Abstract
Objectives
Aldosterone is well recognized as the selective physiological ligand for mineralocorticoid receptor in epithelia. However, in-vitro studies have demonstrated that the affinity of aldosterone and glucocorticoids for mineralocorticoid receptor is similar. We hypothesized that glucocorticoids are involved in the development of renal injury through an mineralocorticoid receptor-dependent mechanism.
Methods and results
Uninephrectomized (UNX) rats were treated with 1% NaCl and divided into three groups: vehicle, bilateral adrenalectomy (ADX) + hydrocortisone (HYDRO; 5 mg/kg/day, s.c.), ADX + HYDRO + eplerenone (0.125% in chow). HYDRO-treated UNX-ADX rats showed increased blood pressure and urinary albumin-to-creatinine ratio with an increase in the expression of the mineralocorticoid receptor target genes, serum and glucocorticoid-regulated kinases-1 and Na+/H+ exchanger isoform-1, in renal tissues. HYDRO treatment induced morphological changes in the kidney, including glomerulosclerosis and podocyte injury. Treatment with eplerenone markedly decreased the gene expression and reduced the albuminuria and renal morphological changes. In contrast, dexamethasone (0.2 mg/kg per day, s.c.) + UNX + ADX induced hypertension and albuminuria in different groups of rats. Eplerenone failed to ameliorate these changes.
Conclusions
Our findings indicate that chronic glucocorticoid excess could activate mineralocorticoid receptor and, in turn, induce the development of renal injury.
Keywords: eplerenone, hydrocortisone, kidney diseases, mineralocorticoid receptor
Introduction
Accumulating evidence indicates an important role for the aldosterone/mineralocorticoid receptor in the pathophysiology of hypertension and renal injury. Aldosterone/salt-treated rats showed progressive hypertension and renal injury, which was suppressed by eplerenone, a selective mineralocorticoid receptor antagonist [1,2]. In-vitro studies found that aldosterone induced proliferation and deformation of rat mesangial cells (RMCs); again, these changes were suppressed by eplerenone [3,4], suggesting that aldosterone has direct deleterious effects on the RMC via activation of mineralocorticoid receptor. Recent studies indicate that high circulating aldosterone levels are not always observed when mineralocorticoid receptor blockers elicit their renoprotective effect; for example, mineralocorticoid receptor blockers prevented renal injury in experimental models that had low or normal plasma aldosterone levels, such as salt-treated Dahl salt-sensitive rats [5–7] and stroke-prone spontaneously hypertensive rats [8]. Furthermore, several clinical studies demonstrated that blockade of mineralocorticoid receptor prevented the progression of proteinuria and renal injury even in patients with normal or low plasma aldosterone levels [9,10], indicating a possible effect of mineralocorticoid receptor that does not depend on high plasma aldosterone level.
The affinities for mineralocorticoid receptor are similar between aldosterone and glucocorticoids (cortisol in humans, corticosterone in rodents) in vitro [11]. The plasma concentration of glucocorticoids is 1000 times greater than that of aldosterone under physiological conditions [12]; however, 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), which is highly expressed in the aldosterone target cells, transforms glucocorticoids into inactive metabolites (cortisone in humans and 11-dehydrocorticosterone in rodents), and prevents the activation of mineralocorticoid receptor by glucocorticoids [13]. Thus, reduced 11βHSD2 activity results in pseudohyperaldosteronism similar to that seen in patients with the syndrome of apparent mineralocorticoid excess (by 11βHSD2 deficiency) and in patients treated with licorice (by glycyrrhizic acid-induced 11βHSD2 inhibition). Kageyama et al. [14] have reported that a physiological dose of corticosterone induces blood pressure elevation in glycyrrhizin-treated bilaterally adrenalectomized rats; the effect was inhibited by a mineralocorticoid receptor antagonist, spironolactone, indicating that corticosterone participated in the mineralocorticoid receptor-dependent pathological responses. However, the contribution of aldosterone-independent mineralocorticoid receptor activation to renal injury has yet to be examined.
Therefore, our purpose was to examine whether hydrocortisone (HYDRO) induces renal injury via activation of mineralocorticoid receptor as observed in aldosterone/salt-treated rats. Studies were conducted to determine whether HYDRO (instead of aldosterone)-treated adrenalectomized rats develop hypertension, and renal injury with significant up-regulation of mineralocorticoid receptor target genes, such as serum and glucocorticoid-regulated kinases-1 (Sgk-1) and Na+/H+ exchanger isoform-1 (NHE1). In addition, we investigated the effect of dexamethasone (DEX), a glucocorticoid that has no apparent in-vivo mineralocorticoid receptor agonistic activity, to elucidate the role of the glucocorticoid receptor. Then, we examined the effects of eplerenone on HYDRO and DEX-induced hypertension and renal injury in these animals.
Methods
Animals
Experiments were performed on male, 6 weeks old Wistar–Kyoto (WKY) rats (SLC, Shizuoka, Japan), weighing 160–183 g. Rats were maintained in a temperature-controlled (24 ± 2°C) room under a 12-h light/dark cycle. All experimental procedures were performed according to the Guidelines for the Care and Use of Animals established by Kagawa University.
