Abstract
Vaccine-elicited cytotoxic T lymphocytes (CTL) should be long-lived memory cells that can rapidly expand in number following re-exposure to antigen. The present studies were initiated to analyze the ability of plasmid interleukin-12 (IL-12) to augment CTL responses in mice when delivered during the peak phase of an immune response elicited by a plasmid human immunodeficiency virus type 1 gp120 DNA vaccine. Delivery of plasmid IL-12 on day 10 postimmunization resulted in a robust expansion of gp120-specific CD8+ T cells, as measured by tetramer, gamma interferon ELISPOT, and functional-killing assays. Interestingly, this delayed administration of plasmid IL-12 had no significant effect on antigen-specific CD4+-T-cell and antibody responses. Phenotypic analyses suggested that administration of plasmid IL-12 near the time of the peak CTL response activated and expanded antigen-specific effector cells, preventing their loss through apoptosis. However, this IL-12-augmented population of gp120-specific CD8+ T cells did not efficiently expand following gp120 boost immunization, suggesting that these effector cells would be of little utility in expanding to contain a viral infection. Analyses of the phenotypic profile and anatomic distribution of the plasmid IL-12-augmented CTL population indicated that these lymphocytes were primarily effector memory rather than central memory T cells. These observations suggest that CTL-based vaccines should elicit central memory rather than effector memory T cells and illustrate the importance of monitoring the phenotype and functionality of vaccine-induced, antigen-specific CTL.
Since cytotoxic T lymphocytes (CTL) have been shown to play a central role in controlling a number of viral infections in humans, vaccine strategies are being developed to elicit populations of long-lived, virus-specific CD8+ T lymphocytes that can contribute to viral containment. Some of the immunogens under evaluation for this purpose include plasmid DNA (4, 6, 9), cytokine-augmented plasmid DNA (2, 14, 24), and recombinant viral vectors (1, 21, 23). These vaccine strategies are primarily being evaluated by assessing the magnitude of vaccine-elicited, virus-specific CD8+-T-lymphocyte populations by intracellular cytokine staining, gamma interferon (IFN-γ) ELISPOT, and tetramer technologies. However, while these technical approaches are of value in quantitating vaccine-elicited memory T-cell responses, they do not assess the functional heterogeneity of these cell populations.
Two subsets of memory T cells have been described on the basis of their anatomic compartmentalization and phenotypic profiles (22). Central memory T cells primarily traffic within lymphoid tissues and are distinguished by their expression of the lymph node (LN) homing receptors CD62L and CCR7. Effector memory T cells are found in peripheral tissues and do not express these cell surface molecules (11, 18, 20, 30). Considerable interest has focused on elucidating functional differences between these memory T-cell subsets and their capacity to confer protection against pathogenic infection. Both central and effector memory CTL in mice demonstrate rapid cytokine secretion and lytic activity following in vitro stimulation (28, 31). However, central memory CTL have recently been shown to have a greater capacity for in vivo expansion following exposure to virus and are thus presumably more efficient in mediating protective immunity than are effector memory CTL (31). Therefore, the subset of memory CTL generated by vaccination may determine the ultimate effectiveness of vaccine-elicited immune protection against infection.
In the present study, tetramer analyses were used to monitor the kinetics, magnitude, and durability of plasmid IL-12-augmented, DNA vaccine-elicited CD8+-T-cell responses in mice. While we demonstrate that delaying the administration of plasmid IL-12 until the peak phase of the immune response substantially increased the pool of vaccine-elicited memory CTL, these cells were primarily effector memory T lymphocytes and lacked the ability to expand efficiently in response to secondary antigen exposure. These findings suggest that it will be important to evaluate the phenotypic subset and functional capacity of memory CTL generated by vaccine candidates as they undergo preclinical evaluation.
MATERIALS AND METHODS
Plasmids.
All plasmids were constructed by using the PMV vector backbone, provided by Wyeth-Lederle Vaccines (Pearl River, N.Y.). A plasmid DNA vaccine expressing human immunodeficiency virus type 1 (HIV-1) IIIB gp120 (PMV-gp120) was used to immunize mice in these experiments. The empty PMV vector was used as a sham plasmid. A bicistronic IL-12 expression plasmid (PMV-IL-12) encoding both the p35 and p40 chains of murine IL-12 was constructed. Separate plasmids encoding murine p35 and p40 cDNAs were provided by Zimra Israel (Wyeth-Lederle Vaccines). The gene for p35 was cloned into the pCITE-2a+ vector (Novagen, Madison, Wis.) downstream of the cap-independent translation enhancer (CITE) region. PCR amplification was used to generate a CITE-p35 fragment that was subsequently cloned into the PMV-p40 plasmid, downstream of the gene for p40. A single cytomegalovirus promoter drives the expression of both genes, with the CITE region facilitating the internal initiation of translation for the downstream p35 chain. Expression of p70 IL-12 protein was confirmed by performing enzyme-linked immunosorbent assays (ELISAs; BD PharMingen, San Diego, Calif.) on supernatants of COS-7 cells transiently transfected with 10 μg of PMV-IL-12 (data not shown). IL-12 bioactivity was further demonstrated by culturing mouse splenocytes with transfection supernatants and measuring IFN-γ production by ELISA as previously described (12). Plasmids for mouse immunization were prepared from large-scale bacterial cultures by alkaline lysis, followed by double CsCl gradient centrifugation as previously described (3).
Mice and immunizations.
Eight- to 10-week-old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, Mass.). Groups of mice were immunized by intramuscular injection with various concentrations of plasmid DNA in 0.15 M sterile saline. The total injection volume was 100 μl, with 50 μl delivered into each quadriceps muscle.
Tetramer staining analysis.
Tetrameric H-2Dd complexes folded with the HIV-1 IIIB gp120 p18 epitope peptide (RGPGRAFVTI) (27) were prepared as previously described (26). Blood was collected from individual mice in RPMI 1640 medium containing 40 U of heparin per ml. Following lysis of red blood cells (RBCs), cells were washed once with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and stained for 30 min with phycoerythrin (PE)-conjugated H-2Dd/p18 tetramer and for 15 min with anti-mouse CD8α allophycocyanin (Caltag, San Francisco, Calif.). The cells were then washed once and fixed with PBS containing 1.5% paraformaldehyde. Samples were acquired with a FACScalibur flow cytometer, and data were analyzed with CellQuest software (BD Biosciences, Mountain View, Calif.). Data are presented as the percentage of gated CD8+ T cells that stained positive with H-2Dd/p18 tetramer. In certain experiments, spleen cells were harvested from immunized mice and tetramer staining was performed as described above. Spleen cells were also stained with anti-CD62L-fluorescein isothiocyanate (FITC), anti-CD11a-FITC, anti-CD54-FITC, or annexin V-FITC (BD Phar-Mingen). Expression of the molecules recognized by these reagents was analyzed on gated CD8+ tetramer-positive lymphocytes.
Isolation of tissue lymphocytes.
