Abstract
Previous research has indicated that the blockade of H3-type histamine receptors may improve attention and memory in normal rodents. The purpose of this study was to determine if ciproxifan, an H3 receptor antagonist, could alleviate the hyperactivity and cognitive deficits observed in a transgenic mouse model (APPTg2576) of Alzheimer’s disease. APPTg2576 mice displayed significantly greater locomotor activity than wild-type mice, but APPTg2576 mice provided with daily ciproxifan treatment showed activity levels that did not differ from wild-type mice. In the swim maze, APPTg2576 mice exhibited significantly longer escape latencies, but the APPTg2576 mice treated daily with ciproxifan had latencies that were indistinguishable from controls. In probe trials conducted one hour after the last training trial, ciproxifan-treated APPTg2576 mice spent more time near the previous platform location and made more crossings of this area than did saline-treated APPTg2576 mice. APPTg2576 mice also demonstrated a significant impairment in the object recognition task that was reversed by acute treatment with ciproxifan (3.0 mg/kg). These data support the idea that modulation of H3 receptors represents a novel and viable therapeutic strategy in the treatment of Alzheimer’s disease.
Keywords: Locomotor activity, swim maze, object recognition, histamine, amyloid-precursor protein, cognitive enhancement
1.1 Introduction
Over the past decade, preclinical research has identified the H3 histamine receptor as a possible target for cognitive-enhancing drugs (Bonaventure, Letavic, Dugovic, Wilson, Aluisio, et al., 2007; Esbenshade, Fox, & Cowart, 2006). The H3 receptor exists as a presynaptic autoreceptor that is expressed in relatively high densities in brain regions associated with memory function, such as the frontal cortex and hippocampus (Pillot, Heron, Cochois, Tardivel-Lacombe, Ligneau et al. 2002). Antagonism (or inverse agonism) of the receptor leads to the release of histamine as well as neurotransmitters involved in learning and memory, such as acetylcholine and dopamine, in the hippocampus and prefrontal cortex (Bacciottini, Passani, Giovannelli, Cangioli, Mannaioni et al., 2002; Clapham & Kilpatrick, 1992; Fox, Esbenshade, Pan, Radek, Krueger, et al., 2005; Ligneau, Lin, Vanni-Mercier, Jouvet, Muir, Ganellin, et al., 1998; Ligneau, Perrin, Landais, Camelin, Calmels, et al., 2007b; Medhurst, Atkins, Beresford, Brackenborough, Briggs, et al., 2007). Moreover, H3 antagonists can generate theta rhythms in the brain (Hajós, Siok, Hoffmann, Li, & Kocsis, 2008) – a form of activity that predicts the onset of new learning (Berry & Seager 2001). On a behavioral level, drugs that act as H3 antagonists, such as the prototypical imidazole-containing compounds, ciproxifan and thioperamide, have been shown to improve memory function in several tasks – in normal rats and mice, as well as in animals treated with anti-cholinergic or anti-glutamatergic drugs (Bardgett, Points, Kleier, LaMontagne, & Griffith, 2010; Fox, Pan, Radek, Lewis, Bitner, et al., 2003; Bernaerts, Lamberty, & Tirelli, 2004; Galici, Boggs, Aluisio, Fraser, Bonaventure, et al., 2009; Ligneau et al., 1998). In addition to their effects on learning and memory, H3 antagonists have also been shown to modulate the elevating effects of psychostimulants on locomotor activity in rats and mice (Clapham & Kilpatrick, 1994, Fox et al., 2005; Ligneau, Landais, Perrin, Piriou, Uguen, et al., 2007a; Morisset, Pilon, Tardivel-Lacombe, Weinstein, Rostene, et al., 2002).