Experimental design
After 1 week acclimatization, male WKY rats were subjected to right uninephrectomy (UNX) and bilateral adrenalectomy (ADX) by flank incision under anesthesia with sodium pentobarbital (50 mg/kg, i.p.). After the operation, all rats were given 1% NaCl in the drinking water, and an osmotic minipump (Alzet, model 2004; DURECT Corporation, Cupertino, California, USA) was implanted subcutaneously at the dorsum of the neck to infuse vehicle, HYDRO (5 mg/kg per day) or DEX (0.2 mg/kg per day) for 8 weeks. After 4 weeks the osmotic minipump was replaced to continue drug infusion. After pump implantation, rats were randomly divided into three groups as follows: group I (n = 10): UNX + vehicle; group II (n = 9): UNX + ADX + HYDRO (5 mg/kg per day, s.c.); and group III (n = 10): UNX + ADX + HYDRO + eplerenone eplerenone (0.125% in chow; approximately 75 mg/kg per day). The dose of HYDRO and eplerenone were determined on the basis of results from previous studies in rats. In another group of experiments, rats were randomly divided into three groups as follows: group I (n = 10): UNX + vehicle; group II (n = 10): UNX + ADX + DEX (0.2 mg/kg per day, s.c.); and group III (n = 10): UNX + ADX + DEX + eplerenone (0.2% in chow; approximately 110 mg/kg per day). The dose of HYDRO and DEX was also determined on the basis of results from previous studies in rats [2,15–17]. The current eplerenone dose could markedly suppress the hypertension induced by 0.75 μg/h of aldosterone for 6 weeks [2,18].
Sample collection
During the 8-week treatment period, systolic blood pressure (SBP) was measured by tail-cuff plethysmography (BP-98A; Softron Co., Tokyo, Japan). Urine samples were collected using metabolic cages. Urine samples were stored at −30°C for urinary total protein, creatinine and albumin analysis. Arterial blood was collected from the abdominal aorta, after anesthesia with sodium pentobarbital (50 mg/kg, i.p.) into chilled tubes containing EDTA. Blood samples were centrifuged, and supernatant was stored at −30°C for further analysis. The left kidney was perfused with chilled saline solution. Kidney sections were fixed in 10% formalin. Renal cortical tissues were snap-frozen in liquid nitrogen and stored at −80°C until processing for RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR) analysis.
Histological examination
Kidneys were embedded in paraffin, sectioned into 4 μm slices, and stained with periodic acid-Schiff (PAS) reagent. Thereafter, glomerular cellularity was determined by counting the nuclear cell total in each glomerulus using light microscopy. The diameters of the glomeruli and percentage of PAS-positive area in each experimental group were also measured using image measurement software, WinROOF (Mitani Corp., Tokyo, Japan). A total of 45–50 glomeruli were examined for each rat and the average percentage of affected lesions were calculated for each rat. The extent of the interstitial fibrotic area was evaluated quantitatively by an automatic image analysis, which determined the area occupied by interstitial tissue positive for Masson’s trichrome-staining as described previously [19], and was analyzed using Image-Pro plus software (Media Cybernetics, Bethesda, Maryland, USA). Twenty consecutive microscopic fields (2500 μm2 in each field) were examined for each rat and the averaged percentages of the collagen-positive lesions were obtained for each rat (×200 magnification). All of the morphometric measurements were performed in a blinded manner to avoid any bias.
Immunohistochemistry
Immunohistochemistry for desmin was performed using the Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences, Tokyo, Japan) [7]. Deparaffinized sections were incubated with 0.1% hydrogen peroxide for 10 min to block endogenous enzymes. After blocking, sections were incubated with primary antibodies (anti-Human Desmin Mouse monoclonal antibody, D33, 1: 500; DAKOCytomation, Glostrup, Denmark) for 10 min at room temperature. Antibodies were visualized by 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate (DakoCytomation); counterstaining was performed with hematoxylin (DAKOCytomation). Sections incubated without primary antibodies were used as controls. The histologic analysis was performed using a color image analyzing system (WinRooF) in a blinded manner.
Laser capture microdissection and mRNA isolation
For glomerular nephrin and podocin mRNA analysis, glomeruli were microdissected by laser capture microdissection methods [7]. Tissue embedded in optimal cutting temperature was subsequently cryosectioned into 8 μm sections. Twenty-five glomeruli were randomly microdissected from each specimen under direct visualization with a laser capture microscope (LM-200; Arcturus Bioscience, Mountain View, California, USA). Finally, glomerular mRNA was extracted using RNAqueous-Micro kits (Ambion, Austin, Texas, USA) according to the manufacturer’s protocol.