Lymphocyte populations from various mouse tissues were isolated as previously described (18). Briefly, the spleen, mesenteric LNs, and peripheral LNs (axillary and inguinal) were harvested from individual mice and placed in single-cell suspensions in Hanks balanced salt solution (HBSS) containing 5% FBS. Bone marrow was harvested from the femurs of mice and treated with Tris-ammonium chloride to remove RBCs. Peritoneal cavity lymphocytes were isolated by peritoneal lavage with 5% HBSS. Lung tissue was finely minced and stirred at 37°C for 30 min in HBSS containing 1.3 mM EDTA. Tissue pieces were then washed once and further stirred at 37°C for 1 h in 5% RPMI medium containing 150 U of collagenase IV per ml and 30 U of DNase I per ml (Sigma, St. Louis, Mo.). The resulting cell suspension was pelleted by centrifugation, resuspended in 44% Percoll (Sigma), layered on 67% Percoll, and centrifuged for 20 min at 600 × g. Lymphocytes at the interface were harvested and washed twice in 5% HBSS. Liver tissue was ground in 5% HBSS with a glass Dounce homogenizer. The resulting suspension was filtered and centrifuged, and the cell pellet was resuspended in 35% Percoll containing 200 U of heparin (Elkins-Sinn, Cherry Hill, N.J.) per ml. Cells were centrifuged for 20 min at 600 × g, and the resulting pellet was treated with Tris-ammonium chloride to remove RBCs.
T-lymphocyte subset depletions.
Spleen cells were harvested from individual mice, stained for 15 min at 4°C with either anti-CD4-PE or anti-CD8-PE monoclonal antibodies (MAbs; BD PharMingen), washed twice, and incubated with magnetic microbeads coated with an anti-PE MAb (Miltenyi Biotec, Auburn, Calif.) for 15 min at 4°C. Cells were then washed once, and separations were performed with an autoMACS cell sorter (Miltenyi Biotec). Cells eluted in the negative fraction were collected and used as effectors in either proliferation or IFN-γ ELISPOT assays. Depletions were 95 to 100% efficient, as determined by flow cytometric analysis.
Proliferation assays.
Spleen cells were harvested from individual mice on day 18 postimmunization and tested in a standard [3H]thymidine incorporation assay as either total splenocytes or splenocytes depleted of CD4+ or CD8+ T cells. Effector cells were resuspended in RPMI 1640 medium (Cellgro, Herndon, Va.) supplemented with 5% FBS, 25 mM HEPES, 2 mM l-glutamine, 20 U of penicillin per ml, 20 μg of streptomycin per ml, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 20 μM 2-mercaptoethanol. Effector cells were cultured in a 96-well flat-bottom plate (4 × 105/well) with recombinant HIV-1 IIIB gp120 protein (Intracel, Cambridge, Mass.) at 1,000, 100, 10, or 0 ng/ml. Cultures were incubated for 4 days at 37°C and pulsed for 16 h with 1 μCi of [3H]thymidine per well. Cells were harvested on glass filter paper, and radioactivity was measured with a Wallac 1450 Microbeta liquid scintillation counter (Perkin-Elmer Life Sciences, Boston, Mass.). The mean number of counts per minute from triplicate wells was used to calculate the stimulation index (SI) as follows: SI = counts per minute with antigen stimulation/counts per minute with medium alone. Data are presented as the mean SI from four animals per group.
IFN-γ ELISPOT assays.
Ninety-six-well Multiscreen HA plates (Millipore, Bedford, Mass.) were coated by overnight incubation (100 μl/well) at 4°C with rat anti-mouse IFN-γ MAb (R4-6A2; BD PharMingen) at 10 μg/ml in PBS. Plates were washed three times with PBS and blocked for 2 h at 37°C with 100 μl/well of RPMI 1640 medium containing 10% FBS. Effector spleen cells were harvested from individual mice on day 18 postimmunization and tested as either total splenocytes or splenocytes depleted of CD4+ or CD8+ T cells. Effector cells were plated in triplicate at 2 × 105/well in a 100-μl final volume with medium alone, 4 μg of p18 epitope peptide per ml, or 4 μg of Env peptide pool per ml. The pool consisted of 47 overlapping 15-mer peptides spanning the HIV-1 IIIB gp120 protein (Centralized Facility for AIDS Reagents, Potters Bar, United Kingdom) and was used such that each peptide was present at a concentration of 4 μg/ml. After 24 h of incubation at 37°C, the plates were washed free of cells with PBS-0.05% Tween 20 and incubated overnight at 4°C with 100 μl of biotinylated rat anti-mouse IFN-γ MAb (XMG1.2; BD PharMingen) per well at 5 μg/ml. Plates were washed four times, and 75 μl of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.) was added at a 1/500 dilution. After a 2-h incubation, plates were washed four times and developed with Nitro Blue Tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce, Rockford, Ill.). Plates were analyzed with an ELISPOT reader (Hitech Instruments, Edgemont, Pa.). The mean number of spots from triplicate wells was calculated for each responder animal and adjusted to represent the mean number of spots per 106 spleen cells. Data are presented as the mean number of spots per 106 spleen cells from four animals per group.
Cytokine secretion assays.
Splenocytes from individual immunized mice were cultured in a 24-well plate (4 × 106/well) with 1 ml of 5% RPMI medium containing 100 ng of recombinant HIV-1 IIIB gp120 protein per ml. After 72 h, supernatants were harvested and cytokine concentrations were measured with commercially available ELISA kits in accordance with the manufacturer's (BD PharMingen) protocols.
51Cr release CTL assays.
Spleen cells were harvested from individual mice on day 18 postimmunization, resuspended in RPMI medium containing 10% FBS, and cultured in a 24-well plate (8 × 106/well) with 10 ng of p18 epitope peptide per ml. IL-2 (Sigma) was added to cultures on day 2 to a final concentration of 10 U/ml. On day 7, cells were harvested, washed once, and used as effectors in a 51Cr release assay with P815 target cells (American Type Culture Collection, Manassas, Va.). P815 cells were cultured overnight in the presence of medium alone or with 100 ng of p18 peptide per ml. Cells (2 × 106) were labeled with 150 μCi of 51Cr for 1 h at 37°C, washed twice, and added to a 96-well round-bottom plate at 104/well in 100 μl of 10% RPMI medium. Titrations of effector cells were added to triplicate wells in 100 μl of medium and incubated for 4 h at 37°C. Spontaneous release and maximum release were measured by incubating target cells with medium alone or 2% sodium dodecyl sulfate, respectively. Supernatants (50 μl) were harvested and mixed with scintillation fluid, and radioactivity was measured with a Wallac 1450 Microbeta liquid scintillation counter. Percent specific lysis was calculated as follows: 100 × (experimental counts per minute − spontaneous counts per minute)/(maximum counts per minute − spontaneous counts per minute). Spontaneous release was <10% of the maximum release. Data are presented as the mean specific 51Cr release from four animals per group.
Antibody titers.
