Abstract
The RNA N-glycosidase ribosome inactivating proteins (RIPs) constitute a ubiquitous family of plant- and bacterium-derived toxins that includes the category B select agents ricin, abrin and shiga toxin. While these toxins are potent inducers of intestinal epithelial cell death and inflammation, very little is known about the mechanisms underlying mucosal immunity to these toxins. In the present study, we report that secretory IgA (SIgA) antibodies are not required for intestinal immunity to ricin, as evidenced by the fact that mice devoid of SIgA, due to a mutation in the polymeric immunoglobulin receptor, were impervious to the effects of intragastric toxin challenge following ricin toxoid immunization. Furthermore, parenteral administration of ricin-specific monoclonal IgGs, directed against either ricin’s enzymatic subunit (RTA) or binding subunit (RTB), to wild type mice were as effective as monoclonal IgAs with comparable specificities in imparting intestinal immunity to ricin. These data are consistent with reports from others demonstrating that immunization of mice by routes known not to induce mucosal antibody responses (e.g., intramuscular and intradermal) are sufficient to elicit protection against both systemic and mucosal ricin challenge.
Keywords: biodefense, vaccine, mucosal, toxin, antibody
1. INTRODUCTION
The intestinal epithelium is constantly exposed to bacterium- and plant-derived toxins, many of which are capable of inducing epithelial destruction, severe mucosal inflammation, and often systemic complications. One of the most notorious classes of epithelial-damaging toxins is the RNA N-glycosidases, or ribosome inactivating proteins (RIPs). Although diverse in structure, the RNA N-glycosidases share a common mode of action: selective depurination of a conserved adenine residue within the so-called sarcin/ricin loop (SRL) of eukaryotic 28S ribosomal RNA[1]. Hydrolysis of the SRL results in an immediate arrest in ribosome progression and complete loss in protein translation [1]. The RNA N-glycosidase family includes shiga toxins (Stx), produced by Shigella dysenteriae and certain strains of foodborne enterotoxigenic Escherichia coli, as well as the plant-derived toxins, ricin and abrin. Ricin, which is found at high concentrations in the seeds of the castor bean plant, Ricinus communis, consists of a 32 kDA enzymatic A subunit (RTA) joined by a disulfide bond to a 34-kDA lectin B subunit (RTB). RTB binds to α(1–3)-linked galactose and N-acetylgalactosamine residues on the surface of almost every cell type, and it mediates toxin internalization via both clathrin-dependent and clathrin-independent mechanisms [2–4]. Once internalized, the toxin exploits multiple endocytic pathways, and traffics in a retrograde fashion from early endosomes to the trans-Golgi network, eventually reaching the endoplasmic reticulum (ER) [5, 6]. In the ER, RTA and RTB dissociate, and the A-subunit is retro-translocated across the ER membrane to the cytoplasm where is gains access to ribosomal RNA [7, 8].
The sensitivity of the intestinal epithelium to ricin, and related RNA N-glycosidases, has been recognized for more than 100 years [9]. Rats challenged with ricin by gavage, for example, develop dose-dependent lesions in the proximal small intestine, including widespread villus atrophy, crypt elongation, sloughing of the epithelium, and occasional infiltration of inflammatory cells, including eosinophils and neutrophils [10–13]. We recently reported that similar histopathologic changes occur in mice following intragastric ricin challenge, although we failed to detect any evidence of mucosal ulceration and only rarely observed neutrophils in the intestinal lumen [14]. Epithelial cells themselves are directly affected by the toxin, based on the fact that application of ricin to the apical surfaces of polarized intestinal epithelial cell monolayers in vitro results in an arrest of protein synthesis within 3–4 hrs [15]. Others have shown that ricin activates cellular stress-activated protein kinase pathways (SAPKs) in intestinal epithelial cells, and induces them to secrete an array of pro-inflammatory cytokines [16–19]. These data suggest that ricin-induced epithelial destruction may be the consequence of direct cytotoxicity in combination with a local, acute inflammatory response.
Although it is well established that immunity to ricin is strictly antibody-mediated, neither the antibody classes nor the antibody specificities involved in protection of the intestinal epithelium has been determined. In a previous report, we demonstrated that mice immunized intragastrically (i.g.) with ricin toxoid (RT) were impervious to the effects of a ricin challenge administered by gavage, and that protection was associated with elevated levels of anti-toxin IgA antibodies in fecal pellets and anti-toxin IgG antibodies in serum. We subsequently produced a collection of RTA-specific and RTB-specific monoclonal IgA and IgG antibodies (MAbs) from the Peyer’s patches and spleens of RT-immunized mice and characterized these MAbs in vitro [15, 20]. Those studies identified a number of RTA-specific and RTB-specific IgA and IgG MAbs that were capable of neutralizing ricin in vitro, as determined using a Vero cell cytotoxicity assay. The MAbs also protected polarized epithelial cell monolayers from toxin-induced cell death, but only when the MAbs were applied to the apical (not basolateral) aspects of the epithelial monolayers [15]. The RTB-specific monoclonal IgGs (e.g., 24B11) and IgAs (e.g., 33G2, 35H6) blocked ricin attachment to polarized epithelial cell monolayers and inhibited binding to the luminal aspects of human duodenum, indicating that this class of antibodies neutralizes ricin by preventing engagement of the toxin with host cell receptors. The RTA-specific monoclonal IgGs (e.g., R70, GD12) and IgAs (e.g., 23D7, 25A4), on the other hand, did not interfere with toxin attachment to cell surfaces, indicating that these MAbs neutralize ricin by an alternative, as of yet unknown, mechanism.
We have postulated, based primarily on our in vitro studies, that only SIgA is present in the intestinal lumen at sufficient concentrations to effectively protect the epithelium from ricin. However, Vitetta and colleagues recently reported that mice immunized with a candidate RTA subunit vaccine by the intramuscular (i.m.) or intradermal (i.d.) routes were protected against a lethal dose of ricin by the oral route [21, 22]. Those mice had high-titer serum antitoxin antibody levels. While those investigators did not measure SIgA in intestinal secretions of vaccinated mice, i.m. or i.d. immunizations generally do not elicit mucosal antibody responses. Therefore, those findings suggest that serum antibodies play a role in protecting the intestinal epithelium from ricin intoxication in vivo. We wished to examine the relative contributions of SIgA and IgG in intestinal immunity to ricin in detail, particularly in light of the fact that relatively little is known in general about toxin-antibody interactions at mucosal surfaces. Taking advantage of a recently developed mouse model of intestinal ricin intoxication, in conjunction with available polymeric immunoglobulin knock-out mice lacking SIgA, as well as our collection of ricin-specific IgA and IgG MAbs, we report here that IgG antibodies directed against either ricin’s enzymatic subunit or binding subunit are sufficient to protect the intestinal epithelium from the effects of ricin. This study contributes to an emerging body of literature demonstrating the importance of serum antibodies in mucosal immunity to a variety of pathogenic and toxic agents, and has implications for the development of vaccines and immunotherapeutics against the family of RNA N-glycosidases, three of which are considered potential biotreat toxins.
2. MATERIALS AND METHODS
2.1 Chemicals and reagents
Ricin (Ricinus communis agglutinin II) was purchased from Vector Laboratories (Burlingame, CA). RiVax™ was kindly provided by Dr. Robert Brey (Soligenix, Inc., Princeton, NJ). Phenylmethylsulphonylfluoride (PMSF) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Tween-20 was obtained from BioRad (Torrance, CA), and protease inhibitor cocktails were purchased from Calbiochem-EMD Biosciences (La Jolla, CA). Paraformaldehyde (16%) was purchased from Electron Microscopy Sciences (Fort Washington, PA), and Bouin’s fixative was obtained from Krackeler Scientific (Albany, NY). Dialysis was performed using Slide-a-lysers from Pierce Chemical (Rockford, IL).