Real-time RT-PCR
The mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), alpha-smooth muscle actin (α-SMA), type 1 collagen, mineralocorticoid receptor, glucocorticoid receptor, Sgk-1, 11βHSD2 and NHE1 in renal cortical tissue, and nephrin and podocin in renal glomeruli were analyzed by RT-PCR using a LightCycler FastStart DNA Master SYBR Green I kit and an ABI Prism 7000 Sequence Detection System (Applied Bio-systems, Foster City, California, USA). The oligonucleotide primer sequences of GAPDH, MR, Sgk-1 and type 1 collagen were as previously described [7,20,21]. The oligonucleotide primer sequences were: rat α-SMA (NM_012893) sense: ACGGCGGCTTCGTCTTCT, antisense: CCAGCTGACTCCATGCCAAT; rat glucocorticoid receptor sense: 5′-ACAGCTCACCCCTACC TTGGT-3′, antisense: 5′-CTTGACGCCCACCTAACA TGT-3′; rat 11βHSD2 (NM_017081) sense: 5′-CTG GCCACTGTGTTGGATTTG-3′, antisense: 5′-TCCA GAACACGGCTGATATCCT-3′; and rat NHE1 (NM_012652) sense: 5′-ACCACAAGATGGAGATG AAGCA-3′, antisense: 5′-GCAAGATGCGCTCTGAAG CT-3′; nephrin sense: 5′-CCAGAGTGGACGAACTAT ATTGGA-3′, antisense: 5′-GACCAGTAACTGCCCGT TATCC-3′; podocin sense: 5′-CCTTTCCATGAGGTG GTAACCA-3′, antisense: 5′-GGATGGCTTTGGACA CATGAG-3′. All data were expressed as the relative differences between UNX + vehicle group and other groups after normalization to GAPDH expression.
Other analytical procedures
Urinary creatinine, urinary albumin and plasma HYDRO were analyzed by a creatinine test kit (micro CRE-test; Wako, Osaka, Japan), an albumin assay kit (Shibayagi, Gunma, Japan) and a cortisol enzyme immunoassay kit (Oxford Biomedical Research, Oxford, Michigan, USA), respectively. The normal value of urinary albumin-to-creatinine ratio (UACR) for rats is around 10–40 mg/g creatinine by using this kit that is almost similar value with previous studies using different kit [22,23]. Plasma and urine sodium and potassium concentration were measured using flame photometry (Hitachi 750; Hitachi, Tokyo, Japan). Plasma aldosterone concentration was analyzed by commercially available kit (SPACK-S aldosterone kit; TFB, Tokyo, Japan).
Statistical analysis
All values are presented as the means ± SEM. Statistical comparisons of the differences were performed using one way analysis of variance combined with the Newman-Keuls post-hoc test. P values below 0.05 were considered statistically significant.
Results
Plasma corticosteroid concentration
Adrenalectomy was confirmed by examining the plasma aldosterone concentration of rats. The plasma aldosterone concentration was found to be within the normal range in vehicle-infused rats. In contrast, the plasma aldosterone concentration was below the detectable range in adrenalectomized rats (less than 13 pg/ml) (Table 1). Plasma HYDRO levels were 36.4 ± 6.3 and 38.5 ± 3.9 ng/ml in UNX + ADX + HYDRO rats and UNX + ADX + HYDRO + eplerenone, respectively.
Table 1.
Biological parameters of HYDRO-induced rats
| Parameters | Treatment groups |
||
|---|---|---|---|
| UNX +vehicle (n =8) | UNX + ADX + HYDRO (n =8) | UNX + ADX + HYDRO + eplerenone (n =8) | |
| Body weight (initial) (g) | 174 ± 3 | 172 ± 4 | 170 ± 2 |
| Body weight (final) (g) | 434 ± 13 | 381 ± 22* | 358 ± 10* |
| Left kidney weight (g) | 2.60 ± 0.26 | 2.43 ± 0.12 | 2.14 ± 0.04* |
| LKW/BW (mg/g) | 5.95 ± 0.45 | 6.44 ± 0.39 | 6.00 ± 0.13 |
| Plasma creatinine level (μmol/l) | 49 ± 2 | 55 ± 6 | 44 ± 4 |
| Plasma aldosterone concentration (nmol/l) | 480 ± 86 | ND | ND |
| Plasma Na+ level (mmol/l) | 144 ± 1 | 141 ± 2 | 144 ± 2 |
| Plasma K+ level (mmol/l) | 4.2 ± 0.1 | 4.5 ± 0.4 | 4.6 ± 0.1 |
| Urinary Na+ level (mEq/24 h) | 3.48 ± 0.40 | 4.43 ± 0.47 | 5.86 ± 1.00 |
| Urinary K+ level (mEq/24 h) | 5.43 ± 0.57 | 5.28 ± 0.62 | 6.80 ± 0.94 |
Values are presented as means ± SE. ADX, bilateral adrenalectomy; BW, body weight; HYDRO, hydrocortisone; LKW, left kidney weight; UNX, uninephrectomy.
P <0.05 vs. UNX + vehicle group.
nd, undetectable (values less than 13 pg/ml).