Serum was collected from individual mice on day 28 postimmunization, and anti-gp120 antibody titers were determined by ELISA as previously described (3). Ninety-six-well plates were coated by overnight incubation at 4°C with 100 μl of PBS containing recombinant HIV-1 IIIB gp120 protein at 500 ng/ml. Plates were washed three times with PBS-0.05% Tween 20 and blocked for 2 h with 200 μl of blocking buffer (Pierce) per well. Plates were then washed three times, and serial dilutions of serum in PBS containing 10% FBS were added at 100 μl/well. After a 2-h incubation, plates were washed five times with PBS-0.05% Tween 20, and 100 μl of peroxidase-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch Laboratories, West Grove, Pa.) per well was added at a 1/5,000 dilution. Plates were incubated for 1 h, washed five times, and developed with TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Reactions were stopped with TMB Stop Solution (Kirkegaard & Perry Laboratories), and plates were analyzed at 450 nm with a Dynatech MR5000 ELISA reader. Data are presented as the geometric mean titer of eight mice per group.
Statistical analysis.
The statistical significance of differences between groups was analyzed by Mann-Whitney t test with the GraphPad Prism software program.
RESULTS
Durable augmentation of HIV-1 gp120 DNA vaccine-elicited CD8+-T-cell response with late administration of plasmid IL-12.
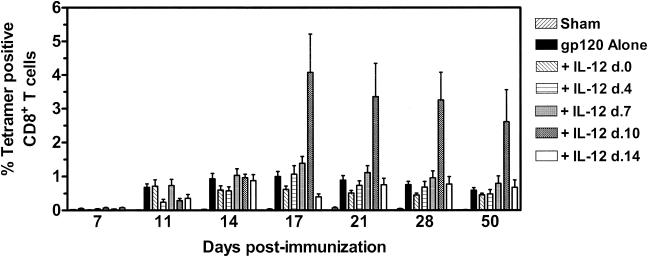
Studies were initiated to evaluate the effect of altering the time of plasmid IL-12 administration on CTL responses elicited by a DNA vaccine expressing HIV-1 IIIB gp120. Groups of mice (six per group) received intramuscular injections of 50 μg of sham PMV plasmid, 50 μg of PMV-gp120, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day 0, 4, 7, 10, or 14 postimmunization. At various times following immunization, peripheral blood was obtained from individual mice and vaccine-elicited cellular immune responses were assessed with an H-2Dd/p18 tetramer to detect a CTL population that recognizes the dominant H-2Dd-restricted gp120 epitope p18. No tetramer-binding CD8+-T-lymphocyte population was detected in mice receiving the sham plasmid (Fig. 1). In mice receiving the gp120 vaccine alone, the p18-specific CTL response was first detected on day 11, peaked at 1.0% of the CD8+ T cells on day 17, and declined to a plateau level of 0.59% between days 28 and 50 postimmunization. The p18-specific CD8+ response in mice receiving plasmid IL-12 on day 0 was diminished compared to that of mice receiving the gp120 DNA alone. These data are consistent with reports demonstrating the suppressive nature of plasmid IL-12 when given at high concentrations concurrently with antigen (5, 8, 15). While further dose titration studies demonstrated that 20 μg of PMV-IL-12 administered concurrently with gp120 DNA could increase the early expansion phase of the p18-specific CTL response, this augmentation was transient, with CTL declining to levels comparable to those observed in mice immunized with gp120 alone as the response reached its plateau phase (data not shown). In mice immunized with the gp120 vaccine plus plasmid IL-12 on day 4, 7, or 14, the p18-specific CD8+-T-cell response was not significantly different from that generated by gp120 DNA immunization alone. Dramatic CTL augmentation was observed, however, in the group of mice receiving PMV-IL-12 on day 10 postimmunization. While levels of p18 tetramer-binding CD8+ T cells in the peripheral blood of these mice were indistinguishable from those of control mice on days 11 and 14 postimmunization, a fourfold increase in p18-specific CD8+ T cells was observed on day 17, 1 week after cytokine administration. Thereafter, levels of peripheral blood p18 tetramer-binding CD8+ T cells began to decline gradually in these mice, with kinetics similar to those observed in mice receiving DNA vaccine alone. Fifty days following gp120 DNA immunization, p18-specific CD8+-T-cell levels in the blood of mice receiving plasmid IL-12 on day 10 were still fourfold higher than those in mice receiving gp120 DNA alone (P < 0.05). The observation that plasmid IL-12 adjuvant activity was demonstrable with cytokine administration on day 10, but not on day 7 or 14, following immunization suggested that the precise timing of IL-12 plasmid delivery was critical for enhancement of CTL responses following vaccination. Experiments performed to further elucidate the optimal time for plasmid IL-12 administration demonstrated that significant CTL expansion occurred only with delivery of plasmid IL-12 between days 10 and 12 postimmunization (data not shown). This adjuvant effect was not observed with similar administration of the sham PMV plasmid (data not shown).
FIG. 1.
p18-specific CD8+-T-cell responses elicited by DNA vaccination with delayed plasmid IL-12 administration. Groups of BALB/c mice were immunized with 50 μg of sham PMV plasmid, 50 μg of PMV-gp120 alone, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 given concurrently or on day (d.) 4, 7, 10, or 14 following vaccine administration. Peripheral blood was obtained from individual mice at the indicated times, and antigen-specific CD8+ T cells were detected with an H-2Dd/p18 tetramer. Data are presented as the percentage of gated CD8+ T cells that bound tetramer, as measured by flow cytometry, and are the means of six mice per group ± SEM.
Enhanced vaccine-elicited CD8+-T-cell effector response with delayed administration of plasmid IL-12.
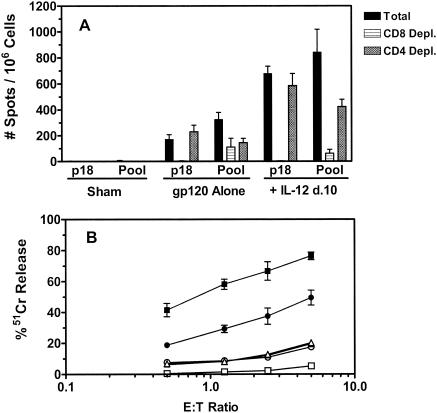
We next sought to determine whether the expansion of p18-specific CD8+ T cells in the peripheral blood of DNA-vaccinated, IL-12-treated mice was associated with increased CTL effector function. Groups of mice were immunized with 50 μg of sham plasmid, 50 μg of PMV-gp120 alone, or PMV-gp120 with 200 μg of PMV-IL-12 on day 10 postimmunization. Spleen cells were harvested on day 18 postimmunization and assessed for p18-specific CTL function by IFN-γ ELISPOT and 51Cr release assays. The percentages of splenic p18-specific CD8+ T cells in mice receiving vaccine alone and those receiving vaccine with plasmid IL-12 were 0.76% (standard error of the mean [SEM], ±0.13%) and 6.1% (SEM, ±0.43%), respectively. The total splenocyte number and CD4+/CD8+-T-cell ratios were not significantly different between these groups of vaccinated mice (data not shown).
