrier (Birder et al., 2008). Low concentrations of oxo-M applied to the serosal surface of the bladder induce large amplitude contractions and increase rhythmic activity (Ng et al., 2006); whereas high intravesical concentrations of oxo-M do not alter baseline bladder tone or smooth muscle contractions indicating that minimal amounts of the drug reached the smooth muscle in the bladder wall after intravesical administration (Kullmann et al., 2008a). Thus the enhancement of afferent firing by oxo-M is most reasonably attributed to activation of receptors on urothelial cells, suburothelial afferent nerves or on myofibroblasts, all of which express muscarinic receptors (Abrams et al., 2006; de Groat, 2006; Finney et al., 2006; Hawthorn et al., 2000; Ikeda and Kanai, 2008; Kanai et al., 2007; Sui et al., 2008) and is not due to stimulation of the smooth muscle which could indirectly induce afferent firing.
However the facilitatory effect of oxo-M on afferent firing may still be indirect because it was suppressed by TNP-ATP a purinergic receptor antagonist. In vivo continuous infusion cystometric studies in the anesthetized rat have also implicated purinergic mechanisms in the facilitatory effects of oxo-M (Kullmann et al., 2008a). In these experiments intravesical infusion of oxo-M increased voiding frequency without eliciting effects directly on the bladder smooth muscle. The facilitatory effects of the drug were blocked by intravesical AMN and reduced by PPADS, a purinergic receptor antagonist. Because oxo-M evokes the release of ATP from cultured urothelial cells (Kullmann et al., 2008b) it was proposed that the enhanced voiding frequency induced by oxo-M was due in part to stimulation of urothelial muscarinic receptors followed by the release of ATP that in turn activates suburothelial afferent nerves (Kullmann et al., 2008a). The present experiments provide direct support for this hypothesis. P2X2/3 receptors which are expressed by bladder afferent nerves (Brady et al., 2004a,b; de Groat and Yoshimura, 2009; Namasivayam et al., 1999; Nishiguchi et al., 2005; Rong et al., 2002) and which are blocked by TNP-ATP (Yu and de Groat, 2008) are presumably involved in the oxo-M induced facilitation of distension-evoked afferent activity.
Although muscarinic agonists can evoke the release of ATP it is clear that the effects of ATP and oxo-M are different. Intravesical administration of high concentrations (30–100 µM) of ATP or another purinergic receptor agonist, α,β-methylene-adenosine 5′-triphosphate induces afferent nerve firing (Birder et al., 2009; Namasivayam et al., 1999; Rong et al., 2002; Yu and de Groat, 2008). On the other hand, intravesical administration of oxo-M did not induce detectable afferent firing in the absence of bladder distension, but rather enhanced the firing induced by bladder distension. This discrepancy might be related to the release of an inhibitory substance (e.g., nitric oxide) in combination with ATP during stimulation of muscarinic receptors (Hanna-Mitchell et al., 2007; Kullmann et al., 2008a) or to lower local concentrations of ATP after endogenous release than the concentrations achieved by exogenous administration of ATP.
A purinergic mechanism is also involved in the effect of oxo-M on afferent activity evoked by electrical stimulation on the serosal surface of the bladder. It is not known if stimulation at this location activates afferent nerve terminals near the urothelium, intramural axons in the bladder wall or only axons on the surface of the bladder (Yu and de Groat, 2008). Based on estimated conduction velocities, the evoked responses consisted of volleys in both myelinated Aδ and unmyelinated C-fibers. Intravesical administration of oxo-M lowered the threshold voltage required to activate the myelinated axons and increased the magnitude of the Aδ and C-fiber evoked volleys. These effects were blocked by TNP-ATP as well as AMN. Previous studies revealed that intravesical administration of a P2X purinergic receptor agonist (α,β-methylene-adenosine 5′-triphosphate) elicited similar changes in the evoked afferent activity; and intravesical administration of TNP-ATP blocked the effects (Yu and de Groat, 2008). Other investigators (Irnich et al., 2001; Lang et al., 2003) have reported that ATP also enhances the excitability of unmyelinated axons in the rat vagus and sciatic nerves. Our results indicate that ATP released by oxo-M can enhance the excitability of both Aδ and C-fiber bladder afferents.