2.2 Production of ricin toxoid (RT)
To produce RT, ricin (1 mg/ml) was dialyzed in a Slide-a-lyzer dialysis unit (MWCO 10,000; Pierce Chemical) against 4% paraformaldehyde for 18 hr at 47°C, followed by 30 hr at 42°C. Dialysis was then continued against 0.1 M glycine for 4 days in the cold room, to quench residual paraformaldehyde in the RT preparations. RT preparations (1 mg/ml) were stored at minus 80°C and were thawed immediately prior to use.
2.3 Hybridomas and MAbs
The hybridomas UNIVAX 70/138 (hereafter referred to as R70), originally described by Lemley et al. [23], and TFTB-1, originally described by Fulton and colleagues [24], were purchased from the ATCC (Manassas, VA) and were maintained in CD Hybridoma serum-free, protein-free, antibiotic-free medium (Gibco-Invitrogen, Carsbad, CA). All other ricin-specific IgG and IgA MAbs were produced in our laboratory and have been described previously [15, 20]. IgG MAbs were purified from serum-free, protein-free hybridoma supernatants by means of a HiTrap Protein G sepharose column (GE Healthcare Life Sciences, Piscataway, NJ). Purity of the MAb preparations was determined by SDS-PAGE, and concentrations determined by absorbance spectroscopy [25]. Antibody preparations were endotoxin-free, as determined by the Limulus Amebocyte Lysate assay (BioWhittaker, Walkersville, MD).
2.4 Intragastric ricin challenge and tissue collection
All animals used in this study were housed under conventional, specific pathogen-free conditions and were treated in strict compliance with guidelines established by the Institutional Animal Care and Use Committee (IACUC) at the Wadsworth Center. Wild-type BALB/c and pIgR knock-out (BALB/c-pIgRtm1 or pIgR−/−) mice (females, 5–6 weeks of age) were purchased from Taconic Laboratories (Hudson, NY). β2 microglobulin deficient (β2−/−) mice (females, 5–6 weeks of age) [26] and C57Bl/6 age and sex-matched control animals were purchased from Jackson Laboratories (Bar Harbor, ME).
For RT immunization studies, groups of mice (6–10 mice per group) received RT (50 µg per animal per immunization) without adjuvant by the i.g. route three times at 10–14 day intervals. For RiVax studies, groups of mice (6–10 mice per group) were immunized with RiVax adsorbed to alum (10 µg/dose) by the subcutaneous route (s.c.) three times at 10–14 day intervals. Ricin-specific serum and fecal antibody titers were determined by ELISA, as described previously [14, 15]. Toxin challenge studies were performed 10–14 days following the last immunization, and involved administration of ricin (5 mg/kg diluted in PBS) to mice by the i.g. route using a 22G × 1.5-inch blunt-end feeding needle (Popper Scientific, New Hyde Park, NY)[14]. Twenty-four hours later, the animals were euthanized by CO2 asphyxiation. Freshly excised segments of the proximal small intestines of the mice were immersed in Bouin’s fixative and embedded in paraffin, or homogenized in ice-cold cell lysis buffer (Cell Signaling, Beverly, MA) supplemented with protease inhibitors, and then frozen at −20°C. MCP-1 levels in intestinal homogenates were determined by the BD cytometric bead array (CBA) flex set (BD Biosciences, San Jose, CA), as described previously [14]. Flow cytometric analysis was done using a FACSCalibur in the Wadsworth Center Immunology Core. Hematoxylin and eosin (H&E) stained sections of the small intestine were scored for ricin intoxication according to a 12-point histologic grading system[14]. Tissue sections were scored for ricin intoxication using a 12-point histological grading system based on the severity and extent of alterations in villus shape (width and height), lamina propria edema, interepithelial swelling, and the presence of cellular infiltrate in the intestinal lumen. Tissue section samples were coded and blinded prior to being provided to investigators for scoring.
2.5 Passive protection studies
MAbs were passively administered to mice by either of two methods. The “backpack tumor” model, used widely as a means to promote pIgR-mediated delivery of dimeric monoclonal IgA antibodies into intestinal secretions [27–30], involved the s.c. implantation of ~2 × 106 MAb-secreting hybridoma cells into the scruff of each mouse, using a 1-cc syringe and 25G needle. The levels of specific MAbs in serum and fecal extracts of these mice were then monitored over the course of the experiment. Mice were challenged with ricin ~10 days after tumor implantation. Alternatively, purified, individual MAbs diluted into endotoxin-free PBS (0.4 ml final vol.) were administered to mice by i.p. injection using a 1 cc syringe equipped with a 22G needle.
2.6 Statistical analysis
Statistical analysis was carried out with Excel 2003 (Microsoft, Redmond, WA) and SigmaStat 3.5 (Systat Software, San Jose, CA). Protection studies were analyzed using Student’s t-tests, one-way ANOVA and/or Tukey’s post-hoc tests.
3. RESULTS
3.1. Intestinal immunity to ricin in the absence of SIgA
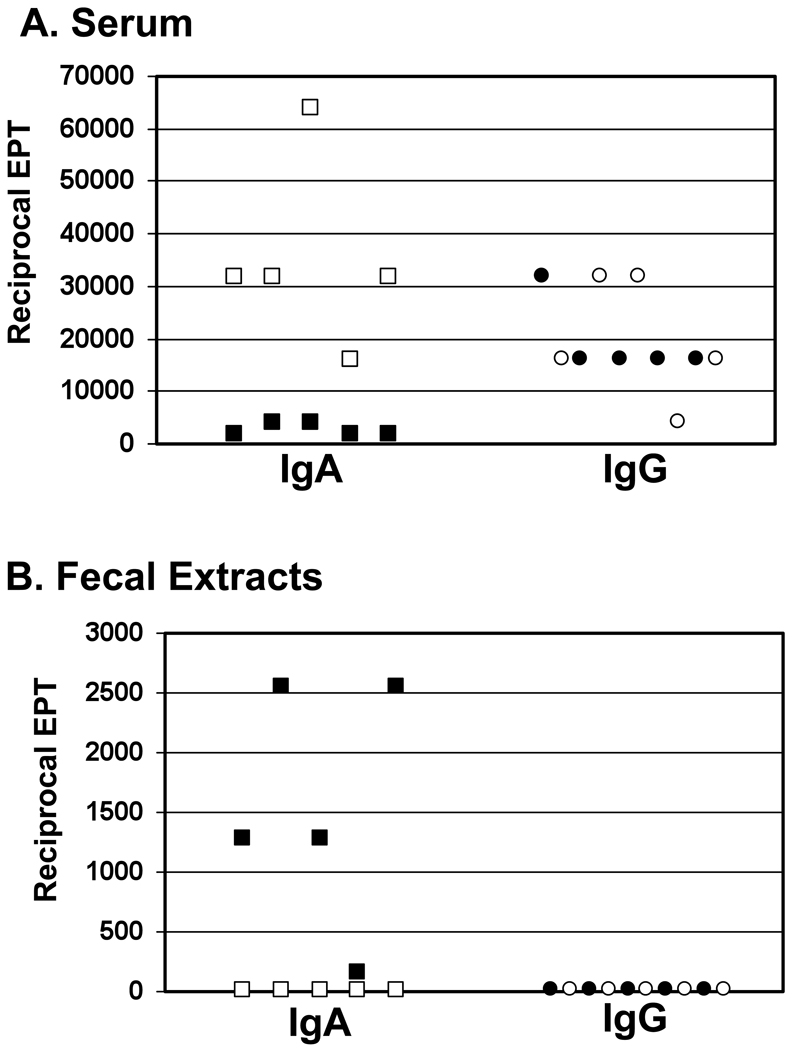
To examine the roles of SIgA and serum IgG in conferring intestinal immunity to ricin, we immunized groups of wild type and polymeric immunoglobulin knock-out (pIgR−/−) mice with RT by the i.g. route three times at 10–14 day intervals. The pIgR−/− mice are effectively devoid of SIgA (and SIgM) due to a targeted deletion in exon 3 of the PIGR locus [31]. Following the third immunization, we measured ricin-specific IgG and IgA titers in serum and fecal extracts of both wild type and pIgR−/− mice. Toxin-specific IgG and IgA antibodies were detected in the sera of both groups of animals (Fig. 1A), demonstrating that wild type and pIgR−/− mice each responded to RT-immunization. Serum IgA titers in pIgR−/− mice were ~10 fold elevated relative to titers in wild type mice, a phenomenon that has been previously been attributed to the fact that pIgR−/− mice are unable to excrete polymeric immunoglobulin into bile [31, 32]. In fecal pellet extracts, toxin-specific IgA antibodies were detected in RT-immunized wild-type mice (reciprocal EPT >160), but not RT-immunized pIgR−/− mice (reciprocal EPT <20) (Fig. 1B), thus confirming the lack of SIgA in pIgR−/− mice. We were unable to detect toxin-specific IgG in the fecal pellet extracts of either RT-immunized wild type or pIgR−/− mice (Fig. 1B), consistent with IgG not being actively transported into intestinal secretions of adult mice [33].