Vaccine-elicited functional immune responses of splenocytes were measured in an IFN-γ ELISPOT assay following stimulation with the H-2Dd-restricted p18 epitope peptide or a pool of 47 overlapping peptides that span the HIV-1 IIIB gp120 protein. The antigen-specific responses of CD8+ and CD4+ T cells were assessed by performing selective depletions of splenocyte subsets prior to assaying these cells. While a potent p18-specific IFN-γ response was detected in mice immunized with gp120 DNA alone, this response was fourfold stronger in animals receiving the IL-12 plasmid (Fig. 2A). As expected, since p18 is a CD8+-T-cell epitope but not a CD4+-T-cell epitope, depletion of CD8+ T cells from the splenocyte populations abrogated the p18-specific response, whereas CD4+-T-cell depletion of the splenocytes had no effect on this response. Splenocytes of mice receiving the IL-12 plasmid also generated greater IFN-γ responses following stimulation with the gp120 peptide pool. Plasmid IL-12-induced immune augmentation reflected increased CD8+-T-cell responses, as almost the entire increase in the ELISPOT response to the peptide pool was lost when splenocytes were depleted of CD8+ T cells. Antigen-specific IFN-γ CD4+-T-cell responses were also detected in both control and IL-12-treated mice following pool peptide stimulation, as diminished responses were observed following CD4+-T-cell depletion. No IFN-γ responses were detected from splenocytes of mice immunized with the sham plasmid.
FIG. 2.
Functional analysis of CD8+ T cells from mice immunized with DNA vaccine with or without delayed plasmid IL-12 administration. Groups of BALB/c mice were immunized with 50 μg of sham PMV plasmid, 50 μg of PMV-gp120 alone, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day (d.) 10 following vaccine administration. Spleen cells were harvested from individual mice on day 18 postimmunization for functional analysis. (A) Total splenocytes or splenocytes depleted (Depl.) of CD8+ or CD4+ T cells were evaluated for IFN-γ production in an ELISPOT assay following stimulation with the gp120-derived p18 epitope peptide or a pool of 47 overlapping peptides spanning the HIV-1 IIIB gp120 protein. Data are presented as the mean number of antigen-specific spots per 106 spleen cells ± SEM with four mice per group. (B) Spleen cells from mice immunized with sham PMV plasmid (triangles), PMV-gp120 alone (circles), or PMV-gp120 plus plasmid IL-12 DNA (squares) were cultured for 7 days in the presence of 10 ng of p18 peptide per ml. Cells were then harvested and used as effectors in a 51Cr release assay to assess lysis of P815 tumor cell targets incubated overnight in the presence of medium alone (open symbols) or p18 peptide (filled symbols). Results are expressed as the percent specific 51Cr release and are the means ± SEM of four mice per group. E:T ratio, effector-to-target ratio.
To assess the cytotoxic potential of these vaccine-elicited CD8+ T cells, splenocytes from immunized animals were cultured with the p18 peptide for 7 days and tested as effectors in a 51Cr release assay with p18-pulsed P815 tumor cell targets. As shown in Fig. 2B, p18-specific cytotoxic activity was detected with effector cells from mice immunized with gp120 DNA alone at effector-to-target ratios of less than 5:1. The cytotoxic activity of splenocytes from mice receiving the DNA vaccine and the IL-12 plasmid was approximately twofold higher at all of the effector-to-target ratios tested. No p18-specific lysis was detected with effector cells from mice immunized with the sham plasmid. These ELISPOT and lysis studies demonstrate that delivery of plasmid IL-12 on day 10 postimmunization significantly enhances the CD8+-T-cell effector response generated by DNA vaccination.
Vaccine-elicited CD4+-T-cell and anti-gp120 antibody responses in mice receiving IL-12 plasmid.
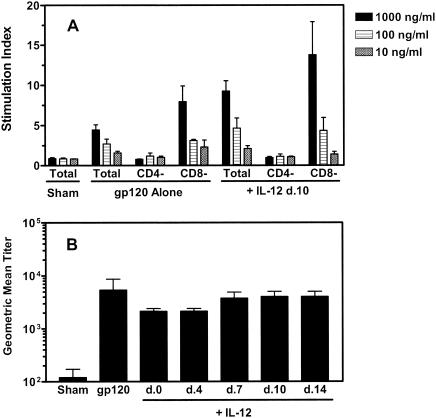
To determine whether late administration of plasmid IL-12 enhances vaccine-elicited CD4+-T-cell responses, splenocytes were assessed for antigen-stimulated proliferation and cytokine production. Splenocytes were harvested from immunized mice as previously described and cultured with various concentrations of recombinant gp120 protein, and proliferative responses were measured by [3H]thymidine incorporation. Antigen-specific, dose-dependent proliferation was observed in splenocytes from mice immunized with plasmid gp120 alone, as well as in those from mice receiving both the vaccine and the IL-12 plasmid (Fig. 3A). While splenocytes from the plasmid IL-12-treated mice exhibited a stronger response to the highest concentration of recombinant gp120 protein than did splenocytes from the mice receiving the vaccine alone (SIs = 9.3 and 4.3, respectively; P < 0.05), the proliferation responses of the two groups of mice stimulated with the lower antigen concentrations were not significantly different. These proliferative responses were CD4+ T cell mediated, as depletion of CD4+ T cells, but not CD8+ T cells, abolished the antigen-specific proliferation. No gp120-specific proliferative response by splenocytes of mice immunized with the sham plasmid was observed.
FIG. 3.
Splenocyte gp120-specific proliferative responses and serum anti-gp120 antibody titers elicited by DNA vaccination with or without delayed plasmid IL-12 administration. (A) Groups of BALB/c mice were immunized with 50 μg of sham PMV plasmid, 50 μg of PMV-gp120 alone, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day (d.) 10 following vaccine administration. Spleen cells from individual mice were harvested on day 18 postimmunization for functional analysis. Total splenocytes or splenocytes depleted of CD4+ or CD8+ T cells were stimulated with the indicated concentrations of recombinant HIV-1 IIIB gp120 protein, and proliferation responses were measured by [3H]thymidine incorporation. Results are presented as the mean SI ± SEM of four mice per group. (B) Groups of BALB/c mice were immunized with 50 μg of sham PMV plasmid, 50 μg of PMV-gp120 alone, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 given on the indicated days. On day 28 postimmunization, serum was collected from individual mice and the titer of gp120-specific antibody was determined by ELISA. Data are presented as the geometric mean titer ± SEM of eight mice per group.
We also examined the profiles of cytokines produced by splenocytes from these mice following culture with 100 ng of recombinant gp120 protein per ml. As shown in Table 1, elevated levels of IFN-γ and IL-2 were detected in the culture supernatants of the splenocytes from mice immunized with gp120 DNA alone, as well as from those of mice receiving both the vaccine and the IL-12 plasmid. While IL-4 levels were slightly higher in splenocyte culture supernatants from both groups of mice, these profiles are consistent with a vaccine-elicited Th1-type immune response. The only significant difference observed between the two groups of immunized mice was higher secretion of IL-2 from splenocytes of mice immunized with gp120 alone (P < 0.03). Together, these proliferation and cytokine data demonstrate that vaccine-elicited CD4+-T-cell responses in animals receiving vaccine and plasmid IL-12 or vaccine alone were for the most part similar. Therefore, while administration of plasmid IL-12 on day 10 postimmunization significantly augmented the CD8+-T-cell response to DNA vaccination, the effect on CD4+-T-cell responses was minimal.