Desensitization of C-fiber bladder afferent nerves by subcutaneous injection of a large dose of capsaicin four days prior to the experiments eliminated the facilitatory effect of oxo-M on both distension-evoked and electrically evoked afferent firing. In cystometry experiments in anesthetized rats, capsaicin pretreatment also blocked the oxo-M enhancement of voiding frequency (Kullmann et al., 2008a). These data indicate that oxo-M targets capsaicin/ATP-sensitive afferent nerves which have been identified immunohistochemically in the suburothelial region of the bladder wall by the co-expression of P2X3 and TRPV1 receptors (Apostolidis et al., 2005; Birder and de Groat, 2007; Birder et al., 2008, 2009; Brady et al., 2004a,b; de Groat and Yoshimura, 2009; Fowler et al., 2008). The contribution of these P2X3/TRPV1 expressing afferents to the excitatory effects of ATP on the bladder has been demonstrated in anesthetized rats using continuous infusion cystometry, where it was shown that the intravesical administration of ATP facilitated voiding frequency and that this effect was eliminated by pretreatment with capsaicin (Nishiguchi et al., 2005). On the other hand, in conscious rats the facilitatory effect of intravesical ATP was not blocked by desensitization of C-fiber bladder afferents with resiniferatoxin, suggesting that ATP also activates capsaicin-insensitive bladder afferents (Zhang et al., 2003). It is usually assumed that capsaicin-sensitive afferent nerves in the bladder are the C-fiber type. However, in the rat bladder Aδ and C-fiber afferents have been shown to respond to the acute excitatory effect of capsaicin (Jiang et al., 1995). It is not known if this population of Aδ afferents expresses P2X3 receptors and if it is desensitized by capsaicin pretreatment. However a study in the in vitro mouse bladder–pelvic nerve preparation indicated that the P2X3 receptor agonists increase the firing of both Aδ and C bladder afferent fibers (Rong et al., 2002).
The elimination of the oxo-M facilitatory effect on afferent firing after capsaicin pretreatment could be due to the degeneration of the suburothelial C-fiber afferent terminals because intravesical resiniferatoxin treatment in humans reduces suburothelial P2X3/TRPV1 nerve staining (Brady et al., 2004a,b; Fowler et al., 2008). However after capsaicin pretreatment the afferent axons responding to electrical stimulation on the serosal surface of the bladder were still intact and the electrical thresholds were not altered even though the facilitatory effect of oxo-M on the electrically evoked responses was eliminated. Thus it is possible that the afferent nerves remaining after capsaicin treatment do not respond to oxo-M because: (1) the nerve terminals are damaged and are therefore unresponsive to chemical or mechanical stimulation or (2) after desensitization of TRPV1 receptors in the urothelium and/or afferent nerves the putative muscarinic–purinergic afferent signaling mechanism identified in the present experiments is disrupted.
The expression of muscarinic receptors has been detected in dorsal root ganglion neurons (Tata et al., 2000) and at various locations near the luminal surface of the bladder (e.g., afferents, urothelial cells and myofibroblasts). The facilitatory effect of intravesical administration of muscarinic agonists on bladder afferent firing and reflex bladder activity in rats (Kullmann et al., 2008a) and cats (de Groat, 2006) raises the possibility that the cholinergic system may have a physiological role in the regulation of sensory mechanisms in the bladder. Acetylcholine might arise at multiple sites including the urothelium and cholinergic nerves distributed in the lamina propria adjacent to the urothelium (Birder et al., 2008, 2009; de Groat, 2006; Hanna-Mitchell et al., 2007; Yoshida et al., 2004). Distension of the bladder or hypotonic stretch of urothelial cells releases acetylcholine (Hanna-Mitchell et al., 2007; Yoshida et al., 2004) indicating that cholinergic receptors might be tonically activated during bladder filling to: (1) directly modulate afferent nerve excitability, (2) act indirectly via the release of ATP or (3) activate intercellular communication via a suburothelial myofibroblast network that in turn influences the afferent nerves (Birder and de Groat, 2007; Birder et al., 2008, 2009; Finney et al., 2006; Kanai et al., 2007; Sui et al., 2008). However under the conditions of our in vitro experiments we did not obtain any evidence for a tonic modulation of afferent nerve activity by activation of muscarinic receptors. AMN in a concentration that blocked the facilitatory effects of oxo-M did not alter the distension-evoked or electrically evoked afferent firing. Other investigators (de Wachter and Wyndaele, 2003) have reported a suppression of bladder afferent nerve activity by muscarinic receptor antagonists, although this effect may be due to action on voltage gated ion channels rather than a block of muscarinic receptors.
