Abstract
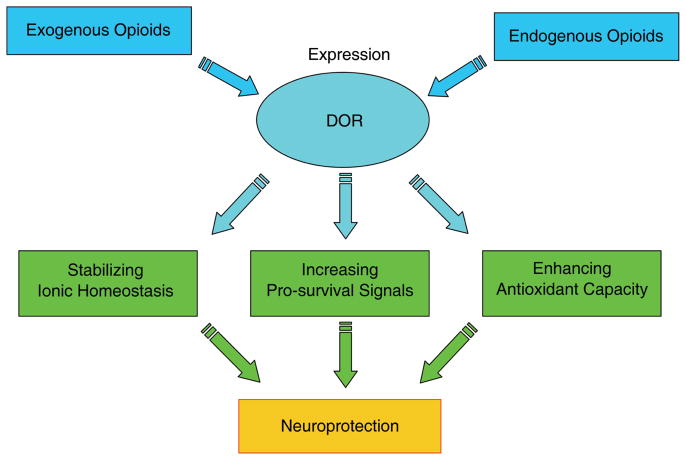
The use of opioid analgesics has a long history in clinical settings, although the functions of opioid receptors, especially their role in the brain, are not well understood yet. Recent studies have generated abundant new data on opioid receptor-mediated functions and the underlying mechanisms. The most exciting finding in the past decade is probably the neuroprotection against hypoxic/ischemic stress mediated by δ-opioid receptors (DOR). An up-regulation of DOR expression and the release of endogenous opioids may increase neuronal tolerance to hypoxic/ischemic stress. The DOR signal triggers, depending on stress duration and severity, different mechanisms at multiple levels to preserve neuronal survival, including the stabilization of ionic homeostasis, an increase in pro-survival signaling (e.g., PKC-ERK-Bcl 2) and the enhanced anti-oxidative capacity. Recent data on DOR-mediated neuroprotection provide us a new concept of neuroprotection against neurological disorders and have a potentially significant impact on the prevention and treatment of some serious neurological conditions, such as stroke.
Keywords: opioids, δ-opioid receptors, neurotransmitters, brain, ionic homeostasis, neuroprotection, hypoxia, ischemia
1 Introduction
Opioids have been widely used as analgesics throughout history to combat pain or induce ecstasy in medical and non-medical situations. Despite the flow of time and the energies of scientists around the world, the function of opioid receptors and their role in the brain still is not well understood to this day.
One of the most interesting findings on opioid receptor function in the past decade is probably the opioid receptor-mediated neuroprotection against hypoxic/ischemic injury. Hypoxia and/or ischemia lead to serious consequences in the clinical setting. Unfortunately, there have been no promising drugs for relieving hypoxic/ischemic injury in the brain despite considerable research efforts that have been made in past several decades. Because of the unique and complex features of neuronal responses to hypoxic/ischemic stress, neuroprotection against hypoxic/ischemic injury must be an exceptional and comprehensive process that is different from neuroprotective strategies against other insults. Accumulating evidence has shown that opioids may protect neuronal tissues/cells via activation of opioid receptors during hypoxic/ischemic stress, providing a novel insight on hypoxic/ischemic injury in the brain. Indeed, the role of opioids and other analgesics in neuroprotection has attracted more and more attention from clinicians and research scientists over the course of time.
2 Historical controversy
The release of opioids increases in the brain during hypoxia[1–3]; however, the role of the opioid system in neuronal responses to hypoxic/ischemic stress remained unclear. Some in vivo studies[4–9] showed that opioid receptor inhibition by intravenous injection of a high dose of naloxone, a non-specific opioid antagonist that interacts with DOR, μ-opioid receptors (MOR) and κ-opioid receptors (KOR), protects the brain from ischemia-induced injury, while others[10–13] suggested that opioid receptor activation with opioid agonists protects the brain from ischemia or extends animal survival time during severe hypoxia. The major controversy that might result from methodological limitations can be laid at the doorstep of ligand selectivity, namely, a majority of these earlier studies were mainly based on the application of opioid ligands with relatively poor in low receptor selectivity and administered at high concentrations intravenously, which complicated the ensuing results; i.e., high doses of opioid ligands may lead to cross-reactivity with various opioid receptor subtypes or induce non-opioid effects. Furthermore, systemic administration of opioids (e.g., DOR ligands) [11,14] may induce multiple extraneous effects, including heart[15] and blood vessels [16], such that the resultant data were difficult to interpret as the results of neuronal action, since DOR protects animals from cardiac injury [17,18] thereby extending survival during severe hypoxia.