Blood pressure, albuminuria and biological parameters
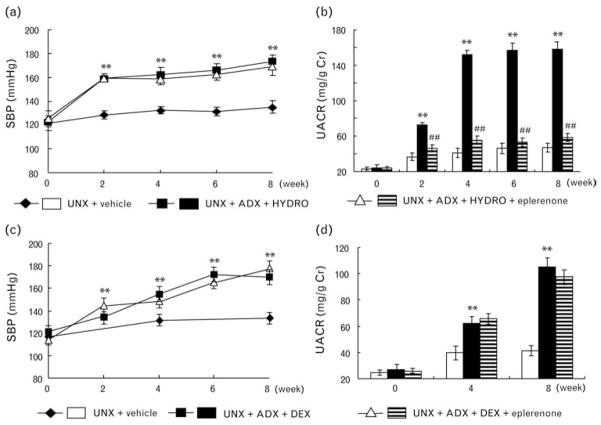
Hydrocortisone infusion into UNX +ADX rats significantly increased SBP compared with UNX controls (Fig. 1a). Eplerenone treatment failed to attenuate the HYDRO-induced increase in SBP (Fig. 1a). During the 8-week treatment period, HYDRO-infused rats showed significantly less body weight gain compared with vehicle-infused rats (Table 1). Eplerenone did not improve the attenuated body weight gain (Table 1). The ratio of left kidney weight to body weight was not significantly different between the groups (Table 1).
Fig. 1.
Both hydrocortisone (HYDRO; a and b) and dexamethasone (DEX; c and d) developed hypertension (a and c) and albuminuria (b and d). Eplerenone affect neither HYDRO nor DEX-induced SBP elevation. Eplerenone markedly ameliorated albuminuria in HYDRO-treated rats but not in DEX-treated rats. **P < 0.01 vs. UNX + vehicle group. ##P < 0.01 vs. UNX + ADX + HYDRO or DEX group (n = 8–10 in each group). ADX, bilateral adrenalectomy; SBP, systolic blood pressure; UNX, uninephrectomy.
Increased UACR (Fig. 1b) was observed in HYDRO-infused UNX + ADX rats. Treatment with eplerenone significantly attenuated the HYDRO-induced increase in UACR (Fig. 1b). Neither HYDRO nor eplerenone treatment significantly affected the plasma creatinine nor plasma as well as urine electrolyte (Na+ and K+) levels (Table 1).
To further eliminate the possibility that the effect of HYDRO was caused through the glucocorticoid receptor, we additionally investigated the effect of DEX. DEX infusion also significantly increased SBP and UACR compared with UNX controls (Fig. 1c), whereas eplerenone failed to suppress both hypertension and albuminuria (Fig. 1c).
Renal histological findings
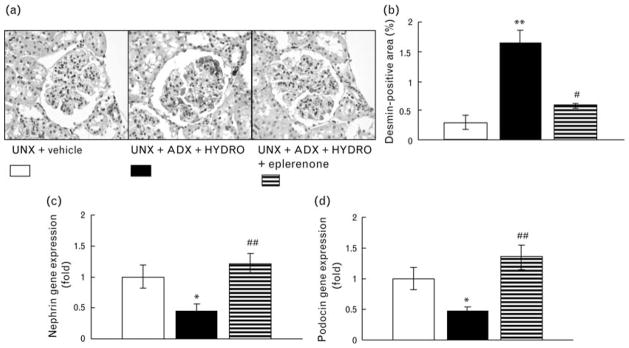
Periodic acid-Schiff staining was performed to analyze glomerular injury (Fig. 2). HYDRO-induced rats exhibited injured glomeruli characterized by glomerular sclerosis (Fig. 2b), hypertrophy (Fig. 2c), and cellularity (Fig. 2d). We evaluated the glomerular podocyte injury in rats by immunostaining for desmin (Fig. 3a and b), and found a significant increase in the antibody-positive area (brown in color) in renal cortical glomeruli of HYDRO-infused rats compared with that of vehicle-treated rats. HYDRO infusion showed significantly down-regulated nephrin and podocin mRNA in glomeruli compared with those of vehicle-treated rats (Fig. 3c and d). In Masson’s trichrome staining, a marker of interstitial fibrosis, of renal sections, HYDRO infusion showed a nonsignificant tendency to increase the percentage of Masson’s trichrome staining (blue) (Fig. 4a). HYDRO-infused rats showed significantly increased profibrotic gene expression, such as α-SMA (Fig. 4b) and type 1 collagen (Fig. 4c) mRNA, in renal cortical tissue compared with vehicle-infused rats. Treatment with eplerenone dramatically attenuated the development of podocyte injury, glomerular sclerotic changes and tubulointerstitial fibrosis.
Fig. 2.