Although P2X2/3 receptor knockout studies indicate that purinergic facilitatory mechanisms in bladder sensory pathways have a physiological role in the regulation of voiding in mice (Cockayne et al., 2005) pharmacological studies with P2X receptor antagonists have failed to detect a physiological role for ATP in the regulation bladder afferent activity or reflex voiding in normal rats (Lu et al., 2007; Yu and de Groat, 2008). However, previous experiments have detected a tonic purinergic facilitation of afferent activity after the induction of chemical cystitis in the rat (Yu and de Groat, 2008) which is known to increase the expression of P2X2/3 receptors in bladder afferent neurons (Dang et al., 2008). Neurogenic detrusor overactivity after spinal cord injury in the rat is also influenced by purinergic mechanisms because administration of a P2X2/3 antagonist suppressed nonvoiding contractions during bladder filling and increased bladder capacity in spinal cord injured rats (Lu et al., 2007). Thus in future experiments it will be important to determine if endogenous cholinergic mechanisms regulating ATP release modulate bladder afferent nerve activity and voiding function in pathological models of bladder overactivity.
4. Experimental procedures
The urinary bladder, urethra, prostate gland, seminal vesicles and innervation including the pelvic nerves and the major pelvic ganglia were removed from ketamine (50 mg/kg, i.m.) anesthetized male Sprague Dawley rats (n = 43, body weight 100–170 g) and placed in a 20 ml bath that was perfused with Krebs solution (1 ml/min) at a temperature of 27 °C and continuously bubbled with 95% O2 and 5% CO2. A temperature below the body temperature was used to prolong the survival of the in vitro preparation. The Krebs solution had the following composition (mM/l): NaCl 128, KCl 1.8, NaHCO3 22, KH2PO4 1.5, MgSO4 1.3, glucose 10, CaCl2 2H2O 0.4, and H2O2 0.4 at pH 7.4. A catheter (PE50) was inserted into the bladder through the urethra and then connected to an infusion pump and to a pressure transducer to monitor bladder activity and to infuse solutions intravesically. The pelvic nerve on one side was placed in an adjacent chamber filled with paraffin oil and positioned on silver bipolar electrodes for recording multiunit afferent nerve activity. Standard electrophysiological methods were used to amplify and analyze the afferent nerve activity (Yu and de Groat, 2008).
Afferent activity was elicited by intravesical infusion of Krebs solution at the rate of 0.04 ml/min for 8 min which also evoked rhythmic contractions of the bladder smooth muscle. After bladder filling was stopped multiunit afferent activity was measured (spikes/s) for 10 min using a pulse height discriminator-ratemeter and displayed on a rectilinear paper recorder and also recorded on a VCR for later off-line analysis. Afferent activity was also evoked by isotonic distension of the bladder with Krebs solution for 30 s periods at pressures of 10, 20, 30 and 40 cmH2O. The effect of intravesical administration of: (1) oxo-M (25 and 50 µM in Krebs solution), (2) a combination of 50 µM and 5 µM AMN or (3) a combination of 50 µM oxo-M and 30 µM TNP-ATP were examined on bladder contractions and afferent firing. Drugs were administered sequentially at intervals of 30 to 60 min after flushing the bladder with Krebs solution.