The ability of H3 antagonists to enhance memory in normal animals and in pharmacological models of memory impairment raises the possibility that such compounds may represent an effective treatment strategy for Alzheimer’s disease. As a way of addressing this possibility, we tested the effects of the H3 antagonist, ciproxifan (Ligneau et al., 1998), on the learning and memory deficits and hyperlocomotion observed in the amyloid precursor protein (APPTg2576) transgenic mouse model of Alzheimer’s disease. Developed by Hsiao and colleagues (1996), APPTg2576 mice express a mutant form of the human APP gene associated with early-onset, familial Alzheimer’s disease. These mice exhibit a phenotype that includes the formation of amyloid plaques with increasing age as well as deficits in spatial learning and memory, and object recognition (Hsiao, Chapman, Nilsen, Eckman, Harigaya, et al., 1996; Taglialatela, Hogan, Zhang, & Dineley, 2009). Some studies of APPTg2576 mice have also reported that mutant mice demonstrate elevated locomotor activity (Golub, Germann, Mercer, Gordon, Morgan, et al., 2008; Tabuchi, Yamaguchi, Iizuka, Imamura, Ikarashi, et al., 2009).
In the first study, the effects of ciproxifan on locomotor activity and performance in the swim maze were compared between APPTg2576 mice and wild-type (WT) littermates of both genders at 12–14 months of age. Using a between-subjects design, approximately half of the mice of each genotype received daily intraperitoneal injections of ciproxifan (3 mg/kg) and the other half received injections of saline. The dose of ciproxifan chosen for study was based on previous research demonstrating its ability to improve attention and memory in normal rats (Bardgett et al., 2010; Fox et al., 2003; 2005; Ligneau et al., 1998) and inhibit stimulant-induced hyperactivity in mice (Morisset et al., 2002). Mice received daily injections for one week prior to testing and daily injections 30 minutes prior to testing over the subsequent three weeks. In a second study using a within-subjects design, a separate cohort of 12–14 month old APPTg2576 and WT mice was tested in a novel object recognition task. Ciproxifan or saline was injected 30 minutes prior to testing in this study.
1.2 Materials and methods
1.2.1. Animals and housing
Offspring of an original crossing of APPTg2576 (Taconic Labs, Hudson, NY) male mice with B6SJLF1/J (Jackson Laboratory, Bar Harbor, ME) female mice were backcrossed with C57Bl6/J mice (Jackson Laboratory, Bar Harbor, ME) for five generations. APPTg2576 mice and their wild-type (WT) littermates of each gender representing the sixth generation of this breeding protocol were used in the present studies. They were housed three to four per cage with free access to food and water. Lighting in the animal colony was maintained on a 12-hour light/dark schedule with lights on at 07:00. All experimental procedures were performed according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Use and Care Committee.
1.2.2. Genotyping
PCR genotyping was performed using DNA isolated from post-weaning tail biopsies. Forward (5′-GTGGATAACCCCTCCCCCAGCCTAGACCA-3′) and reverse (5′-CTGACCACTCGACCAGGTTCTGGGT-3′) primers amplified a 410-base pair product from the amyloid precursor protein transgene (APPTg2576). DNA isolated from non-transgenic littermates did not give a PCR product and served as negative controls.
1.2.3. Ciproxifan dosing and injections
Ciproxifan was kindly provided by the National Institute of Mental Health’s Chemical Synthesis and Drug Supply program and dissolved in saline prior to each injection. In each experiment, mice received intraperitoneal injections 30 minutes prior to testing of either saline or ciproxifan (3.0 mg/kg of body weight). This dose of ciproxifan was based on earlier studies demonstrating that it effectively improved attention, inhibitory avoidance, and social and spatial memory in rats (Bardgett et al., 2010; Fox et al., 2003;2005; Ligneau et al., 1998) and antagonized stimulant-induced hyperactivity in mice (Morisset et al., 2002). Using a between subject design, the first cohort of mice received daily injections beginning one week before locomotor testing and 30 minutes prior to testing each day over the subsequent three weeks. Using a within-subjects, repeated-measures design, the second cohort of mice received injections 30 minutes prior to each object recognition test in a counter-balanced manner.