Fig. 1. Anti-toxin serum and fecal antibody titers in wild type and pIgR−/− mice following immunization with ricin toxoid.
Groups of wild type (solid symbols) and pIgR−/− (open symbols) mice (n=5–8 mice per group) were immunized 3 times with RT at 10–14 day intervals by the i.g. route. Seven days after the final immunization, anti-toxin IgA and IgG levels were measured in (A) serum and (B) fecal extracts. Each symbol represents an individual mouse. EPT, end point titer.
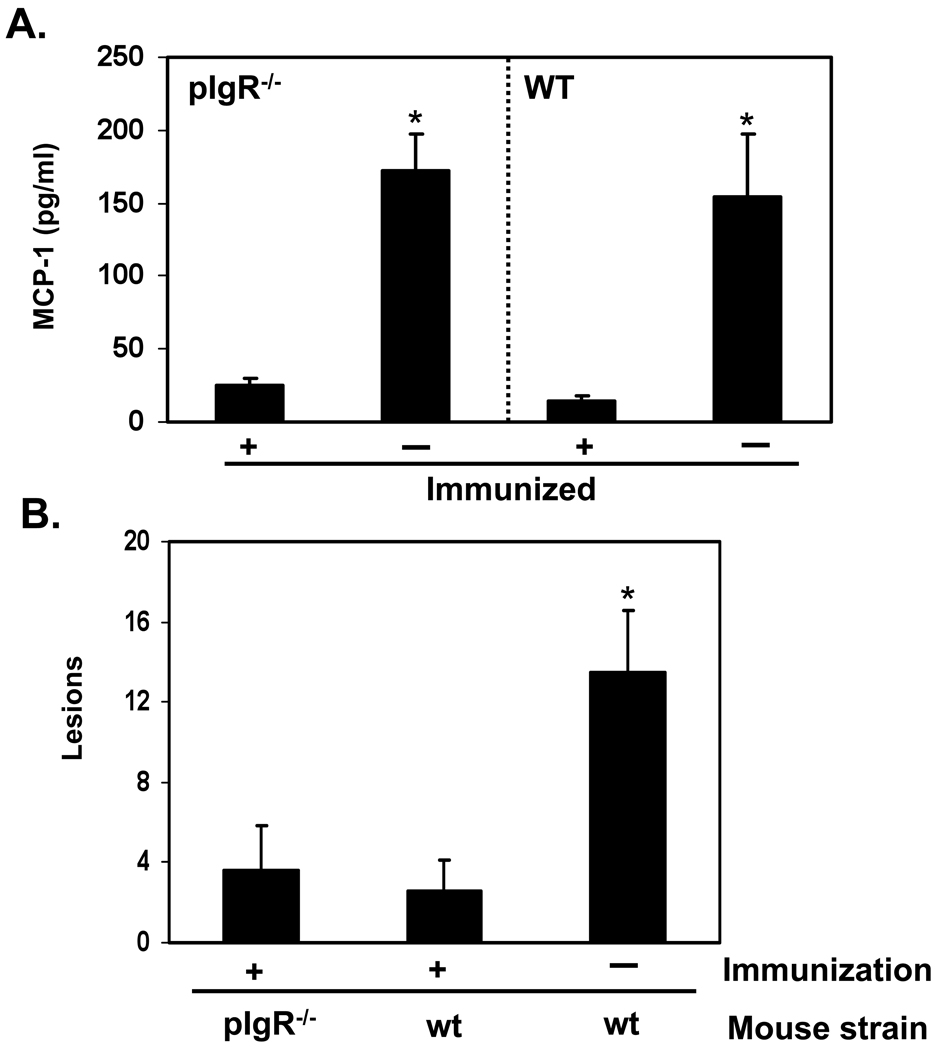
RT-immunized wild-type and pIgR−/− mice were then challenged i.g. with ricin (5 mg/kg per animal). The animals were euthanized 24 hr after challenge, and protection was determined by measurement of MCP-1 levels in intestinal homogenates, and scoring of H&E stained paraffin sections for tissue damage. We previously reported that MCP-1 concentrations in intestinal homogenates serve as a surrogate marker of ricin-induced mucosal damage [14]. Accordingly, MCP-1 levels in intestinal homogenates from ricin-challenged, sham-immunized wild-type and pIgR−/− mice were >160 pg/ml (Fig. 2A), whereas MCP-1 levels in control, unchallenged mice were <20 pg/ml. Wild-type, RT-immunized mice were immune to the effects of ricin challenge, as reflected by the fact that MCP-1 levels in their intestinal homogenates were identical to those observed in unchallenged control mice (<20 pg/ml) (Fig. 2A). Surprisingly, MCP-1 levels in intestinal homogenates from toxin-challenged, RT-immunized pIgR−/− mice were also <20 pg/ml, suggesting that mice devoid of SIgA were protected against toxin-induced mucosal damage. Analysis of H&E stained intestinal tissue sections supported that conclusion: tissues sections from RT-immunized, ricin-challenged wild type and pIgR−/− mice were histologically indistinguishable from unchallenged control animals (Fig. 2B, 3A–B). In contrast, tissue sections from ricin-challenged, sham-immunized mice revealed widespread blunting of intestinal villi, swelling of inter-epithelial spaces, and separation of the epithelium from the lamina propria (Figs. 2B, 3C). These data demonstrate that SIgA is not necessary for conferral of intestinal immunity to ricin, and they suggest that protection is instead mediated by toxin-specific serum IgG.
Fig. 2. Intestinal immunity to ricin in the absence of SIgA.
Groups of wild type and pIgR−/− mice (5–8 mice per group) were immunized (+) or not (−) with RT, as described in the legend of Fig. 1, and then challenged 7–10 days later with ricin (5 mg/kg) by the i.g. route. Intestinal tissues were collected 24 hr later. (A) MCP-1 levels were determined in intestinal homogenates. (B) H&E stained tissue sections were scored for lesions, as described in the Materials and Methods. The asterisks indicate that MCP-1 levels (A) and intestinal lesions (B) in control, toxin-challenged mice were statistically different than RT-immunized mice (p<0.05), as determined by the Student’s t test.