TABLE 1.
Cytokine secretion by splenocytes from DNA-immunized mice following in vitro culture with recombinant HIV-1 IIIB gp120 protein
| Vaccination groupa | Cytokine secretion (pg/ml)b
|
|||
|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-10 | |
| Sham PMV plasmid | 266 ± 47 | 161 ± 34 | 24 ± 2 | 102 ± 22 |
| PMV-gp120 alone | 13,040 ± 2,153 | 446 ± 64 | 54 ± 11 | 138 ± 42 |
| PMV-gp120 + PMV- IL-12 on day 10 | 23,980 ± 7,914 | 238 ± 41 | 47 ± 8 | 122 ± 15 |
Mice were immunized with 50 μg of sham PMV plasmid, 50 μg of PMV-gp120 alone, or 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day 10 following vaccine administration. Spleen cells from individual animals were harvested on day 18 postimmunization.
Spleen cells were cultured with 100 ng of recombinant HIV-1 IIIB gp120 protein per ml; and 72 h later, supernatants were harvested and tested for cytokine production by ELISA. Data are presented as the mean cytokine concentration produced by eight animals per group ± SEM.
We next measured anti-gp120 antibody titers in the sera of these immunized mice to determine whether delayed administration of the IL-12 plasmid augmented vaccine-elicited antibody responses. As shown in Fig. 3B, no significant differences in gp120-specific antibody titers were detected between mice immunized with the gp120 plasmid alone and those receiving vaccine and 200 μg of PMV-IL-12 on day 0, 4, 7, 10, or 14 postimmunization.
Phenotypic analysis of HIV-1 gp120 DNA vaccine-elicited CD8+ T lymphocytes following delayed administration of plasmid IL-12.
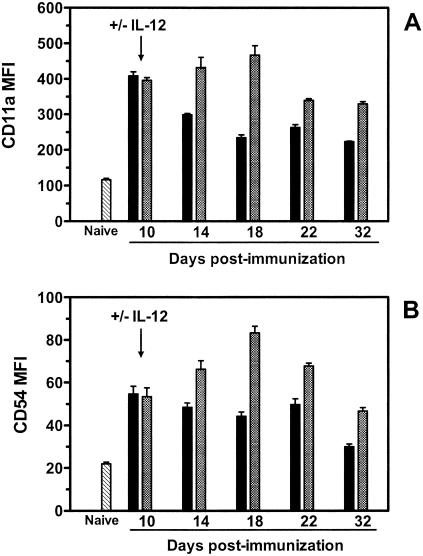
To elucidate the mechanism by which late administration of plasmid IL-12 augments vaccine-elicited CTL responses, we examined p18-specific CD8+ T cells for phenotypic evidence of activation and apoptosis. Groups of mice were immunized with 50 μg of PMV-gp120 alone or PMV-gp120 with 200 μg of PMV-IL-12 delivered on day 10 postimmunization. Spleen cells were harvested from individual mice at multiple times through day 32, and expression of CD11a and CD54 on tetramer-positive CD8+ T lymphocytes was evaluated. CD11a and CD54 are adhesion molecules whose expression is upregulated on T lymphocytes following activation (25). Consistent with this biology, on day 10 post DNA immunization, expression of CD11a and CD54 was higher on p18-specific CD8+ T lymphocytes than on naive CD8+ T lymphocytes (Fig. 4A and B, respectively). Following day 10, expression of these molecules on p18-specific CD8+ T lymphocytes from mice immunized with plasmid gp120 alone began to decline gradually. In contrast, the p18-specific CD8+ T lymphocytes in mice receiving the IL-12 plasmid continued to upregulate CD11a and CD54, with expression peaking on day 18 postimmunization. On day 32 postimmunization, tetramer-positive CD8+ T cells in mice receiving vaccine and the IL-12 plasmid still exhibited higher expression of CD11a and CD54 compared to tetramer-positive CD8+ T cells in mice receiving vaccine alone, and these differences were still detectable more than 100 days postimmunization (data not shown).
FIG. 4.
Phenotypic analysis of p18-specific CD8+ T cells elicited by DNA vaccination with or without delayed administration of plasmid IL-12. BALB/c mice were immunized with 50 μg of PMV-gp120 alone (black bars) or with 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day 10 following vaccine administration (checkered bars). Spleen cells were harvested from individual mice at the indicated times, and the expression of CD11a (A) and CD54 (B) on gated H-2Dd/p18 tetramer-positive CD8+ T cells was measured by flow cytometry. Expression of these molecules on CD8+ T cells from naive animals is also indicated (hatched bars). Data are presented as the mean fluorescence intensity (MFI) and represent the means of four mice per group ± SEM.
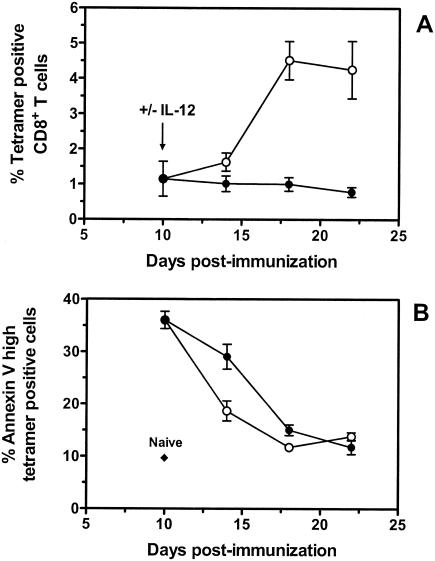
We also examined whether delayed IL-12 plasmid administration influenced the rate of apoptosis in DNA vaccine-elicited CD8+ T cells. Annexin V staining was used to identify p18-specific CD8+ T cells undergoing the early changes of apoptosis (29). In mice immunized with gp120 DNA alone, the peak of annexin V staining on tetramer-positive CD8+ T cells was observed on day 10 (35%) and gradually declined to background levels between days 18 and 22 postimmunization (Fig. 5B). In mice receiving plasmid IL-12 on day 10, a substantial decrease in annexin V-positive, p18-specific CD8+ T cells was measured on day 14 postimmunization compared with mice receiving vaccine alone (19 versus 29%, respectively). This decrease was observed prior to the dramatic expansion of the vaccine-elicited, p18-specific CD8+ T cells that occurred between days 14 and 18 following immunization (Fig. 5A). By day 18 postimmunization, there was an approximately fourfold increase in tetramer-positive CD8+ T cells in plasmid IL-12-treated mice, and annexin V staining of these cells had declined to background levels. Together, these results demonstrate that the delayed administration of plasmid IL-12 postimmunization resulted in an increase in the activation of vaccine-elicited CD8+ T lymphocytes and a decrease in apoptosis of these cells.
FIG. 5.