To ascertain the role of opioid receptors in neuronal response to hypoxic stress, it is important to differentiate the role of opioid receptor subtypes in specific neurons/regions, since opioid receptors are differentially distributed in the brain. One earlier report using morphine, U50,488H and naloxone provided no significant protection in the hippocampus subjected to ischemia[19]. Another study revealed that a variety of μ-opioid ligands at excessively high concentrations (0.1–3 mmol/L) reduced neuroexcitotoxicity in cortical neurons, which could not be blocked by naloxone, suggesting a “non-opioid effect”[20]. Those results have added to the complexity of the role played by opioids on neurons during conditions of stress. To clarify the potential mode of action of opioids and their receptors in hypoxic/ischemic neuronal injury, we demonstrated that DOR is neuroprotective against hypoxic/excitotoxic stress in the brain, especially cortical neurons[21–32].
3 δ-opioid receptor mediated neuroprotection
In the late 1980s, we pursued the mechanisms underlying the major differences in neuronal sensitivity to hypoxic stress between the turtle and rat. In early 1989, an observation based on receptor autographical analysis indicated that turtle brain had a higher density of DOR than rat brain, while MOR density was lower in the former than in the latter, which was confirmed in 1997 and eventually published in 2001[33]. Since turtle brains are more tolerant to hypoxic/ischemic stress[34,35], this unique phenomenon was linked to a potential mechanism of neuroprotection [21,33].
The confirmation of that observation prompted the further elucidation of the role of DOR in neuroprotection against hypoxic/ischemic injury. To clarify the action of various opioid receptors, especially DOR in neuronal responses to excitotoxicity, opioid agonists and/or antagonists were directly applied to cultured neurons to investigate whether opioids protect from glutamate-induced[21] neuronal injury and death due to the fact that DOR is highly distributed in the cortex[36].
Initially, glutamate was added to cultured cortical neurons to mimic neuroexcitotoxicity since it is a key mediator of ischemic/hypoxic injury and induces neuronal injury and cell death in a dose- and time-dependent manner. After 8–10 days in vitro, neurons exposed to 100 μmol/L glutamate for 4 hours daily showed substantial neuronal injury as assessed by morphologic determination and LDH release. Activation of DOR with 10 μmol/L DADLE reduced glutamate-induced injury by almost half, and naltrindole (10 μmol/L) completely blocked this protective effect. In sharp contrast, administration of MOR and KOR agonists (5–10 μmol/L DAMGO and U50,488H, respectively) did not induce appreciable neuroprotection, and MOR or KOR antagonists had no significant effect on glutamate-induced injury. These data demonstrate that activation of DOR, but not MOR and KOR, protected cultured neocortical neurons from glutamate excitotoxicity[21,22]. DOR neuroprotection from hypoxic stress, ischemic insult or excitotoxic injury was shown by our studies[21,22,24,37] and substantiated in other laboratories[38–52].
Similarly, we observed that following the microinjection and expression of DOR mRNA into Xenopus oocytes, 1–5 μmol/L of the selective and potent δ-agonist UFP-512[53] not only significantly attenuated anoxic decrease in extracelluar [Na+], but also enhanced the recovery in the anoxia-induced decline due to the change in [Na+], which was blocked by naltrindole[32]. Furthermore, with the expression of DOR, UFP-512 reduced sodium currents, permitting us to conclude that DOR is directly involved in the inhibition of Na+ channel regulation[32].
Interestingly, we also observed that electroacupuncture attenuated ischemic injury via DOR system[37], which is now well documented[27–29,54]. In addition, we demonstrate that neuronal preconditioning induced neuroprotection is dependent on the activity of DOR[25,55], which is now also confirmed in another model[51].
Based on these accumulative data, the previous controversies that existed in the literature can now be addressed. The reason why μ-ligands had little or no protective effect on hippocampal neurons[19] is likely due to the low density of MOR in the hippocampus, and that high concentrations of μ-ligands reduced neuroexcitotoxicity in cortical neurons[20] might be, at least partially, attributed to activation of DOR, which is highly expressed in the cortex, through use of opioid ligands with limited receptor selectivity and specificity.