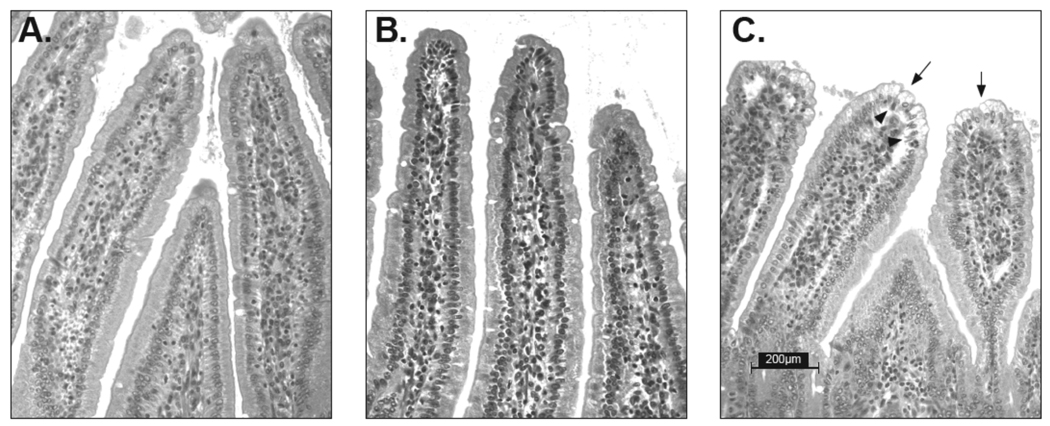
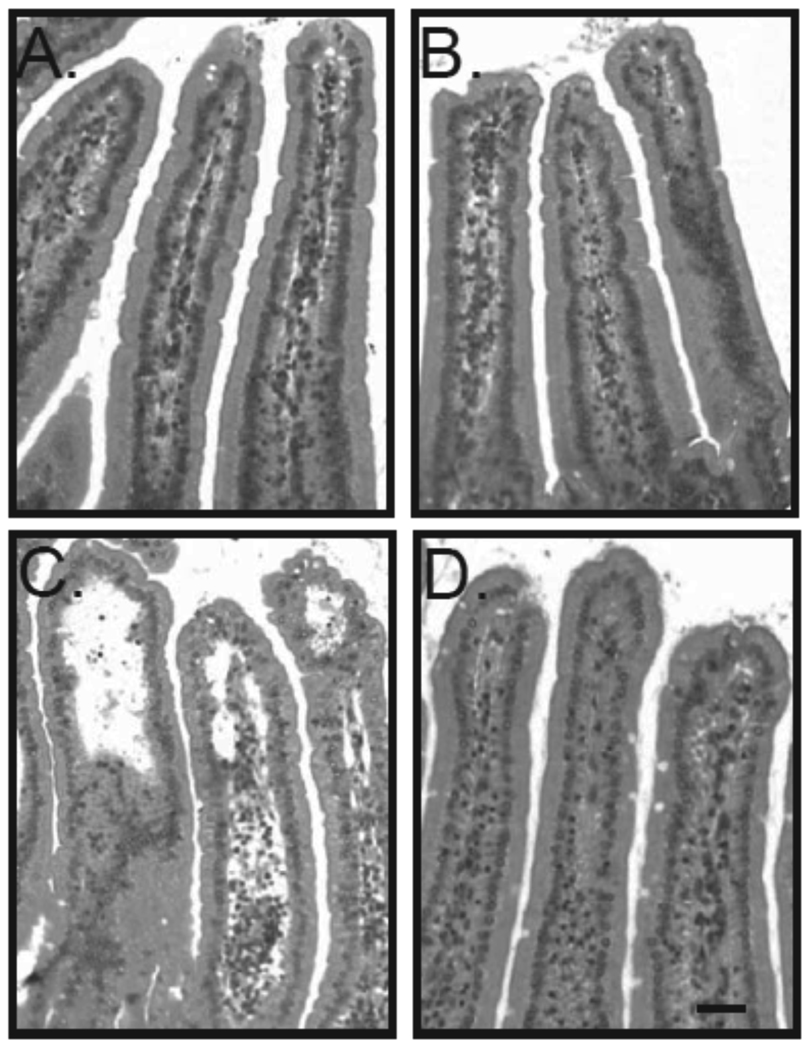
Fig. 3. Absence of ricin-induced epithelial damage in pIgR knock-out mice.
Representative images of H&E stained tissue sections from ricin-challenged control, and RT-immunized wild type and pIgR−/− mice, as described in the legends of Figures 1 and 2. (A) RT-immunized, ricin-challenged wild type Balb/c mice; (B) RT-immunized, ricin-challenged pIgR−/− mice; (C) ricin-challenged, control Balb/c mice. Arrows indicate evidence of epithelial cell vacuolization; arrowheads indicated sites of epithelial detachment. The images were originally captured using a 25 × objective. Scale bar equals 200 µm.
A second series of experiments was performed that supported the above conclusion. Specifically, groups of wild type and pIgR−/− mice were immunized three times with RT by intraperitoneal injection (i.p.), a delivery route known not to elicit SIgA antibodies in intestinal secretions, rather than the i.g. route used for the experiments described above. We found that i.p. immunization with ricin elicited high titer anti-toxin IgG antibodies in serum, but no measurable toxin-specific SIgA or IgG in fecal extracts (data not shown). The two groups of RT-immunized mice were then challenged i.g. with ricin and examined for intestinal damage 24 hr later. In agreement with the results described above, we observed no evidence of epithelial or mucosal damage in either RT-immunized wild type or RT-immunized pIgR−/− mice.
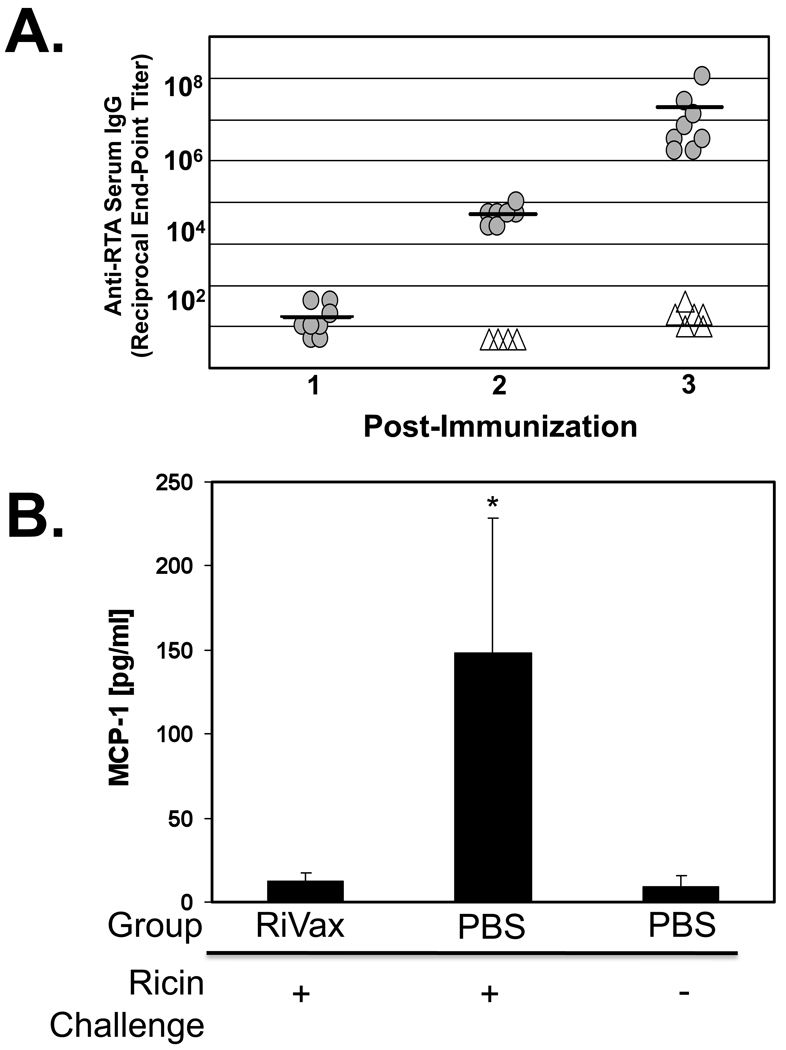
We performed a final set of experiments to examine whether a candidate ricin vaccine, namely RiVax, administered parenterally by a route known not to stimulate mucosal antibodies was sufficient to confer intestinal immunity to ricin. Groups of mice were immunized subcutaneously (s.c.) three times at two week intervals with RiVax (10 µg/dose), and then challenged 7 days later with ricin administered by gavage. RiVax immunization stimulated anti-toxin IgG antibodies in serum (Fig. 4A) but no detectable IgG or IgA in fecal pellets (data not shown). RiVax immunized mice proved to be immune to the effects of intragastric ricin challenge (Fig. 4B), supporting the conclusion that serum IgG is sufficient to confer intestinal immunity to ricin. Moreover, these data confirm the findings of Vitetta and colleagues who previously demonstrated in a lethal dose challenge model that parenteral immunization with RiVax elicits both systemic and mucosal immunity to toxin challenge [21, 22].