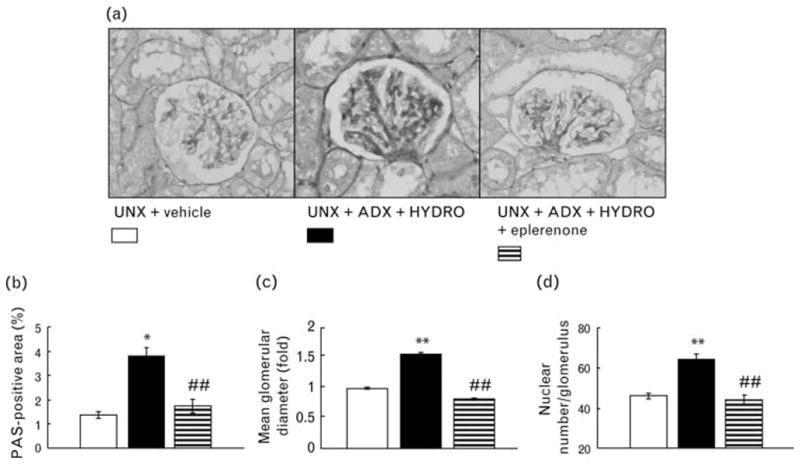
Representative micrographs of periodic acid-Schiff (PAS)-stained renal sections (a), the PAS-positive area within total glomerular area (b) and the mean glomerular diameter (c) and cell number of the glomeruli (d). Rats receiving HYDRO exhibited glomerular sclerotic changes. Treatment with eplerenone significantly attenuated HYDRO-induced glomerular sclerotic changes. **P < 0.01, **P < 0.05 vs. UNX + vehicle group. ##P < 0.01, #P < 0.05 vs. UNX + ADX + HYDRO group (n =8 in each group).
Fig. 3.
Representative renal sections of immunostaining for desmin (a) and percentage positive area of immunostaining for desmin in glomeruli within the total glomerular area (b). Gene expression of nephrin (c) and podocin (d) in the glomeruli. HYDRO-treated rats exhibited a wider desmin positive area, and down-regulation of nephrin and podocin mRNA in the glomeruli than UNX rats, suggesting that HYDRO increased glomerular podocyte injury. Treatment with eplerenone significantly reduced the HYDRO-induced increase in the desmin positive area and prevents the down-regulation of gene expressions. **P < 0.01 vs. UNX + vehicle group. #P <0.05 vs. UNX + ADX + HYDRO group (n =8 in each group).
Fig 4.
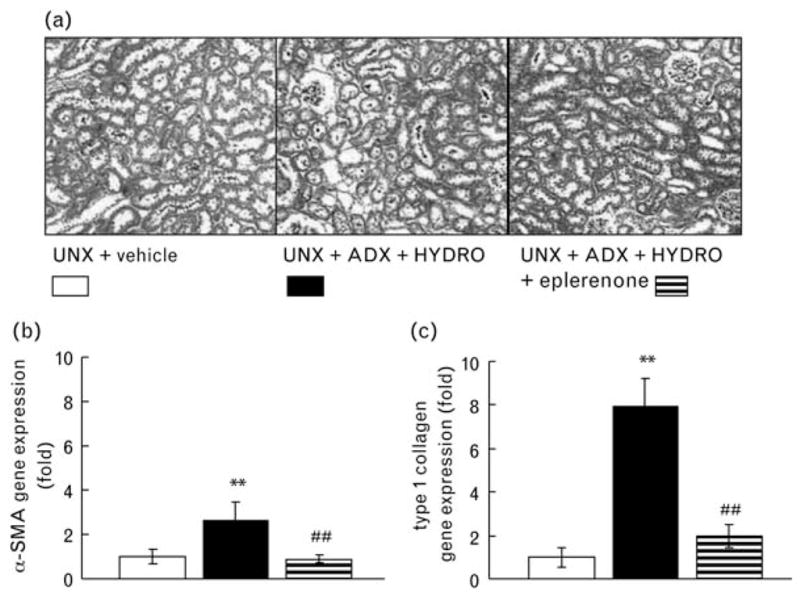
Representative micrographs of Masson’s trichrome-stained renal sections (a) and gene expression of α-smooth muscle actin (α-SMA; b) and type 1 collagen (c). HYDRO-treated rats exhibited interstitial fibrosis and markedly up-regulated α-SMA and type 1 collagen gene expression in renal cortical tissue. Treatment with eplerenone significantly attenuated HYDRO-induced interstitial fibrosis and upregulation of fibrotic gene expressions (n =8 in each group).
Mineralocorticoid receptor target gene expression
Hydrocortisone-infused rats showed markedly up-regulated mineralocorticoid receptor target gene, Sgk-1 in renal cortical tissue (Fig. 5a). Since Sgk-1 is reported to respond to the other stimulations [24,25], we also evaluated another mineralocorticoid receptor target gene, NHE1, and observed that HYDRO-infused rats had greater renal NHE1 expression (Fig. 5b). Treatment with eplerenone prevented the HYDRO-induced up-regulation of Sgk-1 and NHE1 gene expression in renal cortical tissue. HYDRO-infused rats also showed significantly increased mineralocorticoid receptor mRNA expression in renal cortical tissue compared with vehicle-infused rats (Fig. 5c), but glucocorticoid receptor and 11βHSD2 mRNA expression were not affected by HYDRO infusion (Fig. 5d, e). Treatment with eplerenone significantly attenuated the HYDRO-induced increase in mineralocorticoid receptor expression.
Fig 5.