4 Mechanisms of DOR neuroprotection
Acute hypoxic/ischemic stress causes an immediate loss of ionic homeostasis based on the functional response of inherent membrane proteins, while prolonged stress may lead to major alterations in signal transduction (especially death/survival signal systems), gene expression (e.g., DOR expression[55]) and even cellular structure[56–59]. DOR signals may trigger, depending on the duration of stress and different modes of action at multiple levels, a preservation of neuronal survival. Recent progress on the mechanistic exploration is briefly summarized below.
4. 1 Up-regulation of DOR expression
DOR expression is influenced by multiple factors including development[36], hypoxic stress[25,55] and neurotransmitters. There is evidence showing that brain DOR is sensitive to hypoxia/ischemia and significantly decreases after a few hours of ischemic stress or prolonged hypoxia. For example, exposure to hypoxia for seven days down-regulated DOR, but not MOR and KOR[60].
Since the hypoxia-resistant turtle has a high density of DOR in the brain, we asked whether DOR expression plays a role in neuroprotection against hypoxic/ischemic stress. Our recent studies provide strong evidence for the involvement of DOR expression in neuronal survival under hypoxic condition. In cortical neurons, rapid hypoxia preconditioning (HPC) increased DOR protein density with no appreciable changes in mRNA level of DOR[55]. However, delayed HPC increased DOR expression at both mRNA and protein levels[25]. The DOR up-expression is associated with an increase in neuronal survival[25,55]. In sharp contrast to neuronal preconditioning with short-term hypoxia, prolonged hypoxia caused serious neuronal injury with a significant decrease in DOR protein and mRNA[25].
Furthermore, we showed that transgenic over-expression of DOR alone made the cortex more tolerant to hypoxic stress[26]. Indeed, an expression of DOR itself without DOR agonists inhibits Na+ channel function[32], which serves as a neuroprotective strategy against hypoxic stress[32,65,66]. These data show that DOR expression is a critical factor determining neuronal tolerance to hypoxia/ischemia.
4.2 Stabilization of ionic homeostasis
Opioids may modulate electrical activity and excitatory transmission which are based on the changes in the activities of ionic channels and ion flux[61,62]. Under hypoxia/ischemia, ionic homeostasis is greatly disrupted, which is characterized by the enhanced Na+, Ca2+ influx and K+ efflux[31,32]. Activation of DOR, but not MOR, remarkably attenuates K+ leakage in hypoxia or simulated ischemia[26,63–66], suggesting that the stabilization of K+ homeostasis is an important mechanism of DOR neuroprotection, since the enhanced K+ leakage is a characteristic response of neurons to hypoxia/ischemia[31,67] and initiates cellular apoptosis[68–73]. Furthermore, data suggest that DOR activation attenuates anoxic K+ leakage partially through inhibition of Ca2+ loading[63]. Additional results revealed that DOR-mediated inhibition on Na+ entry through voltage-gated Na+ channels and NMDA receptor channels constituted a major mechanism underlying the DOR protection against anoxic K+ derangement in the cortex because DOR protection against anoxic disruption of K+ homeostasis was largely abolished by low Na+ perfusion, either with impermeable N-methyl-D-glucamine or permeable Li+ substitution, Na+ channel blocker TTX, and NMDA receptor channel blocker MK 801[65,66]. In support of this notion, activation of DOR indeed attenuated anoxic Na+ influx in the cortical slices, which can be abolished by naltrindole[32]. Taken together, these results indicate that DOR signal stabilizes hypoxic/ischemic disruption of ionic homeostasis to protect neurons against hypoxic/ischemic insults.
4.3 Intracellular transduction
The post-receptor signaling following opioid receptor activation is an intricate dance among various intracellular signaling pathways. In hypoxic neurons, we found that DOR-mediated protection against hypoxic injury was inhibited by the treatment with a protein kinase C (PKC) inhibitor[25], suggesting the involvement of PKC in the DOR protection. Furthermore, we found that DOR activation attenuated anoxic disruption of K+ homeostasis and this DOR-mediated event also relies on a PKC-dependent, but PKA-independent pathway[64]. Down-stream, the extracellular signal-regulated kinase (ERK) stimulated by the mitogen-activated protein kinase kinase (MEK) is important in the signaling cascades of DOR neuroprotection because we observed that this DOR-mediated activity was largely inhibited by the treatment with an ERK inhibitor in the hypoxic neurons[25]. Narita et al. [41] also showed the importance of PKC and MEK in DOR-mediated neurogenesis and neuroprotection. Therefore, PKC-MEK-ERK signaling may form the central pathway for DOR protection.