Fig. 4. Intestinal immunity to ricin following parenteral immunization with RiVax.
Groups of Balb/c mice (n= 6–9/group) were immunized by the s.c. route three times with RiVax (10 µg/immunization) or PBS. Serum was collected 7–10 days following each immunization. (A) Anti-ricin serum IgG antibody titers were determined by ELISA, as described in Materials and Methods. Shaded circles reflect immunized mice, whereas open triangles reflect sham-immunized controls. Each symbol represents a single mouse. The solid, horizontal bars reflect the average of the groups. (B) Groups of RiVax or sham immunized mice were challenged with ricin (5 mg/kg) by gavage. A third group of mice not immunized or challenged served as negative controls for this experiments. MCP-1 levels in intestinal tissues from all three groups of mice were determined 24 hr following intragastric ricin challenge, as described in Materials and Methods. The asterisks indicate that MCP-1 levels in control, toxin-challenged mice were statistically different from RiVax-immunized mice (p<0.05), as determined by the Student’s t test.
3.2 Passively administered IgG MAbs are sufficient to confer intestinal immunity to ricin
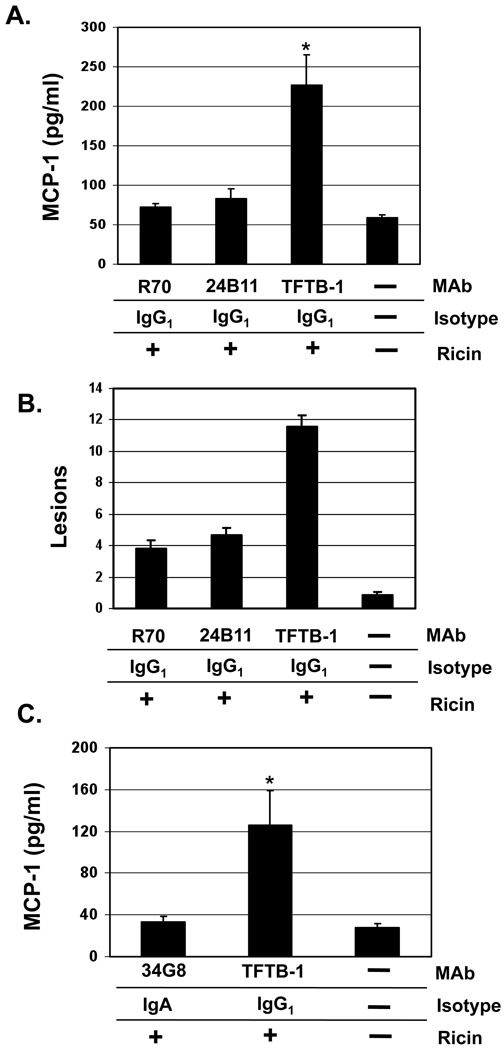
To more fully investigate the possibility that serum IgG is sufficient to confer intestinal immunity to ricin, we took advantage of our collection of well-characterized ricin-specific IgG and IgA MAbs to perform passive protection studies in wild type mice. BALB/c mice were passively administered MAbs R70, 24B11, or TFTB-1, using the so-called backpack tumor model (see Materials and Methods) [28–30, 34, 35], and were then challenged i.g. with ricin. Proximal small intestines from these animals were collected 24 hr after ricin challenge, and were assayed for MCP-1 and scored for damage. MAbs R70 and 24B11 are IgG1s directed against RTA and RTB, respectively, that have been shown to neutralize ricin [20]. TFTB-1 is an IgG1 specific for RTB that does not neutralize ricin, and was therefore used as a negative control in these studies [20].
MCP-1 levels in intestinal tissues from TFTB-1 treated mice 24 hr after ricin challenge were significantly elevated, as compared to levels from unchallenged, control mice, confirming that mice treated with this negative control MAb were susceptible to the effects of ricin (Fig. 5A). In contrast, MCP-1 levels in intestinal tissues from R70-treated or 24B11-treated mice collected 24 hr after toxin challenge were at baseline (Fig. 5A), indicating that R70- treated and 24B11-treated animals were immune to ricin intoxication. Comparative analysis of intestinal tissue sections supported that result: widespread epithelial damage was observed on sections derived from TFTB-1-treated, ricin-challenged mice, whereas intestinal tissues from R70- treated or 24B11-treated, ricin-challenged animals were largely indistinguishable from control tissues (Figs. 5B, 6). Furthermore, R70 and 24B11 were no less effective than 34G8, a ricin-neutralizing, RTB-specific IgA MAb directed against the RTB, in protecting the intestinal epithelium from the effects of ricin (Fig. 5C). Histologic examination of tissues from 34G8 mice confirmed the integrity of the epithelium following ricin challenge (data not shown).
Fig. 5. Passively administered toxin-specific IgG MAbs protect the intestinal epithelium from ricin.
Groups of female BALB/c mice bearing hybridoma backpacks secreting the indicated IgG or IgA MAbs were challenged i.g. with ricin (5 mg/kg). 24 hr later, the mice were euthanized and intestinal tissues were assessed for MCP-1 (panels A, C) or scored for mucosal lesions (panel B). Each column represents the average values (+/− s.d.) of a single experiment with a total 5–8 mice per group. The asterisks in panels A and C indicate that the mean MCP-1 concentration is significantly greater (p<0.05) than the mean concentration determined from untreated control animals (far right columns in panels A, C).
Fig. 6. Anti-ricin IgG MAbs protect against toxin-induced epithelial damage.
Representative images of intestinal tissues collected 24 hr after mice carrying backpack tumors secreting IgG MAbs (A) R70, (B) 24B11, or (C) TFTB-1 were challenged with ricin. Panel D shows tissues from a control mouse that had not been challenged with ricin. Scale bar equals 200 µm.
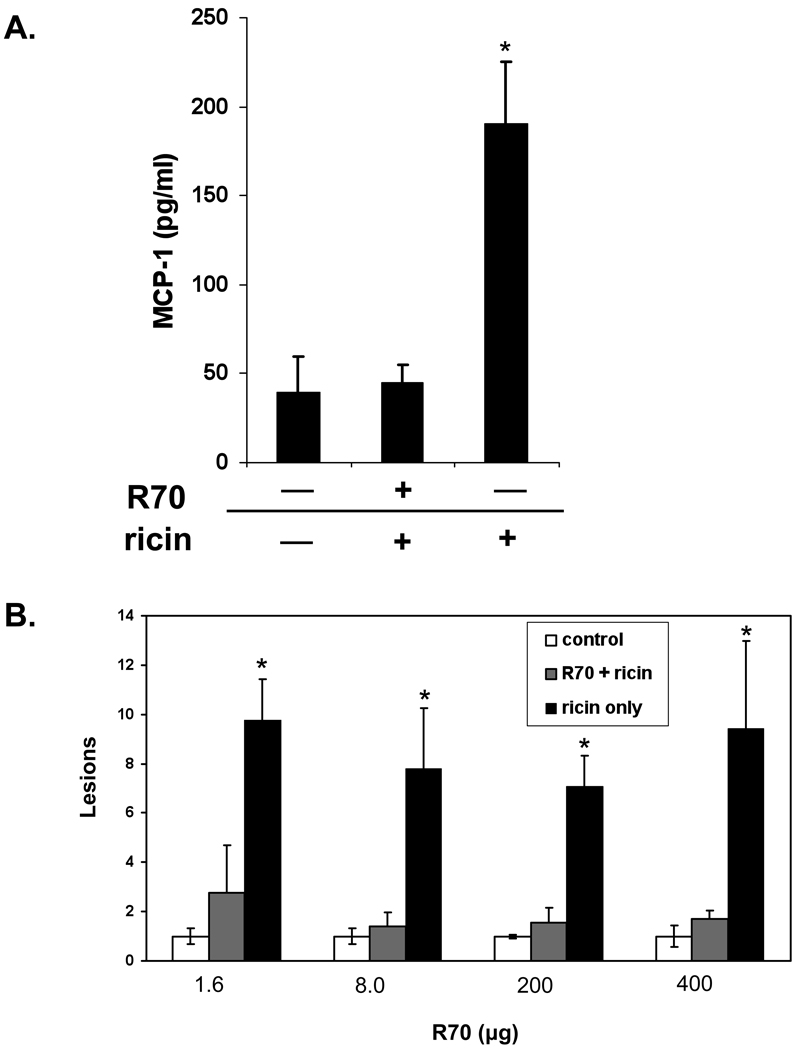
While the backpack tumor studies demonstrated that each of the neutralizing ricin-specific IgGs (i.e., 24B11 and R70) were sufficient to confer intestinal immunity to ricin, these experiments were confounded by the fact that serum antibody concentrations in these animals at the time of challenge was highly variable (range 1.0 to 1000 µg/ml), and were dependent on the growth of the hybridomas in individual mice (data not shown). This fact raised the concern that the observed protection could be the result of abnormally high serum antibody levels associated with the backpack tumors. To address that concern, we performed passive protection experiments in which fixed amounts of purified, endotoxin-free MAb R70 were administered to mice by i.p. injection 24 hr prior to ricin challenge. Twelve or 24 hr after i.g. ricin challenge, the animals were euthanized, and protection was assessed via measurement of MCP-1 levels in intestinal extracts, and visual inspecting H&E stained tissue sections.