Annexin V staining of p18-specific CD8+ T cells elicited by DNA vaccination with or without delayed administration of plasmid IL-12. Groups of BALB/c mice were immunized with 50 μg of PMV-gp120 alone (closed circles) or with 50 μg of PMV-gp120 plus 200 μg of PMV-IL-12 on day 10 following vaccine administration (open circles). Spleen cells from individual mice were harvested at the indicated times and stained with anti-CD8 MAb, H-2Dd/p18 tetramer, and annexin V. Data are presented as the percentage of gated CD8+ T cells that bound tetramer (A) and the percentage of gated tetramer-positive CD8+ T cells that bound high levels of annexin V (B), as measured by flow cytometry. The background level of annexin V binding to gated CD8+ T cells from naive animals is also indicated. Data are the mean of four mice per group ± SEM.
p18-specific memory CTL expansion in mice receiving HIV-gp120 DNA vaccine with delayed administration of plasmid IL-12.
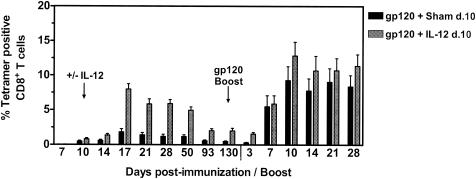
Having demonstrated that delayed administration of plasmid IL-12 significantly enhances the memory pool of plasmid gp120-elicited CD8+ T cells, we sought to assess the ability of these CTL to expand in response to a plasmid gp120 boost immunization. Groups of mice were immunized with 50 μg of PMV-gp120 plus either 200 μg of sham plasmid or 200 μg of PMV-IL-12 delivered on day 10 postimmunization, and all mice were subsequently boosted on day 130 with 50 μg of plasmid PMV-gp120 alone. As previously observed, mice receiving the DNA vaccine with delayed plasmid IL-12 administration exhibited a durable fourfold increase in their primary p18-specific CTL response compared with mice receiving the vaccine plus the sham plasmid (Fig. 6). At the time of boosting on day 130 postimmunization, the percentages of p18-specific CD8+ T cells in the peripheral blood of the plasmid IL-12- and sham plasmid-treated groups of mice were 2.0 and 0.48%, respectively (P < 0.001). Surprisingly, no significant difference between these groups of mice was observed in the magnitude of the peripheral blood p18-specific CTL expansion following the plasmid gp120 boost immunization. On day 10 following the boost immunization, p18-specific CTL responses comprised 12.8 and 9.3% of the CD8+ T cells in the plasmid IL-12- and sham plasmid-treated groups of mice, respectively, and these levels were durable through day 28 postboosting. Comparable p18-specific responses were further observed in these groups of mice following a third plasmid gp120 boost immunization (data not shown).
FIG. 6.
p18-specific memory CD8+-T-cell expansion elicited in mice primed by DNA vaccination with or without delayed plasmid IL-12 administration. Groups of BALB/c mice were immunized with 50 μg of PMV-gp120 plus either 200 μg of sham PMV plasmid or 200 μg of PMV-IL-12 given on day (d.) 10 following vaccine administration. On day 130 postimmunization, mice were boosted with 50 μg of PMV-gp120 plasmid alone. Peripheral blood was obtained from individual mice at the indicated times, and antigen-specific CD8+ T cells were detected with an H-2Dd/p18 tetramer. Data are presented as the percentage of gated CD8+ T cells that bound tetramer, as measured by flow cytometry, and are the means of eight mice per group ± SEM.
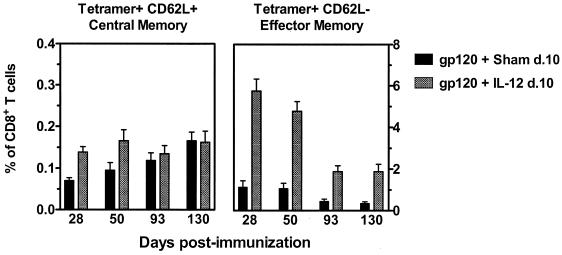
Previous studies have suggested that antigen-specific CD8+ T cells with a central memory phenotype (CD62L+) have a greater capacity to expand in vivo during a recall immune response than do CD8+ T cells with an effector memory phenotype (CD62L−) (31). We therefore examined whether the absence of an increased p18-specific CTL expansion in mice receiving delayed administration of plasmid IL-12 could be explained by the type of memory CTL induced during priming. Phenotypic analysis of peripheral blood p18-specific CD8+ T cells on day 28 post primary immunization demonstrated a significant increase in tetramer-positive CD62L− CD8+ T cells in mice receiving the delayed IL-12 plasmid administration (5.75% of CD8+ T cells) compared with mice receiving the delayed sham plasmid (1.14%) (Fig. 7). While a substantial decay in CTL was observed over time in all vaccinated mice, those receiving the delayed plasmid IL-12 administration still retained fivefold more p18-specific CD62L− CTL at the time of boosting on day 130 than did those receiving the sham plasmid (P < 0.001). In contrast, levels of p18-specific CD62L+ CD8+ T cells were much lower than levels of the p18-specific CD62L− CD8+ T cells and remained relatively constant between days 28 and 130 postimmunization. Importantly, no significant difference in the levels of p18-specific CD62L+ CTL was observed between animals receiving the delayed plasmid IL-12 or the sham plasmid at the time of boosting on day 130. These phenotypic analyses of peripheral blood CTL suggest that delayed administration of plasmid IL-12 resulted in an increase in p18-specific effector memory CD8+ T cells while having no significant effect on the levels of p18-specific central memory CTL.
FIG. 7.
Analysis of p18-specific CD8+-T-cell central memory and effector memory subsets elicited by DNA vaccination with or without delayed administration of plasmid IL-12. Groups of BALB/c mice were immunized as described in the legend to Fig. 6. Peripheral blood was obtained from individual mice at the indicated times, and expression of CD62L on gated H-2Dd/p18 tetramer-positive CD8+ T cells was measured by flow cytometry. Data are presented as the percentage of CD8+ T cells that were either tetramer positive and CD62L+ or tetramer positive and CD62L− and are the means of eight mice per group ± SEM. d., day.