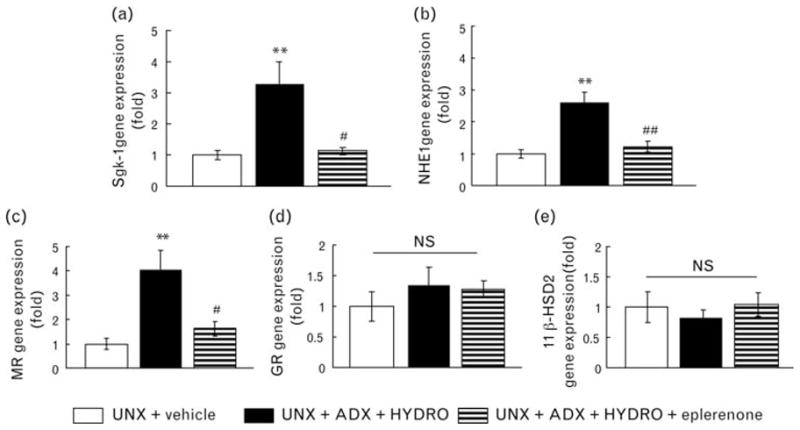
Gene expression of serum and glucocorticoid-regulated kinases-1 (Sgk-1; a), Na+/H+ exchanger isoform-1 (NHE1; b), mineralocorticoid receptor (MR) (c), glucocorticoid receptor (GR; d) and 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2; e). HYDRO-treated rats exhibited up-regulation of MR target gene expression and MR in renal cortical tissue. Treatment with eplerenone significantly attenuated the HYDRO-induced gene up-regulation. GR and 11βHSD2 gene expression remained unchanged. **P <0.01 vs. UNX + vehicle group. ##P <0.01, #P <0.05 vs. UNX + ADX + HYDRO group (n =8 in each group).
Discussion
The epithelial mineralocorticoid receptor is predominantly stimulated by a mineralocorticoid, aldosterone, under physiological conditions. Eplerenone, however, showed a renoprotective effect in patients with normal or low aldosterone levels, indicating that there may be other possible ligands for mineralocorticoid receptor under pathological conditions. Our present results showed that hydrocortisone increased the transcription of two mineralocorticoid receptor target genes, Sgk-1 and NHE1, suggesting that HYDRO stimulates mineralocorticoid receptor in the kidney. In addition, eplerenone markedly attenuated HYDRO-induced, but not DEX-induced, renal injury without affecting blood pressure, indicating that glucocorticoids can promote renal injury via both mineralocorticoid receptor-dependent and mineralocorticoid receptor-independent pathways in some pathophysiological conditions.
The present study showed that renal 11βHSD2 gene expression was not altered by HYDRO excess. Since activation of epithelial mineralocorticoid receptor by a glucocorticoid is normally prevented by 11βHSD2 [26], it is possible that exogenous HYDRO is also converted into an inactivated state. Our current study, however, showed that eplerenone attenuated the HYDRO, but not DEX-induced renal injury and that HYDRO induced the eplerenone-inhibitable increase in Sgk-1 transcription, showing that administered HYDRO stimulated mineralocorticoid receptor. There are several possible mechanisms by which HYDRO stimulates mineralocorticoid receptor. First, exogenous HYDRO might overcome the protective role of 11βHSD2 and lead to the activation of mineralocorticoid receptor-dependent signaling. However, it may not be the case because the plasma HYDRO level did not exceed the normal plasma glucocorticoid level observed in rats, whereas it is still possible that the abolition of diurnal variation on glucocorticoid might play a role under the effect of exogenous glucocorticoid treatments, although the mechanism of which is unclear. Second, HYDRO acted as partial agonists under adrenal-ectomized condition (lack of intrinsic aldosterone) as has been shown by in-vitro studies [27–29]. Finally, HYDRO might have caused renal injury, such as glomerulosclerosis and podocyte injury, by stimulating the nonepithelial mineralocorticoid receptor rather than epithelial mineralocorticoid receptor due to the influence of 11βHSD2 in epithelial cells, and induced subsequent albuminuria as those have been demonstrated in aldosterone-infused animals. [18,30] Also, this may explain why eplerenone showed little protective effect against the HYDRO-induced hypertension even by the dose that could prevent aldosterone-induced hypertension [2,18] despite the fact that the aldosterone-induced hypertension was rather greater than HYDRO-induced hypertension. Since sodium retention through epithelial mineralocorticoid receptor activation is one of the major causes of the mineralocorticoid receptor-dependent hypertension, 11βHSD2-dependent inactivation of HYDRO in epithelial cells might attenuate the HYDRO-induced blood pressure elevation.
High concentrations of glucocorticoid hormones, as in patients with Cushing’s syndrome or patients treated with a glucocorticoid, are known to be associated with alteration in body water homeostasis, hypertension and osteoporosis [11,31]. Koh et al. [32] reported that patients with Cushing’s syndrome showed albuminuria, which was almost completely reversed after successful surgical correction of hypercortisolemia, indicating that the contribution of hypercortisolemia to the progression of albuminuria. They also showed that urinary albumin excretion rate was correlated with the blood pressure and plasma glucose levels; however, the increased urinary albumin excretion in Cushing’s syndrome cannot be simply explained by the blood pressure and glucose level since the changes in urinary albumin excretion after surgery were not correlated with the changes in these factors. In addition, Bailey et al. [33] reported that exogenous adrenocorticotropic hormone (ACTH) infusion induced blood pressure elevation and sodium retention as a result of epithelial sodium channel activation, and that glucocorticoid receptor blockade, in addition to mineralocorticoid receptor blockade, was required to prevent the responses, indicating that either glucocorticoid receptor or mineralocorticoid receptor participates in the ACTH-induced Cushing’s syndrome-like responses. Taken together with our present findings, it is possible to speculate that glucocorticoid-induced mineralocorticoid receptor activation could be one of the leading causes of urinary albumin excretion and hypertension in patients with Cushing’s syndrome.