As shown in Figure 7A, mice treated with R70 MAb (8 µg/mouse) and then challenged 12 hr later were protected against intestinal ricin intoxication, as determined by measuring local MCP-1 levels. Additional MAb titration-toxin challenge studies coupled with direct examination of intestinal tissues revealed that protection was achieved across a wide range of R70 MAb concentrations (Fig. 7B). It should be noted that parallel experiments failed to detect R70 in intestinal secretions and fecal pellets of mice that had received even 400 µg of MAb (data not shown). Collectively, these data demonstrate that IgG antibodies are sufficient to confer intestinal immunity to ricin and that this protection is unlikely due to simply IgG-transudation into the intestinal lumen.
Fig. 7. IgG MAb R70 administered systemically confers intestinal immunity to ricin.
MAb R70 (8 µg) was administered by i.p injection to a single group of BALB/c mice. 12 hr later, the R70-treated mice, as well as a control group of mice, were challenged by gavage with ricin (5 mg/kg) diluted into PBS. A third group of animals, not treated with R70, received PBS only by gavage. All three groups of mice were euthanized 18 hr following challenge, and MCP-1 levels were assessed in intestinal homogenates. Each column represents the average (+/− s.d.) of a single experiment, with a total of 6 mice per group. The asterisk indicates that MCP-1 in sham-treated, toxin-challenged mice were statistically different from unchallenged controls (p<0.05), as determined by the Student’s t test. (B) Groups of mice (n=5–6) were administered R70 at the indicated doses by i.p injection then challenged 24 hr later with ricin by gavage. Intestinal tissues were collected 24 hr later and scored for damage. The asterisks indicate lesion scores that were significantly greater (p<0.05) than control, unchallenged mice,
IgG-mediated intestinal immunity to ricin, independent of FcRn
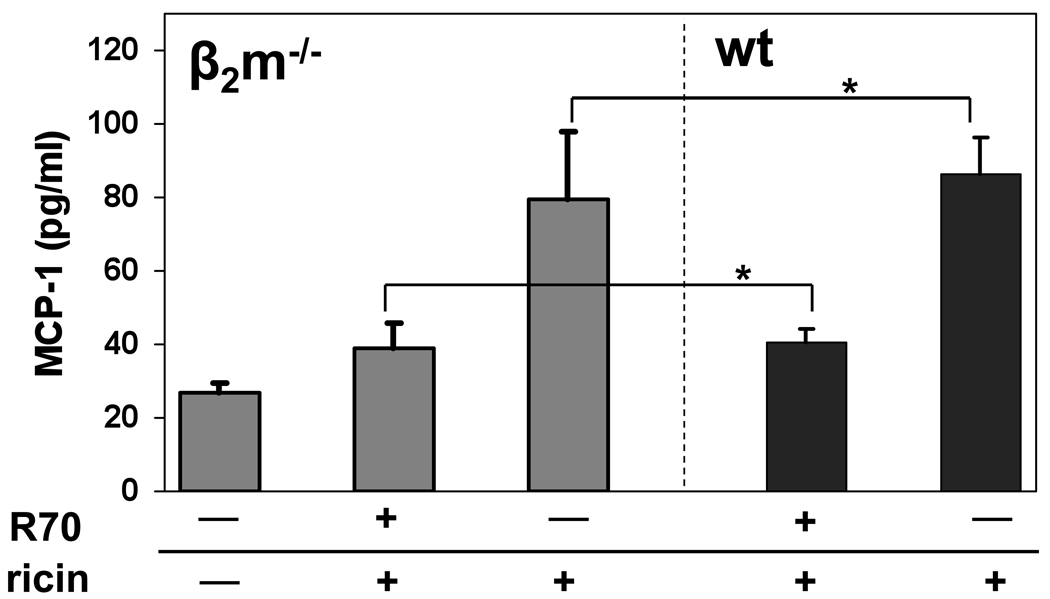
The neonatal Fcγ receptor, FcRn, mediates the apical-to-basolateral, as well as basolateral-to-apical, transport of IgG across epithelia [36, 37]. While it is well-established that FcRn is developmentally down-regulated in the murine intestinal epithelium during weaning, and functionally absent in the intestinal epithelium of adult mice[38], it has recently been suggested that FcRn expression (and possibility activity) is transiently up-regulated in response to toxin-induced signals or inflammation [39]. To examine whether FcRn is required for IgG-mediated immunity to ricin in our model, we passively administered R70 to wild type C57BL/6 and β2-microglobulin deficient (β2m−/−) mice, and then examined MCP-1 levels and tissue damage 18 hr after ricin challenge. β2m−/− mice are used as a model for FcRn deficiency because the association of FcRn with β2-microglobulin is essential for FcRn function in IgG recognition and transport [40, 41].
We first confirmed that β2m−/− mice were susceptible to intestinal ricin intoxication. A group of β2m−/− mice (n=6) was challenged i.g. with ricin, and then examined for intestinal damage 24 hr later. We found that β2m−/− mice had elevated levels of MCP-1 in their intestinal homogenates after ricin challenge, and that the levels were indistinguishable from those observed in ricin-challenged C57BL/6 control mice (Fig. 8). These data indicate that the β2m−/− mouse strain responds to toxin challenge in a manner identical to C57BL/6 control animals. To examine the role of FcRn in IgG-mediated intestinal immunity to ricin, groups of wild type and β2m−/− mice were passively administered MAb R70 (n=6) by i.p. injection, and then challenged 18 hr later with ricin. Analysis of MCP-1 levels in intestinal homogenates revealed that both groups of mice were equally protected against ricin intoxication (Fig. 8), indicating that FcRn is not required for IgG-mediated immunity to ricin in this model.
Fig. 8. IgG-mediated intestinal immunity to ricin is independent of FcRn.
MAb R70 (10 µg) was administered by i.p injection to β2M−/− mice or C57Bl/6 age and sex-matched controls. Twenty-four hours later, the mice were challenged i.g. with ricin (5 mg/kg). The animals were then euthanized 24 hr following challenge, and MCP-1 levels were assessed in intestinal homogenates. Each column represents the average values (+/− s.d.) of a single experiment, with a total 6 mice per group. The asterisks indicate that the respective MCP-1 values between groups of identically treated β2M−/− and C57BL/6 mice were not statistically significant.