Our studies show that G proteins are an important component of signal transduction and the activation of the G protein-PKC-ERK pathway enhances the activity of Bcl-2 and suppresses cytochrome C release, thus protecting neurons from severe hypoxic stress[25]. Interestingly, the crosstalk between ERK and p38 displays a “Yin-Yang” antagonism under the regulation of the DOR-G protein-PKC pathway[25]. In addition, Narita et al. [41] demonstrated that DOR signaling involves the activation of Trk-dependent tyrosine kinase, which could be linked to PI3K and CaMKII in addition to MEK and PKC. The neuroprotective property of DOR may be partly due to its ability to potently block Bax-related apoptotic processes[74].
4.4 Attenuation of oxidative injury
Since ERK and cytochrome c are differentially involved in caspase signaling of oxidative injury that significantly contributes to neuronal damage in ischemia/reperfusion, the question arose if DOR activation protected the ischemic brain by attenuating oxidative injury. Recent data[30] show that in the model of cerebral ischemia with middle cerebral artery occlusion, DOR activation increased the activity of major antioxidant enzymes, glutathione peroxidase and superoxide dismutase, and decreased malondialdehyde and nitric oxide levels in the cortex exposed to cerebral ischemia/reperfusion. Moreover, DOR activation reduced caspase-3 expression in ischemic regions, such as the hippocampus. PD98059, an inhibitor of MAPK extracellular signaling-regulated kinase, accelerated animal death during ischemia/reperfusion. Therefore, DOR activation greatly enhanced the activity of antioxidant enzymes and reduced free radicals, MDA and NO, thereby attenuating oxidative injury by enhancing antioxidant ability and inhibiting caspase activity in the brain after cerebral ischemia and reperfusion.
Taken together, the DOR-mediated signal is very likely a regulator at multiple levels in neurons and protects brains from hypoxic/ischemic stress through complex mechanisms. The major mechanisms are schematically shown in the accompanying Fig. 1.
Fig. 1.
Schematic diagram of major mechanisms for DOR neuroprotecion.
5 Concluding remarks
The research on the DOR-mediated neuroprotection provides us a new concept of how the brain responds to hypoxic/ischemic stress. The underlying mechanisms include the stabilization of ionic homeostasis in acute stage of hypoxic/ischemic stress, and up-regulation of DOR expression, survival molecules and anti-oxidative capacity, and the down-regulation of apoptotic signaling during the long-term stress of hypoxia/ischemia. These latest findings may have clinical importance because hypoxic/ischemic insult (e.g., stroke) is a major cause of neurological morbidity and mortality in the world as a consequence of neuronal dysfunction and death, while therapeutic modality directed against hypoxic/ischemic injury and death have had limited success.
At present, however, many questions remain unknown regarding the mechanism of DOR neuroprotection. For example, does DOR activity affect cellular pH in hypoxia/ischemia? Since we have shown that DOR expression is down-regulated in the brain with null mutation of Na+/H+ exchanger 1[75], and Na+/H+ exchangers are involved in the pH regulation in neurons[76], it is likely that DOR plays a role in pH homeostasis and protein stability[77] in hypoxic/ischemic conditions. It is our belief that more mechanistic research on DOR protection may eventually lead to novel clues for better solutions of hypoxic/ischemic injury in the brain.
Acknowledgments
This work was supported by the grants of NIH-HD34852, NIH-AT004422, AHA-0755993T, STCSM-05DZ19745, 973 Program-2006CB504509, and in part by the Intramural Research Program of the NIH and NIEHS.