Multiunit compound action potentials were also elicited by electrical stimulation (0.15 ms pulse duration) using a pair of silver electrodes (diameter: 0.25 mm) positioned on the serosal surface close to the neck of the bladder. Individual responses were averaged with a computer (Lab View program, National Instrument Company). Based on the latencies of evoked action potentials in the pelvic nerve and the distance between stimulus and recording sites the conduction velocities of the afferent nerves were calculated. The distance between stimulating and recording electrodes ranged between 11 and 13 mm. Changes in the electrically evoked action potentials were studied after intravesical infusion (rate of 0.04 ml/min for 8 min) or following isotonic bladder distention with: (1) Krebs solution, (2) a single drug solution or (3) a solution containing a combination of drugs.
In some experiments (n = 5) capsaicin (125 mg/kg) was administered on two consecutive days, 25 mg/kg on the first day and 100 mg/kg on the second day) was injected subcutaneously 4 days prior to the experiments to desensitize C-afferent fibers (Cheng et al., 1993). All procedures utilized in this study were approved by the University of Pittsburgh, Institutional Animal Care and Use Committee.
4.1. Analysis of data
Multiunit recordings of afferent activity are presented as peak firing frequency in spikes/s recorded under isovolumetric or isotonic conditions. The resting activity of afferent nerves was measured for 1 min before the start of bladder filling. The Lab View program (National Instrument Company) was used to analyze the area of evoked action potentials. All data are expressed as mean±SE. Results were evaluated using two-way ANOVA followed by Bonferroni post tests using Prism 4 program (GraphPad software Inc, San Diego, CA).
4.2. Drugs
oxo-M, TNP-ATP and AMN (all obtained from Sigma) were diluted to final concentrations in Krebs solution and the pH was adjusted to 7.4. Capsaicin (obtained from Sigma) dissolved in 10% Tween 80, 10% alcohol and 80% saline was injected subcutaneously in a concentration of 20 mg/ml.
Acknowledgments
This work was supported by the National Institutes of Health Grant DK49430.
Abbreviations
- oxo-M
oxotremorine-M
- TNP-ATP
2′,3′-0-trinitrophenyl-ATP
- BAN
bladder afferent nerve
- AP
action potential
- AMN
atropine methyl nitrate
REFERENCES
- Abrams P, Andersson K-E, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems and the implications for treating overactive bladder. Br. J. Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K-E. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- Andersson K-E, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC, Tannenbaum C, Wein AJ. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr. Opin. Urol. 2009;19:380–394. doi: 10.1097/MOU.0b013e32832ce8a4. [DOI] [PubMed] [Google Scholar]
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford APDW, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinumtoxin for human detrusor overactivity. J. Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, de Groat WC, Apodaca G. Physiology of the urothelium. Chapter 3. In: Shick E, Corcos J, editors. Textbook of the Neurogenic Bladder. second ed. London, UK: Taylor and Francis; 2008. pp. 19–39. [Google Scholar]
- Birder LA, Drake M, de Groat WC, Fowler C, Mayer E, Morrison J, Paton J, Griffiths D, Mills I, Thor K. Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. fourth ed. Paris, France: Health Publications Ltd; 2009. pp. 169–253. [Google Scholar]
- Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibers in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur. Urol. 2004a;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis A, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004b;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J. Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rat. Am. J. Physiol. 1993;265:R132–R138. doi: 10.1152/ajpregu.1993.265.1.R132. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F, Guimaraes M, Silva C, Rio ME, Coimbra A, Reis M. Desensitization of bladder sensory fibers by intravesical capsaicin has long lasting clinical and urodynamic effects in patients with hyperactive or hypersensitive bladder dysfunction. J. Urol. 1997;157:585–589. [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohan M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J. Neurophysiol. 2008;99(1):49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50 suppl 6A:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64 suppl 6A:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br. J. Pharmacol. 2006;147 Supplement 2:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu. Rev. Pharmacol. Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- deGroat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb. Exp. Pharmacol. 2001;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 2010;29:63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System: Nervous Control of the Urogenital System. Vol. 3. London: Harwood Academic Publishers; 1993. pp. 227–289. [Google Scholar]