4. DISCUSSION
The intestinal epithelium is under a constant barrage of microbial- and plant-derived toxins, many of which are capable of inducing epithelial damage, mucosal inflammation, and/or systemic complications. One major roadblock to developing vaccines and antibody-based therapeutics against a large number of these toxins, particularly those that have been identified by the Centers for Disease Control and Prevention (CDC) as being possible biothreat agents, is the simple fact that the respective contributions of SIgA and serum IgG in protecting the intestinal epithelium from toxin-induced damage remains unresolved. While eliciting antitoxin antibodies in serum can be achieved relatively easily using conventional vaccine formulations (e.g., alum salts) and parenteral delivery routes (e.g., i.m.), stimulating IgA antibodies in mucosal secretions poses considerably greater challenges, including the need to immunize orally or intranasally, to protect the antigen from degradation in the gut or respiratory tract, and the requirement for a safe and effective mucosal adjuvant. Therefore, defining the “division of labor” that exists between SIgA and IgG in the intestinal mucosa not only represents a fundamental issue in the field of mucosal immunology, but has practical implications for the development of countermeasures against an array of medically important pathogenic agents.
In the present study, we used a recently developed mouse model to examine the roles of SIgA and serum IgG in mediating intestinal immunity to ricin, a member of the RNA N-glycosidase family of toxins that also includes abrin and Stx. We found that toxin-specific IgG, either induced by vaccination with RT or else administered passively by parenteral injection, were sufficient to protect the intestinal epithelium from the effects of ricin. Moreover, we demonstrated that IgG-mediated immunity to ricin was independent of FcRn, which has been shown to be capable of moving IgG in a basolateal to apical (and vice versa) direction across the intestinal epithelium. To our knowledge, the present study is only one of two in vivo experimental demonstrations that serum IgG antibodies are sufficient to protect intestinal epithelial cells from toxin-induced death [42], and the first to show that this activity is independent of FcRn.
Robbins and colleagues have argued that baseline IgG antibody levels in mucosal secretions are sufficient to neutralize toxins before they interact with the epithelial surfaces [43]. However, it is clear from studies by Lycke and colleagues using J chain knock-out (J chain−/−) mice and cholera toxin (CT), that this is not necessarily the case [44]. J chain−/− mice, due to their inability to produce polymeric immunoglobulins, have severely reduced levels SIgA (and SIgM) in intestinal secretions. Immunization of groups of J chain−/− mice with CT elicited high titers of anti-toxin IgG and IgA antibodies in the sera, and normal levels of IgA plasma cells in the lamina propria of the small intestine, but only negligible amounts of anti-toxin SIgA in intestinal secretions [44]. An intraluminal toxin challenge assay revealed that the CT-immunized wild type mice were largely immune to CT, whereas the J chain−/− mice were not, as evidenced by significant toxin-induced intraluminal fluid accumulation. Other investigators repeated these experiments using pIgR−/− mice and came to the same conclusion: serum IgG antibodies are not sufficient to protect the intestinal epithelium from the effects of CT [45]. Subsequent analysis of IgA MAbs produced from the Peyer’s patches of CT-immunized mice reveled that protective antibodies were directed against CT’s binding subunit (CTB), and that these MAbs blocked toxin attachment to the receptors on the apical surfaces of intestinal epithelial cells [34]. Presumably the levels of CTB-specific IgG antibodies in intestinal secretions are insufficient interfere with toxin adherence.
On the other hand, serum IgG antibodies are sufficient to protect the intestinal epithelium from the A (CdtA) and B (CdtB) toxins of Clostridium difficile, the causative agent of pseudomembranous colitis [46]. CdtA, like ricin, has both enterotoxic and cytotoxic activities, whereas CdtB is primarily cytotoxic in nature. Two groups have demonstrated that protection against C. difficile-induced death and diarrhea is most effectively imparted by passive transfer of anti-toxin serum IgG antibodies [42]. While it has not been determined where (or how) CdtA and CdtB are neutralized in the intestinal mucosa, it has been proposed that circulating antitoxin IgG antibodies gain access to the gut lumen through transudation as a consequence of toxin-induced inflammation. This model is supported by the fact that both CdtA and CdtB are known to disrupt epithelial barrier functions and increase paracellular permeability [47–49]. However, it was noted that serum IgG must gain access to the intestinal lumen in the absence of observable fluid loss, since immune animals do not develop any measurable signs of diarrhea [42].
Simple transudation is unlikely to explain how IgG mediates intestinal immunity to ricin. Unlike CdtA and CdtB, RNA N-glycosidases do not immediately affect epithelial permeability or barrier function, despite the fact that cellular protein synthesis is arrested within a few hours of toxin exposure [50]; E. McCarthy and N. Mantis, unpublished observations). Moreover, levels of IgG and other serum components like albumin measured 24 hr following toxin exposure were only nominally elevated (<4 fold) over background in intestinal fluids of ricin challenged mice (E. McCarthy and N. Mantis, unpublished results). This is in contrast to CdtA–CdtB, which have been shown to cause a >300 fold rise in intestinal IgG levels [51]. Taken together, these data suggest that a mechanism other than serum transudation must account for the ability of IgG to protect the intestinal epithelium from the effects of ricin.
Although the exact mechanism by which IgG protects the intestinal epithelium from ricin intoxication has yet to be determined, we have demonstrated in the present study that that protection occurs independent of the neonatal Fc receptor, FcRn. While FcRn is developmentally down-regulated in the intestinal epithelium of adult rodents, we were prompted to examine a role for this receptor in intestinal immunity to ricin in light of a recent report demonstrating that FcRn expression is up-regulated in response to toxin-induced signals [39]. While we found that intestinal immunity to ricin was unaffected by the absence of FcRn in mice, we cannot exclude the possibility that this receptor might facilitate mucosal immunity to toxins such as ricin in humans, considering that FcRn may be expressed in the human adult intestinal epithelium, and has been shown, in a transgenic mouse model, to bind IgG-immune complexes in the intestinal lumen and to transport them to underlying dendritic cells [52–54].
Based on what is known about the pathogenesis of ricin and Stx, we speculate that anti-ricin IgG antibodies may function in the lamina propria to suppress toxin-induced mucosal inflammation, rather than neutralizing ricin in the intestinal lumen. Ricin is known to be internalized by villus enterocytes, where it actives epithelial stress-activated protein kinases (e.g., p38 MAPK) and promotes secretion of pro-inflammatory chemokines (e.g., MCP-1) [15, 16, 55]. At the same time, ricin (and stx) is likely transcytosed across the epithelium [15, 55], thereby intoxicating lamina propria macrophages. Macrophages have been shown to release an array of pro-inflammatory cytokines and chemokines, including TNF-α, in response to ricin [19, 56, 57] that are known to regulate epithelial permeability and detachment [58, 59]. Based on this model, we hypothesize that ricin-induced epithelial damage is primarily the consequence of an inflammatory response emanating from the lamina propria, not a direct result of enterocyte intoxication. Therefore ricin-specific IgG antibodies may abrogate mucosal inflammation and epithelial destruction by simply blocking inflammation local inflammatory responses initiated by macrophages or other cells within the lamina propria.
In conclusion, the results of the present study are consistent with the findings of Smallshaw and colleagues who reported that parenteral immunization of mice with a recombinant RTA subunit vaccine, known as RiVax, was sufficient to protect the animals against an intragastric toxin challenge [21, 22]. Based on our work, we would propose that the RTA-specific serum IgG antibodies elicited by RiVax were in fact responsible for conferring intestinal immunity to ricin. These data are significant in that they suggest that a single parenteral vaccine capable of eliciting high titer anti-toxin antibody titers in serum may be sufficient to impart both systemic and mucosal immunity to ricin challenge in humans. It remains to be determined whether serum IgG antibodies are sufficient to protect non-human primates and humans from ricin and other RNA glycosidases. It also remains to be determined whether IgG is sufficient to protect other mucosal surfaces, notably the respiratory tract, from the effects of ricin.