References
- 1.Shankar V, Armstead WM. Opioids contribute to hypoxia-induced pial artery dilation through activation of ATP-sensitive K+ channels. Am J Physiol. 1995;269(3 Pt 2):H997–H1002. doi: 10.1152/ajpheart.1995.269.3.H997. [DOI] [PubMed] [Google Scholar]
- 2.Yan S, Zhang C, Laferrière, Moss IR. Met-enkephalin-like immunoreactivity in microdialysates from nucleus tractus solitarii in piglets during normoxia and hypoxia. Brain Res. 1995;687(1–2):217–220. doi: 10.1016/0006-8993(95)00541-w. [DOI] [PubMed] [Google Scholar]
- 3.Armstead WM. Nitric oxide contributes to opioid release from glia during hypoxia. Brain Res. 1998;813(2):398–401. doi: 10.1016/s0006-8993(98)01022-1. [DOI] [PubMed] [Google Scholar]
- 4.Hosobuchi Y, Baskin DS, Woo SK. Reversal of induced ischemic neurologic deficit in gerbils by the opiate antagonist naloxone. Science. 1982;215(4528):69–71. doi: 10.1126/science.6274019. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Olinger CP, Barsan WG, Butler MJ, Graff-Radford NR, Brott TG, Biller J, Damasio H, Tomsick T, Goldberg M. A dose-escalation study of large doses of naloxone for treatment of patients with acute cerebral ischemia. Stroke. 1986;17(3):404–409. doi: 10.1161/01.str.17.3.404. [DOI] [PubMed] [Google Scholar]
- 6.Skarphedinsson JO, Thorén P. Endorphin mechanisms are responsible for the beneficial effects of opioid antagonists on cerebral function during relative cerebral ischaemia in rats. Acta Physiol Scand. 1988;132(3):281–288. doi: 10.1111/j.1748-1716.1988.tb08331.x. [DOI] [PubMed] [Google Scholar]
- 7.Olinger CP, Adams HP, Jr, Brott TG, Biller J, Barsan WG, Toffol GJ, Eberle RW, Marler JR. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke. 1990;21(5):721–725. doi: 10.1161/01.str.21.5.721. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Cheng FC, Liao SL, Chen WY, Lin NN, Kuo JS. Effects of naloxone on lactate, pyruvate metabolism and antioxidant enzyme activity in rat cerebral ischemia/reperfusion. Neurosci Lett. 2000;287(2):113–116. doi: 10.1016/s0304-3940(00)01151-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. Neuroreport. 2001;12(6):1245–1249. doi: 10.1097/00001756-200105080-00038. [DOI] [PubMed] [Google Scholar]
- 10.Hayward NJ, McKnight AT, Woodruff GN. Neuroprotective effect of the kappa-agonist enadoline (CI-977) in rat models of focal cerebral ischaemia. Eur J Neurosci. 1993;5(7):961–967. doi: 10.1111/j.1460-9568.1993.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 11.Mayfield KP, D’Alecy LG. Delta-1 opioid agonist acutely increases hypoxic tolerance. J Pharmacol Exp Ther. 1994;268 (2):683–688. [PubMed] [Google Scholar]
- 12.Endoh H, Taga K, Yamakura T, Sato K, Watanabe I, Fukuda S, Shimoji K. Effects of naloxone and morphine on acute hypoxic survival in mice. Crit Care Med. 1999;27(9):1929–1933. doi: 10.1097/00003246-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Summers RL, Li Z, Hildebrandt D. Effect of a delta receptor agonist on duration of survival during hemorrhagic shock. Acad Emerg Med. 2003;10(16):587–593. doi: 10.1111/j.1553-2712.2003.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 14.Bofetiado DM, Mayfield KP, D’Alecy LG. Alkaloid delta agonist BW373U86 increases hypoxic tolerance. Anesth Analg. 1996;82(6):1237–1241. doi: 10.1097/00000539-199606000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Schultz JE, Hsu AK, Gross GJ. Ischemic preconditioning in the intact rat heart is mediated by delta1- but not mu- or kappa-opioid receptors. Circulation. 1998;97(13):1282–1289. doi: 10.1161/01.cir.97.13.1282. [DOI] [PubMed] [Google Scholar]
- 16.Sun FY, Zhang AZ, Xia Y. Mechanism of dynorphin inhibition on vasoconstriction in-vitro. Acta Physiol Sin. 1989;41(4):354–360. [PubMed] [Google Scholar]
- 17.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35(7):709–718. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 18.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94(7):960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 19.Iwai T, Niwa M, Nakashima M, Kambara T, Yamada H, Tsurumi K, Nozaki M. Effect of opioids on delayed neuronal death in the gerbil hippocampus. Life Sci. 1992;50(26):239–244. doi: 10.1016/0024-3205(92)90580-i. [DOI] [PubMed] [Google Scholar]