- de Wachter S, Wyndaele JJ. Intravesical oxybutynin: a local anesthetic effect on bladder C afferents. J. Urol. 2003;169:1892–1895. doi: 10.1097/01.ju.0000049903.60057.4b. [DOI] [PubMed] [Google Scholar]
- Finney SM, Andersson K-E, Gillespie JI, Stewart LH. Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions? BJU Int. 2006;98:503–507. doi: 10.1111/j.1464-410X.2006.06258.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat. Rev. Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J. Neurocytol. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–2302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorn MH, Chapple CR, Cock M, Chess-William R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br. J. Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am. J. Physiol. Renal Physiol. 2008;295:F454–F461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irnich D, Burgstahler R, Bostock H, Grafe P. ATP affects both axons and Schwann cells of unmyelinated C fibers. Pain. 2001;92:343–350. doi: 10.1016/S0304-3959(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kibble A, Morrison JFB. The effects of capsaicin on pelvic afferent inputs from the urinary bladder of anaesthetized rats. J. Physiol. 1995:487. 119P. [Google Scholar]
- Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder LA, Griffiths D, de Groat WC, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am. J. Physiol. Renal Physiol. 2007;292:F1065–F1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yoshimura N, Masuda H, de Miguel F, Chancellor MB. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathway. Urology. 2005;65:238–242. doi: 10.1016/j.urology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J.Neurosci. 2008a;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann F, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor mediated Ca2+ responses in cultured urothelial cells from rat. Am. J. Physiol. Renal Physiol. 2008b;294:F971–F981. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Cross RL, Chess-Williams R, Chapple CR. Recent advances in basic science for overactive bladder. Curr. Opin. Urol. 2005;15:222–226. doi: 10.1097/01.mou.0000172393.52857.92. [DOI] [PubMed] [Google Scholar]
- Lang PM, Sippel W, Schmidbauer S, Irnich D, Grafe P. Functional evidence for P2X receptors in isolated human vagus nerve. Anesthesia. 2003;99:232–235. doi: 10.1097/00000542-200307000-00038. [DOI] [PubMed] [Google Scholar]
- Lu SH, de Groat WC, Lin AT, Chen KK, Chang LS. Evaluation of purinergic mechanism for the treatment of voiding dysfunction: a study in conscious spinal cord-injured rats. J. Chin. Med. Assoc. 2007;70:439–444. doi: 10.1016/s1726-4901(08)70035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom I, Abrams P, Cardozo L, Roberts RG, Th�roff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- Namasivayam S, Eardley L, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1049–R1059. doi: 10.1152/ajpregu.00236.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology. 2005;66:1332–1337. doi: 10.1016/j.urology.2005.06.099. [DOI] [PubMed] [Google Scholar]
- Rong W, Spyer M, Burnstock G. Activation and sensitization of low and high threshold afferent fibers mediated by P2X receptors in the mouse urinary bladder. J. Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J. Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J. Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH. Modulation of bladder myofibroblast activity: implications for bladder function. Am. J. Physiol. Renal Physiol. 2008;295:F688–F697. doi: 10.1152/ajprenal.00133.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J. Urol. 2004;171:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- Tata AM, Vilaro MT, Mengod G. Muscarinic receptor subtypes expression in rat and chick dorsal root ganglia. Mol. Brain Res. 2000;82:1–10. doi: 10.1016/s0169-328x(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during age. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Yu YB, de Groat WC. Sensitization of pelvic afferent nerve in the in vitro rat urinary bladder–pelvic nerve preparations by purinergic agonists and cyclophosphamide pretreatment. Am. J. Physiol. Renal Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Igawa Y, Ishizuka O, Nishizawa O, Andersson K-E. Effects of resiniferatoxin desensitization of capsaicin-sensitive afferents on detrusor over-activity induced by intravesical capsaicin, acetic acid or ATP in conscious rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2003;367:473–479. doi: 10.1007/s00210-003-0748-x. [DOI] [PubMed] [Google Scholar]