ACKNOWLEDGEMENTS
We would like to thank Dr. Robert Brey for providing us with RiVax, and for his helpful insights over the course of the study. We would like to thank Helen Johnson (Animal Pathology Core, Wadsworth Center) for tissue processing, Dr. Karen Chave (Protein Expression Core, Wadsworth Center) for MAb purification, and Renjie Song (Immunology Core, Wadsworth Center) for assistance with flow cytometry. This work was supported by grants from the National Institutes of Health (R21 AI058147) and the Northeast Biodefense Center (U54-AI057158-Lipkin) to NJM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 2.Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. JBiolChem. 1979;254(19):9795–9799. [PubMed] [Google Scholar]
- 3.Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326(6113):624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- 4.Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978 Sep 26;536(1):18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 5.Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, et al. Pathways followed by ricin and Shiga toxin into cells. HistochemCell Biol. 2002;117(2):131–141. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- 7.Argent RH, Parrott AM, Day PJ, Roberts LM, Stockley PG, Lord JM, et al. Ribosome-mediated folding of partially unfolded ricin A-chain. J Biol Chem. 2000 Mar 31;275(13):9263–9269. doi: 10.1074/jbc.275.13.9263. [DOI] [PubMed] [Google Scholar]
- 8.Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexner S. The histological changes produced by ricin and abrin intoxications. Journal of Experimental Medicine. 1897;2:197–220. doi: 10.1084/jem.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiguro M, Harada H, Ichiki O, Sekine I, Nishimori I, Kikutani M. Effects of ricin, a protein toxin, on glucose absorption by rat small intestine. (Biochemical studies on oral toxicity of ricin. II) Chem Pharm Bull. 1984;32(8):3141–3147. doi: 10.1248/cpb.32.3141. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro M, Nakashima H, Tanabe S, Sakakibara R. Interaction of toxic lectin ricin with epithelial cells of rat small intestine in vitro. Chemical & Pharmaceutical Bulletin. 1992;40(2):441–445. doi: 10.1248/cpb.40.441. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro M, Tanabe S, Matori Y, Sakakibara R. Biochemical studies on oral toxicity of ricin. IV. A fate of orally administered ricin in rats. Journal of Pharmacobio-Dynamics. 1992;15(4):147–156. doi: 10.1248/bpb1978.15.147. [DOI] [PubMed] [Google Scholar]
- 13.Sekine I, Kawase Y, Nishimori I, Mitarai M, Harada H, Ishiguro M, et al. Pathological study on mucosal changes in small intestine of rat by oral administration of ricin. I. Microscopical observation. Acta Pathologica Japonica. 1986;36(8):1205–1212. doi: 10.1111/j.1440-1827.1986.tb02840.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoder JM, Aslam RU, Mantis NJ. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect Immun. 2007 Apr;75(4):1745–1750. doi: 10.1128/IAI.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006 Jun;74(6):3455–3462. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jandhyala DM, Ahluwalia A, Obrig T, Thorpe CM. ZAK: a MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008 Apr 27; doi: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe CM, Hurley BP, Lincicome LL, Jacewicz MS, Keusch GT, Acheson DW. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. InfectImmun. 1999;67(11):5985–5993. doi: 10.1128/iai.67.11.5985-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorpe CM, Smith WE, Hurley BP, Acheson DW. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. InfectImmun. 2001;69(10):6140–6147. doi: 10.1128/IAI.69.10.6140-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki C, Nishikawa K, Zeng XT, Katayama Y, Natori Y, Komatsu N, et al. Induction of cytokines by toxins that have an identical RNA N-glycosidase activity: Shiga toxin, ricin, and modeccin. Biochim Biophys Acta. 2004 Mar 17;1671(1–3):44–50. doi: 10.1016/j.bbagen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006 Jun;74(6):3463–3470. doi: 10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine. 2010 Jul 19;28(32):5315–5322. doi: 10.1016/j.vaccine.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007 Oct 16;25(42):7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–421. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 24.Fulton RJ, Uhr JW, Vitetta ES. The effect of antibody valency and lysosomotropic amines on the synergy between ricin A chain- and ricin B chain-containing immunotoxins. JImmunol. 1986 Apr 15;136(8):3103–3109. [PubMed] [Google Scholar]
- 25.Grimsley GR, Pace NC. Spectrophotometric Determination of Protein Concentration. In: Coligan JE, Dunn BM, Speicher DW, Wingfield PT, editors. Current Protocols in Protein Science. Hoboken, NJ: John Wiley & Sons, Inc; 2003. [Google Scholar]
- 26.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 27.Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effects of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 28.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60(5):1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007 Nov 15;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J Virol. 2001 Nov;75(22):10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. Journal of Experimental Medicine. 1999;190(7):915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrickson BA, Conner DA, Ladd DJ, Kendall D, Casanova JE, Corthesy B, et al. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. Journal of Experimental Medicine. 1995;182(6):1905–1911. doi: 10.1084/jem.182.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meckelein B, Externest D, Schmidt MA, Frey A. Contribution of serum immunoglobulin transudate to the antibody immune status of murine intestinal secretions: influence of different sampling procedures. Clin Diagn Lab Immunol. 2003 Sep;10(5):831–834. doi: 10.1128/CDLI.10.5.831-834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apter FM, Lencer WI, Finkelstein RA, Mekalanos JJ, Neutra MR. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993;61(12):5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neal LM, O'Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010 Jan;78(1):552–561. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004 Apr;15(4):1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. The Journal of Cell Biology. 1984;99(1):159S–164S. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida M, Masuda A, Kuo TT, Kobayashi K, Claypool SM, Takagawa T, et al. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Semin Immunopathol. 2006 Dec;28(4):397–403. doi: 10.1007/s00281-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 39.Verdin-Teran SL, Vilches-Flores A, Moreno-Fierros L. Immunization with Cry1Ac from Bacillus thuringiensis increases intestinal IgG response and induces the expression of FcRn in the intestinal epithelium of adult mice. Scand J Immunol. 2009 Dec;70(6):596–607. doi: 10.1111/j.1365-3083.2009.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Praetor A, Hunziker W. beta(2)-Microglobulin is important for cell surface expression and pH-dependent IgG binding of human FcRn. J Cell Sci. 2002 Jun 1;115(Pt 11):2389–2397. doi: 10.1242/jcs.115.11.2389. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. Journal of Immunology. 2001;166(5):3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, et al. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999 Feb;67(2):527–538. doi: 10.1128/iai.67.2.527-538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995 Jun;171(6):1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 44.Lycke N, Erlandsson L, Ekman L, Schon K, Leanderson T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J Immunol. 1999 Jul 15;163(2):913–919. [PubMed] [Google Scholar]
- 45.Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. EurJImmunol. 2005 Jan;35(1):180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- 46.Belyi Y, Aktories K. Bacterial toxin and effector glycosyltransferases. Biochim Biophys Acta. 2010 Feb;1800(2):134–143. doi: 10.1016/j.bbagen.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992 Feb;102(2):416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 49.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001 Mar;69(3):1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acheson DW, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, et al. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infection & Immunity. 1996;64(8):3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corthier G, Muller MC, Elmer GW, Lucas F, Dubos-Ramare F. Interrelationships between digestive proteolytic activities and production and quantitation of toxins in pseudomembranous colitis induced by Clostridium difficile in gnotobiotic mice. Infect Immun. 1989 Dec;57(12):3922–3927. doi: 10.1128/iai.57.12.3922-3927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999 Oct;104(7):903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004 Jun;20(6):769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006 Aug;116(8):2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Deurs B, Hansen SH, Petersen OW, Melby EL, Sandvig K. Endocytosis, intracellular transport and transcytosis of the toxic protein ricin by a polarized epithelium. EurJCell Biol. 1990;51(1):96–109. [PubMed] [Google Scholar]
- 56.Gonzalez TV, Farrant SA, Mantis NJ. Ricin induces IL-8 secretion from human monocyte/macrophages by activating the p38 MAP kinase pathway. Mol Immunol. 2006 Apr;43(11):1920–1923. doi: 10.1016/j.molimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Korcheva V, Wong J, Lindauer M, Jacoby DB, Iordanov MS, Magun B. Role of apoptotic signaling pathways in regulation of inflammatory responses to ricin in primary murine macrophages. Mol Immunol. 2007 Apr;44(10):2761–2771. doi: 10.1016/j.molimm.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009 Nov;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 59.Weber B, Saurer L, Mueller C. Intestinal macrophages: differentiation and involvement in intestinal immunopathologies. Semin Immunopathol. 2009 Jul;31(2):171–184. doi: 10.1007/s00281-009-0156-5. [DOI] [PubMed] [Google Scholar]