- 20.Choi DW, Viseskul V. Opioids and non-opioid enantiomers selectively attenuate N-methyl-D-aspartate neurotoxicity on cortical neurons. Eur J Pharmacol. 1988;155(1–2):27–35. doi: 10.1016/0014-2999(88)90399-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JH, Xia Y, Haddad GG. Activation of δ-opioid receptors protects cortical neurons from glutamate excitotoxic injury. Soc Neurosci Abstract. 1999;28:736. [Google Scholar]
- 22.Zhang JH, Haddad GG, Xia Y. δ-, but not μ- and κ-opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885(2):143–153. doi: 10.1016/s0006-8993(00)02906-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JH, Gibney GT, Xia Y. Effect of prolonged hypoxia on expression of Na+ channel mRNA subtypes in the developing rat cortex. Brain Res Mol Brain Res. 2001;91(1–2):154–158. doi: 10.1016/s0169-328x(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JH, Gibney GT, Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol. 2002;282(6):C1225–C1234. doi: 10.1152/ajpcell.00226.2001. [DOI] [PubMed] [Google Scholar]
- 25.Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen sensitive δ-opioid receptor-regulated survival and death signals: Novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280(16):16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- 26.Chao D, Qian H, Ghassemi F, Chen JS, Xia Y. Transgenic over-expression of δ-opioid receptors protects the cortex from anoxic disruption of ionic homeostasis. Soc Neurosci Abstract. 2006 Program No. 87.19/MM68. [Google Scholar]
- 27.Tian XS, Zhou F, Yang R, Xia Y, Wu GC, Guo JC. Effects of intracerebroventricular injection of delta-opioid receptor agonist TAN-67 or antagonist naltrindole on acute cerebral ischemia in rat. Acta Physiol Sin. 2008;60(4):475–484. (Chinese, English abstract) [PubMed] [Google Scholar]
- 28.Tian XS, Zhou F, Yang R, Xia Y, Wu GC, Guo JC. Electroacupuncture protects the brain against acute ischemic injury via up-regulation of delta-opioid receptor in rats. J Chin Integr Med. 2008;6(6):632–638. doi: 10.3736/jcim20080617. [DOI] [PubMed] [Google Scholar]
- 29.Tian XS, Zhou F, Yang R, Xia Y, Wu GC, Guo JC. Role of δ-opioid receptors in cumulative electro-acupuncture induced protection from ischemic injury in the rat brain. Shanghai J TCM. 2008;42:71–74. [Google Scholar]
- 30.Yang YL, Xia XW, Zhang Y, Wang Q, Li L, Luo GH, Xia Y. Delta-opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009;7:55. doi: 10.1186/1741-7007-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung JH, Chao DM, Xia Y. Neuronal responses to hypoxia. In: Wang, Ying, editors. New Frontiers in Neurological Research. Kerala: Research Signpost; 2008. pp. 73–153. [Google Scholar]
- 32.Kang X, Chao D, Gu Q, Ding G, Wang Y, Balboni G, Lazarus LH, Xia Y. δ-opioid receptors protect from anoxic disruption of Na+ homeostasis via Na+ channel regulation. Cell Mo Life Sci. 2009;66(21):3505–3516. doi: 10.1007/s00018-009-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Y, Haddad GG. Major difference in the expression of δ- and μ-opioid receptors between turtle and rat brain. J Comp Neurol. 2001;436(2):202–210. [PubMed] [Google Scholar]
- 34.Sick TJ, Rosenthal M, LaManna JC, Lutz PL. Brain potassium ion homeostasis, anoxia, and metabolic inhibition in turtles and rats. Am J Physiol. 1982;243(3):R281–R288. doi: 10.1152/ajpregu.1982.243.3.R281. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Jiang C, Haddad GG. Oxidative and glycolytic pathways in rat (newborn, adult) and turtle: Role in anoxia. Am J Physiol. 1992;262 (4Pt2):R595–R603. doi: 10.1152/ajpregu.1992.262.4.R595. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549(2):181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhao P, Guo JC, Xia Y, Hong SS, Bazzy-Asaad A, Cheng JS, Xia Y. Electro-acupuncture and brain protection from cerebral ischemia: The role of delta-opioid receptor. Soc Neurosci Abstract. 2002 Program No. 490.13. [Google Scholar]