While central memory CTL are believed to traffic primarily within lymphoid compartments, effector memory CTL have been demonstrated predominantly in nonlymphoid tissues (18, 20). To elucidate further whether delayed administration of plasmid IL-12 specifically augmented the generation of effector memory CTL, we analyzed lymphocyte populations isolated from various lymphoid and parenchymal tissues of vaccinated mice for p18-specific CTL. Groups of mice were immunized with 50 μg of PMV-gp120 plus either 200 μg of sham plasmid or 200 μg of PMV-IL-12 administered on day 10, and tissues were harvested on day 34 postimmunization. As shown in Table 2, only a small percentage of p18-specific CD8+ T cells were detected in lymphocytes harvested from peripheral and mesenteric LNs, and these CTL were almost exclusively CD62L+. No significant differences in p18-specific CTL levels were detected in these lymphoid compartments when mice receiving the vaccine plus either IL-12 or sham plasmids were compared. In contrast, a dramatic increase in p18-specific CD8+ T cells was observed in mice receiving delayed plasmid IL-12 treatment in lymphocyte populations isolated from the spleen, blood, bone marrow, peritoneum, lung, and liver. While both CD62L+ and CD62L− p18-specific CTL were detected in these nonlymphoid tissues, substantial augmentations were only observed in the CD62L− p18-specific CTL in the mice receiving delayed plasmid IL-12 administration, ranging from 1.3-fold (peritoneum) to 5.3-fold (blood and lung). Despite these increased levels of CD62L− CTL, the expansions of p18-specific CD8+ T cells in the peripheral tissues of plasmid IL-12- and sham plasmid-treated mice following a plasmid gp120 boost immunization were found to be similar (Table 3). These data further support the hypothesis that delayed plasmid IL-12 administration drives the expansion of vaccine-elicited CD8+ T cells with an effector memory phenotype while having no significant impact on the levels of central memory CTL.
TABLE 2.
Phenotypic analysis of p18-specific CD8+ T cells isolated from various lymphoid and nonlymphoid tissues following DNA vaccination with or without delayed administration of plasmid IL-12
| Tissuea | % of CD8+ T-cell populationb
|
|||
|---|---|---|---|---|
| Tetramer+ CD62L+ central memory
|
Tetramer+ CD62L− effector memory
|
|||
| Sham plasmid | Plasmid IL-12 | Sham plasmid | Plasmid IL-12 | |
| Lymphoid | ||||
| Peripheral LN | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Mesenteric LN | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Spleen | 0.15 ± 0.05 | 0.21 ± 0.03 | 0.53 ± 0.12 | 1.50 ± 0.22 |
| Nonlymphoid | ||||
| Blood | 0.08 ± 0.01 | 0.13 ± 0.04 | 0.71 ± 0.10 | 3.75 ± 0.78 |
| Bone marrow | 0.23 ± 0.07 | 0.31 ± 0.11 | 1.21 ± 0.32 | 2.99 ± 0.56 |
| Peritoneum | 1.12 ± 0.16 | 1.07 ± 0.07 | 4.71 ± 0.89 | 6.06 ± 0.67 |
| Lung | 0.17 ± 0.02 | 0.30 ± 0.04 | 2.43 ± 0.48 | 12.82 ± 1.46 |
| Liver | 0.52 ± 0.13 | 0.64 ± 0.04 | 1.84 ± 0.31 | 6.20 ± 1.04 |
Mice were immunized with 50 μg of PMV-gp120 plus 200 μg of sham PMV plasmid or 200 μg of PMV-IL-12 on day 10 following vaccine administration. The indicated lymphoid and nonlymphoid tissues were harvested from animals on day 34 postimmunization, and lymphocytes were isolated as described in Materials and Methods.
Expression of CD62L on gated H-2Dd/p18 tetramer-positive CD8+ T cells from the indicated tissues was analyzed by flow cytometry. Data are presented as the percentage of CD8+ T cells that were tetramer positive CD62L+ or tetramer positive CD62L− and represent the means of four mice per group ± SEM.
TABLE 3.
p18-specific CD8+ T cells isolated from various lymphoid and nonlymphoid tissues following plasmid gp120 boost immunization
| Tissuea | % Tetramer+ CD8+ T cellsb
|
|
|---|---|---|
| Sham plasmid | Plasmid IL-12 | |
| Lymphoid | ||
| Peripheral LN | 0.46 ± 0.02 | 0.34 ± 0.10 |
| Mesenteric LN | 0.66 ± 0.04 | 0.63 ± 0.19 |
| Spleen | 8.70 ± 0.24 | 10.70 ± 1.06 |
| Nonlymphoid | ||
| Blood | 12.94 ± 1.38 | 15.50 ± 0.08 |
| Bone marrow | 8.76 ± 0.05 | 14.02 ± 2.38 |
| Peritoneum | 29.54 ± 3.29 | 22.53 ± 2.70 |
| Lung | 34.80 ± 3.07 | 53.80 ± 3.56 |
| Liver | 29.70 ± 1.71 | 35.12 ± 1.91 |
Mice were immunized with 50 μg of PMV-gp120 plus 200 μg of sham PMV plasmid or 200 μg of PMV-IL-12 on day 10 following vaccine administration. On day 145 postimmunization, mice were boosted with 50 μg of PMV-gp120. The indicated lymphoid and nonlymphoid tissues were harvested from animals on day 10 following boost immunization, and lymphocytes were isolated as described in Materials and Methods.
Antigen-specific CD8+ T cells were detected by using an H-2Dd/p18 tetramer. Data are presented as the percentage of gated CD8+ T cells that bound tetramer, as measured by flow cytometry, and are the means of four mice per group ± SEM.
DISCUSSION
Long-term immunological protection is determined by both the quantity and quality of memory T cells produced following initial antigen exposure. As the number of memory T cells generated is largely dependent on the magnitude of the primary immune response, CTL-based vaccine strategies have focused on eliciting as large an expansion of antigen-specific CD8+ T cells as possible. One of the approaches being evaluated in this effort is the use of plasmid-encoded cytokines or costimulatory molecules to enhance the proliferation of vaccine-elicited CTL. In the present study, we illustrate the potential for vaccine adjuvants to modulate the formation of central memory and effector memory T-cell subsets, and thus potentially the efficacy of vaccine-elicited immune protection.
We have demonstrated that administration of plasmid IL-12 during the peak phase of an immune response elicited by a DNA vaccine encoding HIV-1 gp120 resulted in a robust expansion of antigen-specific effector CD8+ T cells and a durable increase in the pool of persisting CTL. The time period in which late delivery of plasmid IL-12 could elicit this effect, however, was found to be very restricted. Optimal CTL expansion occurred with cytokine plasmid delivery between days 10 and 12 postimmunization, just prior to the peak CTL response. Cytokine plasmid delivery within this time period may provide optimal levels of IL-12 at the peak of the vaccine-elicited immune response, causing an expansion of activated CTL.
We studied in detail the mechanism by which delayed IL-12 administration augmented antigen-specific CTL responses. It has been suggested that apoptosis induced by extrinsic factors such as cytokine starvation may play a major role in the downregulation of effector T-cell responses (13). The present data suggest that providing IL-12 within the time period in which activated CD8+ T cells are susceptible to elimination may provide an activating signal that induces cellular proliferation, inhibits induction of apoptosis, and allows long-term survival. These data are supported by observations that IL-12 can induce the proliferation of previously activated T cells (7) and also prevent Fas-mediated apoptosis (16, 17, 19).
Why late administration of IL-12 specifically augmented antigen-specific CTL yet had minimal effects on CD4+ T helper responses remains uncertain. However, accumulating evidence suggests that the expansion and contraction phases of CD8+- and CD4+-T-cell responses are differentially regulated. A study comparing the responses of these two lymphocyte subsets in the setting of lymphocytic choriomeningitis virus infection demonstrated that the primary expansion of CD8+ T cells vastly exceeded that of CD4+ T cells. This CD8+-T-cell population subsequently underwent rapid contraction within a 1- to 2-week period, forming a stable population of memory cells. In contrast, antigen-specific CD4+ T cells had a more delayed and protracted phase of downregulation that was characterized by a persistent decline in the memory T-cell population (10). Thus, the stimulatory effect of late IL-12 administration may have a larger impact on the preservation and maintenance of effector CD8+ T cells than on CD4+ T cells.