Okada et al. [34] found that renal biopsy samples in patients with minimal change nephrotic syndrome and lupus nephritis who had steroid pulse therapy showed increased connective tissue growth factor (CTGF), a fibrogenetic molecule, and its protein expression. Furthermore, DEX infusion into mice stimulated the CTGF production via transcriptional regulation, suggesting that excessive (chronic) glucocorticoid receptor stimulation also facilitated the fibrotic changes in the kidney [34]. Therefore, we could not eliminate the possibility that the changes in the kidney were partly glucocorticoid receptor-dependent responses to HYDRO in the present study. The limitation is that there is no specific glucocorticoid receptor antagonist that can be used in vivo to examine the involvement of glucocorticoid receptor activation in rats. We, however, did not observe significant fibrotic changes in the kidney of HYDRO-infused rats and eplerenone almost normalized the gene expression of fibrotic molecules, suggesting that glucocorticoid receptor-dependent signaling might not play an important role in the renal fibrosis in the current experimental conditions.
It is known that glucocorticoids induce blood pressure elevation; however, the mechanism by which glucocorticoid, either cortisol (corticosteroid) or synthetic glucocorticoid, raises blood pressure is not fully understood [35]. Mineralocorticoid receptor-dependent salt retention hypothesis cannot explain the case of synthetic glucocorticoids, such as dexamethasone, since the glucocorticoids, which do not induce mineralocorticoid receptor activation in vivo, elevated blood pressure without salt loading in rats [36], and, rather, dexamethasone induced natriuresis [37]. Taken together, the glucocorticoids-induced hypertension in the present study might be caused through mineralocorticoid receptor-independent action of steroids.
In conclusion, our present study suggests that mineralocorticoid receptor could be activated by HYDRO, but not DEX, and that the HYDRO-induced mineralocorticoid receptor activation participates in the development of renal injury. Steroids are widely used as immunosuppressants for patients. Our current results suggest that excessive steroid treatment, especially with steroids that stimulate mineralocorticoid receptor in vivo, may induce renal dysfunction as well as blood pressure elevation, a well known side effect of steroids. Moreover, the present study also suggested a possible pharmacotherapy for glucocorticoid-induced albuminuria; mineralocorticoid receptor blockers may have renoprotective effects in patients treated with steroids that activate mineralocorticoid receptor or in Cushing’s syndrome patients with albuminuria.
Acknowledgments
We are grateful to Pfizer, Inc. for supplying us with eplerenone.
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590253) and Kagawa University Project Research Fund 2009 (to A.N.).
Abbreviations
- 11βHSD2
11β-hydroxysteroid dehydrogenase type 2
- α-SMA
alpha-smooth muscle actin
- ACTH
adrenocorticotropic hormone
- ADX
bilateral adrenalectomy
- CTGF
connective tissue growth factor
- DEX
dexamethasone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HYDRO
hydrocortisone
- NHE1
Na+/H+ exchanger isoform-1
- PAS
periodic acid-Schiff
- RMC
rat mesangial cells
- RT-PCR
reverse transcription-polymerase chain reaction
- SBP
systolic blood pressure
- Sgk-1
serum and glucocorticoid-regulated kinases-1
- UACR
urinary albumin per creatinine ratio
- UNX
uninephrectomized
- WKY
Wistar–Kyoto
Footnotes
There are no conflicts of interest.