- 38.Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D-Ala2,D-Leu5) enkephalin: linking hibernation and neuroprotection. Front Biosci. 2004;9:3392–3398. doi: 10.2741/1490. [DOI] [PubMed] [Google Scholar]
- 39.Borlongan CV, Hayashi T, Oeltgen PR, Su TP, Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. 2009;17(7):31. doi: 10.1186/1741-7007-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim YJ, Zheng S, Zuo Z. Morphine preconditions purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. 2004;100(3):562–568. doi: 10.1097/00000542-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97(5):1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65(10):945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- 43.Su DS, Wang ZH, Zheng YJ, Zhao YH, Wang XR. Dose-dependent neuroprotection of delta opioid peptide [D-Ala2,D-Leu5] enkephalin in neuronal death and retarded behavior induced by forebrain ischemia in rats. Neurosci Lett. 2007;423(2):113–117. doi: 10.1016/j.neulet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 44.Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Effects of delta-opioid receptor stimulation and inhibition on hippocampal survival in a rat model of forebrain ischaemia. Br J Anaesth. 2007;99(4):538–546. doi: 10.1093/bja/aem220. [DOI] [PubMed] [Google Scholar]
- 45.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93(3):502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Kao TK, Ou YC, Liao SL, Chen WY, Wang CC, Chen SY, Chiang AN, Chen CJ. Opioids modulate post-ischemic progression in a rat model of stroke. Neurochem Int. 2008;52(6):1256–1265. doi: 10.1016/j.neuint.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Horiuchi T, Kawaguchi M, Sakamoto T, Kurita N, Inoue S, Nakamura M, Konishi N, Furuya H. The effects of the delta-opioid agonist SNC80 on hind-limb motor function and neuronal injury after spinal cord ischemia in rats. Anesth Analg. 2004;99 (1):235–240. doi: 10.1213/01.ANE.0000130389.77859.1C. [DOI] [PubMed] [Google Scholar]
- 48.Horiuchi T, Kawaguchi M, Kurita N, Inoue S, Sakamoto T, Nakamura M, Konishi N, Furuya H. Effects of delta-opioid agonist SNC80 on white matter injury following spinal cord ischemia in normothermic and mildly hypothermic rats. J Anesth. 2008;22(1):32–37. doi: 10.1007/s00540-007-0576-0. [DOI] [PubMed] [Google Scholar]
- 49.Govindaswami M, Brown SA, Yu J, Zhu H, Bishop PD, Kindy MS, Oeltgen PR. Delta 2-specific opioid receptor agonist and hibernating woodchuck plasma fraction provide ischemic neuroprotection. Acad Emerg Med. 2008;15(3):250–257. doi: 10.1111/j.1553-2712.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 50.Pamenter ME, Buck LT. δ-opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. 2008;211(Pt21):3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- 51.Peng PH, Huang HS, Lee YJ, Chen YS, Ma MC. Novel role for the δ-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108(3):741–754. doi: 10.1111/j.1471-4159.2008.05807.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhu M, Li M, Tian X, Ou X, Zhu C, Guo J. Neuroprotective role of δ-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 2009;1252:183–191. doi: 10.1016/j.brainres.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 53.Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45 (25):5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- 54.Xiong LZ, Yang J, Wang Q, Lu ZH. Involvement of delta-and mu-opioid receptors in the delayed cerebral ischemic tolerance induced by repeated electroacupuncture preconditioning in rats. Chin Med J (Engl) 2007;120(5):394–399. [PubMed] [Google Scholar]
- 55.Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through δ-opioid receptor. Stroke. 2006;37(4):1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- 56.Brouwer M, Larkin P, Brown-Peterson N, King C, Manning S, Denslow N. Effects of hypoxia on gene and protein expression in the blue crab, Callinectes sapidus. Mar Environ Res. 2004;58 (2–5):787–792. doi: 10.1016/j.marenvres.2004.03.094. [DOI] [PubMed] [Google Scholar]