While delayed administration of plasmid IL-12 generated a three- to fourfold increase in the pool of vaccine-elicited memory CTL, no significant difference in the magnitude of the secondary antigen-specific CTL response was observed between mice primed with gp120 alone and those primed with gp120 and plasmid IL-12. The absence of a difference between these animal groups in their CTL expansion following a boost immunization can most likely be explained by our observation that the IL-12-treated mice had increased levels of CTL with an effector memory phenotype, while levels of CTL with a central memory phenotype were comparable to those observed in mice that did not receive cytokine. While previous studies have demonstrated that central and effector memory CD8+ T cells in mice have similar profiles of cytokine secretion and cytolytic activity, central memory CTL have been shown to have a greater capacity to expand rapidly in vivo following a pathogenic infection and are able to confer better immune protection than effector memory CTL (28, 31). This difference has been attributed to the ability of central memory CD8+ T cells to produce higher levels of IL-2 than effector memory CTL (20, 31). The findings in the present study are consistent with this previously described inability of effector memory CTL to expand efficiently in response to secondary antigen exposure. While substantial clonal expansion of memory CTL is critical for the containment of many pathogenic infections, effector memory CTL present at the site of initial infection may contribute as an immediate line of defense. Whether the increased levels of effector memory CTL in plasmid IL-12-treated mice can significantly enhance protection against a pathogenic-virus challenge is under investigation.
A recent study has provided evidence supporting a model of CTL differentiation in which newly activated CD8+ T cells follow a stereotypic pathway of evolution: naive→effector→effector memory→central memory (31). This model suggests that effector memory CD8+ T cells are not a distinct subset but a transitional cell population that will, with time, reacquire the ability to produce IL-2 as it evolves to central memory CTL. While we observed a progressive decline in antigen-specific CD62L− effector memory CTL between days 28 and 130 postimmunization in the peripheral blood of plasmid IL-12-treated and untreated mice, an increase in the central memory subset of CTL was observed only in mice immunized with gp120 alone. Mice immunized with the DNA vaccine plus delayed plasmid IL-12 demonstrated consistent levels of antigen-specific central memory CTL, even when the analysis period was extended to day 240 postimmunization (data not shown). These data suggest that activation of CD8+ T cells during the peak phase of the immune response may drive their terminal differentiation into effector memory CTL.
Current methods for evaluating cellular immune responses elicited by vaccination primarily rely on quantitating antigen-specific T cells, usually by tetramer staining and peptide IFN-γ ELISPOT assays. Our results suggest that the type of memory CTL generated following immunization is also an important parameter to be monitored in studies analyzing various vaccine strategies. Since a robust expansion of vaccine-elicited memory CTL is critical for controlling viral replication following infection, vaccination strategies that augment the generation of central memory CD8+ T cells are likely to be most effective in conferring protection. It will be important to gain a better understanding of the mechanisms that influence the generation of central and effector memory CD8+ T cells and to develop strategies that specifically amplify populations of central memory CTL.
Acknowledgments
We thank Kristi Martin, Carol Lord, and Ayako Miura for excellent technical assistance and Dan H. Barouch for critical review of the manuscript.
This work was supported by NIH grant CA-50139 and by funds provided by Wyeth-Lederle vaccines. The HIV-1 IIIB gp120 overlapping peptides were provided by the EU Program EVA/MRC Centralized Facility for AIDS Reagents, National Institute for Biological Standards and Control, United Kingdom (grants QLK2-CT-1999-00609 and GP828102).
REFERENCES
- 1.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J.-H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed]
- 2.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M. E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875-1882. [PubMed] [Google Scholar]
- 4.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. W., C. H. Pan, H. W. Huan, M. Y. Liau, J. R. Chiang, and M. H. Tao. 2001. Suppression of immune response and protective immunity to a Japanese encephalitis virus DNA vaccine by coadministration of an IL-12-expressing plasmid. J. Immunol. 166:7419-7426. [DOI] [PubMed] [Google Scholar]
- 6.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gately, M. K., B. B. Desai, A. G. Wolitzky, P. M. Quinn, C. M. Dwyer, F. J. Podlaski, P. C. Familletti, F. Sinigaglia, R. Chizonnite, U. Gubler, et al. 1991. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J. Immunol 147:874-882. [PubMed] [Google Scholar]
- 8.Gherardi, M. M., J. C. Ramírez, and M. Esteban. 2000. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 Env antigen in a DNA prime/vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. J. Virol. 74:6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 10.Homann, D., L. Teyton, and M. B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 11.Iezzi, G., D. Scheidegger, and A. Lanzavecchia. 2001. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J. Exp. Med. 193:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki, A., B. J. Stiernholm, A. K. Chan, N. L. Berinstein, and B. H. Barber. 1997. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J. Immunol. 158:4591-4601. [PubMed] [Google Scholar]
- 13.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. J., N. N. Trivedi, L. K. Nottingham, L. Morrison, A. Tsai, Y. Hu, S. Mahalingam, K. Dang, L. Ahn, N. K. Doyle, D. M. Wilson, M. A. Chattergoon, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 28:1089-1103. [DOI] [PubMed] [Google Scholar]
- 15.Koblish, H. K., C. A. Hunter, M. Wysocka, G. Trinchieri, and W. M. Lee. 1998. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J. Exp. Med. 188:1603-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. W., Y. Park, J. K. Yoo, S. Y. Choi, and Y. C. Sung. 2003. Inhibition of TCR-induced CD8 T cell death by IL-12: regulation of Fas ligand and cellular FLIP expression and caspase activation by IL-12. J. Immunol. 170:2456-2460. [DOI] [PubMed] [Google Scholar]
- 17.Marth, T., M. Zeitz, B. R. Ludviksson, W. Strober, and B. L. Kelsall. 1999. Extinction of IL-12 signaling promotes Fas-mediated apoptosis of antigen-specific T cells. J. Immunol. 162:7233-7240. [PubMed] [Google Scholar]
- 18.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, E. M., L. Farrokh-Siar, J. Maguire van Seventer, and G. A. van Seventer. 2001. IL-12 decreases activation-induced cell death in human naive Th cells costimulated by intercellular adhesion molecule-1. I. IL-12 alters caspase processing and inhibits enzyme function. J. Immunol. 167:749-758. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 21.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 23.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 24.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]
- 25.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 26.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H. X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 167:5386-5394. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 28.Unsoeld, H., S. Krautwald, D. Voehringer, U. Kunzendorf, and H. Pircher. 2002. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 169:638-641. [DOI] [PubMed] [Google Scholar]
- 29.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 30.Weninger, W., M. A. Crowley, N. Manjunath, and U. H. von Andrian. 2001. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194:953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]