References
- 1.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 3.Terada Y, Kobayashi T, Kuwana H, Tanaka H, Inoshita S, Kuwahara M, Sasaki S. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J Am Soc Nephrol. 2005;16:2296–2305. doi: 10.1681/ASN.2005020129. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–716. doi: 10.1161/01.HYP.0000154681.38944.9a. [DOI] [PubMed] [Google Scholar]
- 5.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant. 2007;22:1314–1322. doi: 10.1093/ndt/gfl780. [DOI] [PubMed] [Google Scholar]
- 6.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–1093. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Fan YY, Hitomi H, Kiyomoto H, Kimura S, Kong CZ, et al. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am J Physiol Renal Physiol. 2009;297:F802–F808. doi: 10.1152/ajprenal.00197.2009. [DOI] [PubMed] [Google Scholar]
- 8.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT., Jr Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. doi: 10.1038/sj.ki.5001854. [DOI] [PubMed] [Google Scholar]
- 10.White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021–1026. doi: 10.1161/01.HYP.0000067463.13172.EA. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 12.Funder J, Myles K. Exclusion of corticosterone from epithelial mineralocorticoid receptors is insufficient for selectivity of aldosterone action: in vivo binding studies. Endocrinology. 1996;137:5264–5268. doi: 10.1210/endo.137.12.8940344. [DOI] [PubMed] [Google Scholar]
- 13.Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama Y, Suzuki H, Saruta T. Role of glucocorticoid in the development of glycyrrhizin-induced hypertension. Clin Exp Hypertens. 1994;16:761–778. doi: 10.3109/10641969409078024. [DOI] [PubMed] [Google Scholar]
- 15.Otsuki M, Okabayashi Y, Ohki A, Suehiro I, Nakamura T, Fujii M, et al. Effects of hydrocortisone on glucose- and cholecystokinin-induced insulin release from the isolated perfused rat pancreas. Pancreas. 1988;3:459–464. doi: 10.1097/00006676-198808000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Vackova Z, Vagnerova K, Libra A, Miksik I, Pacha J, Staud F. Dexamethasone and betamethasone administration during pregnancy affects expression and function of 11 beta-hydroxysteroid dehydrogenase type 2 in the rat placenta. Reprod Toxicol. 2009;28:46–51. doi: 10.1016/j.reprotox.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Wang W, Summer SN, Falk S, Schrier RW. Downregulation of UT-A1/UT-A3 is associated with urinary concentrating defect in glucocorticoid-excess state. J Am Soc Nephrol. 2008;19:1975–1981. doi: 10.1681/ASN.2008010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 19.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–927. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diah S, Zhang GX, Nagai Y, Zhang W, Gang L, Kimura S, et al. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp Cell Res. 2008;314:3654–3662. doi: 10.1016/j.yexcr.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Gong D, Lu J, Chen X, Reddy S, Crossman DJ, Glyn-Jones S, et al. A copper(II)-selective chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic TGF-beta activation in the kidneys of rats used as a model of diabetes. Diabetologia. 2008;51:1741–1751. doi: 10.1007/s00125-008-1088-7. [DOI] [PubMed] [Google Scholar]
- 23.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallon V, Lang F. New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr Opin Nephrol Hypertens. 2005;14:59–66. doi: 10.1097/00041552-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa H, Yanagisawa J, Fuse H, Ogawa S, Yogiashi Y, Okuno A, et al. Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol. 2002;22:3698–3706. doi: 10.1128/MCB.22.11.3698-3706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Rogerson FM, Fuller PJ. Interdomain interactions in the mineralocorticoid receptor. Mol Cell Endocrinol. 2003;200:45–55. doi: 10.1016/s0303-7207(02)00413-6. [DOI] [PubMed] [Google Scholar]
- 29.Hultman ML, Krasnoperova NV, Li S, Du S, Xia C, Dietz JD, et al. The ligand-dependent interaction of mineralocorticoid receptor with coactivator and corepressor peptides suggests multiple activation mechanisms. Mol Endocrinol. 2005;19:1460–1473. doi: 10.1210/me.2004-0537. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A, Abe Y. Aldosterone and renal injury. Nippon Yakurigaku Zasshi. 2004;124:101–109. doi: 10.1254/fpj.124.101. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari P. Cortisol and the renal handling of electrolytes: role in glucocorticoid-induced hypertension and bone disease. Best Pract Res Clin Endocrinol Metab. 2003;17:575–589. doi: 10.1016/s1521-690x(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 32.Koh JM, Kim JY, Chung YE, Park JY, Shong YK, Hong SK, et al. Increased urinary albumin excretion in Cushing’s syndrome: remission after correction of hypercortisolaemia. Clin Endocrinol (Oxf) 2000;52:349–353. doi: 10.1046/j.1365-2265.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 33.Bailey MA, Mullins JJ, Kenyon CJ. Mineralocorticoid and glucocorticoid receptors stimulate epithelial sodium channel activity in a mouse model of cushing syndrome. Hypertension. 2009;54:890–896. doi: 10.1161/HYPERTENSIONAHA.109.134973. [DOI] [PubMed] [Google Scholar]
- 34.Okada H, Kikuta T, Inoue T, Kanno Y, Ban S, Sugaya T, et al. Dexamethasone induces connective tissue growth factor expression in renal tubular epithelial cells in a mouse strain-specific manner. Am J Pathol. 2006;168:737–747. doi: 10.2353/ajpath.2006.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong SL, Zhang Y, Whitworth JA. Reactive oxygen species and glucocorticoid-induced hypertension. Clin Exp Pharmacol Physiol. 2008;35:477–482. doi: 10.1111/j.1440-1681.2008.04900.x. [DOI] [PubMed] [Google Scholar]
- 36.Tonolo G, Fraser R, Connell JM, Kenyon CJ. Chronic low-dose infusions of dexamethasone in rats: effects on blood pressure, body weight and plasma atrial natriuretic peptide. J Hypertens. 1988;6:25–31. [PubMed] [Google Scholar]
- 37.Whitworth JA, Gordon D, Andrews J, Scoggins BA. The hypertensive effect of synthetic glucocorticoids in man: role of sodium and volume. J Hypertens. 1989;7:537–549. doi: 10.1097/00004872-198907000-00005. [DOI] [PubMed] [Google Scholar]