- 57.Storey KB. Adventures in oxygen metabolism. Comp Biochem Physiol B Biochem Mol Biol. 2004;139(3):359–369. doi: 10.1016/j.cbpc.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Bindra RS, Schaffer PJ, Meng A, Woo J, Måseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM. Alterations in DNA repair gene expression under hypoxia: elucidating the mechanisms of hypoxia-induced genetic instability. Ann N Y Acad Sci. 2005;1059:184–195. doi: 10.1196/annals.1339.049. [DOI] [PubMed] [Google Scholar]
- 59.Appenzeller O, Minko T, Qualls C, Pozharov V, Gamboa J, Gamboa A, Wang Y. Gene expression, autonomic function and chronic hypoxia: lessons from the Andes. Clin Auton Res. 2006;16(3):217–222. doi: 10.1007/s10286-006-0338-3. [DOI] [PubMed] [Google Scholar]
- 60.Mayfield KP, Kozak W, Malvin GM, Porreca F. Hypoxia decreases opioid delta receptor expression in mouse brain. Neuroscience. 1996;72:785–789. doi: 10.1016/0306-4522(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 61.Fujita T, Kumamoto E. Inhibition by endomorphin-1 and endomorphin-2 of excitatory transmission in adult rat substantia gelatinosa neurons. Neuroscience. 2006;139(3):1095–1105. doi: 10.1016/j.neuroscience.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Wayne NL, Kuwahara K. Beta-endorphin alters electrical activity of gonadotropin releasing hormone neurons located in the terminal nerve of the teleost medaka (Oryzias latipes) Gen Comp Endocrinol. 2007;150(1):41–47. doi: 10.1016/j.ygcen.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Chao D, Bazzy-Asaad A, Balboni G, Xia Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212(1):60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- 64.Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2007b;27(2):356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- 65.Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. 2008;18(9):2217–2227. doi: 10.1093/cercor/bhm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of δ-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol Life Sci. 2009;66(6):1105–1115. doi: 10.1007/s00018-009-8759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 68.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enchancement of outward potassium current. Science. 1997;278(5335):114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 69.Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmer E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86 (4):966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- 70.Wei L, Yu SP, Gottron F, Snider BJ, Zipfei GJ, Choi DW. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke. 2003;34(5):1281–1286. doi: 10.1161/01.STR.0000065828.18661.FE. [DOI] [PubMed] [Google Scholar]
- 71.Mongin AA. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology. 2007;14 (3–4):183–193. doi: 10.1016/j.pathophys.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nistico R, Piccirilli S, Sebastianelli L, Nistico G, Bernardi G, Mercuri NB. The blockade of K+-ATP channels has neuroprotective effects in an in vitro model of brain ischemia. Int Rev Neurobiol. 2007;82:383–395. doi: 10.1016/S0074-7742(07)82021-6. [DOI] [PubMed] [Google Scholar]
- 73.Karki P, Seong C, Kim JE, Hur K, Shin SY, Lee JS, Cho B, Park IS. Intracellular K+ inhibits apoptosis by suppressing the Apaf-1 apoptosome formation and subsequent downstream pathways but not cytochrome c release. Cell Death Differ. 2007;14(12):2068–2075. doi: 10.1038/sj.cdd.4402221. [DOI] [PubMed] [Google Scholar]
- 74.Tsao LI, Su TP. Hibernation-induction peptide and cell death: [D-Ala2,D-Leu5]enkephalin blocks Bax-related apoptotic processes. Eur J Pharmacol. 2001;428(1):149–151. doi: 10.1016/s0014-2999(01)01346-2. [DOI] [PubMed] [Google Scholar]
- 75.Zhao P, Ma MC, Qian H, Xia Y. Decreased density of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53(4):442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Yao H, Gu XQ, Douglas RM, Haddad GG. Role of Na+/H+ exchanger during O2 deprivation in mouse CA1 neurons. Am J Physiol (Cell Physiol) 2001;281(4):C1205–1210. doi: 10.1152/ajpcell.2001.281.4.C1205. [DOI] [PubMed] [Google Scholar]
- 77.Chan P, Warwicker J. Evidence for the adaptation of protein pH-dependence to subcellular pH. BMC Biol. 2009;7:69. doi: 10.1186/1741-7007-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]