1.2.4. Locomotor testing
A total of 39 twelve - fourteen month old mice were tested for locomotor activity: 10 WT and 9 APPTg2576 mice treated with saline, and 9 WT and 11 APPTg2576 mice were treated with ciproxifan. Of the 39 mice tested, nine were male (4 WT and 5 APP) and 30 were female (15 WT and 15 APP). Testing equipment consisted of 12 Hamilton-Kinder (HK) Cage Rack - high density activity frames with 12 polypropylene cages (28cm x 17cm x 12.5cm h) tracked by (HK) MotorMonitor software (Kinder Scientific, Poway, CA). Drug injections occurred 30 minutes prior to testing. Mice were placed in the testing apparatus with the lights off for 60 minutes. Testing was conducted between 8:00 a.m. and 2:00 p.m. for 5 consecutive days.
1.2.5. Swim maze training and testing
A total of 38 mice tested for locomotor activity were trained and tested in the hidden and visible versions of swim maze task: 9 WT and 8 APPTg2576 mice treated with saline, and 9 WT and 11 APPTg2576 mice treated with ciproxifan. Of the 38 mice tested, nine were male (4 WT and 5 APP) and 29 were female (15 WT and 14 APP). Mice were placed in the testing room 30 minutes prior to testing on each test day. The swim maze consisted of a 1.2 m high circular stainless steel tank measuring 114 cm in diameter. Data were collected and analyzed using San Diego Instruments SmartTrack software (San Diego, CA). The platform consisted of a weighted PVC pipe base that was 45.7 cm tall with a Plexiglas platform that was 12 cm in diameter. The platform was maintained at approximately 2 cm below the surface of the water for habituation and the hidden platform conditions. White non-toxic poster paint was added to the pool to make the water opaque. The water was kept at room temperature. All drug injections occurred 30 minutes prior to training and testing.
Habituation
The mice were habituated to the swim maze by placing the platform in the center of the pool. Mice were placed in the water near the platform and guided to it. They were then required to climb upon the platform and stay there for 10 seconds. This procedure was repeated three times. All mice demonstrated an ability to climb and stay on the platform for 10 seconds by the end of the third trial. Following all trials, mice were placed in a holding cage that had a 60 watt lamp centered immediately above it to provide warmth and an absorbent pad placed on its floor to absorb excess water.
Hidden Platform Condition
For training, each mouse was placed in the swim maze at a randomly chosen starting point and was required to find the platform within 60 seconds. The same start point was used for each trial on a given day, but switched each day. Escape was defined as climbing onto the platform, and each mouse was left on the platform for 10 seconds after escape. If the platform was not found within 60 seconds, the mouse was manually placed on the platform for 10 seconds and then returned to a holding cage. Five days of training were conducted, and each day of testing consisted of four test trials with a five-minute intertrial interval. During each training trial, the following measures were recorded: latency to find the platform, path length, swim speed, and average distance from the platform.
Probe Trial
A probe trial was conducted one hour after training on the last day of the hidden platform condition. The platform was removed from the pool. Each mouse was placed in the pool at a starting point opposite the original platform location and allowed to swim for 60 seconds. A second probe trial was conducted 48 hours later. During each trial, the following measures were recorded: the number of crossings over the previous platform location and the time spent swimming in an area (2232.56 cm2) that included the former platform location.
Visible Platform Condition
Two days after the completion of hidden platform testing, the ability to locate a visible platform was tested in each mouse over three consecutive days. A Styrofoam ball wrapped in aluminum foil was attached to a bolt sticking 7 cm above the platform. Each day of testing consisted of four test trials with a five-minute intertrial interval. Prior to each trial, the platform was moved to a new location. At the beginning of each trial, the mouse was placed into the water at a randomly chosen starting point that remained constant within each day of testing. During each trial, the following measures were recorded: latency to find the platform, path length, swim speed, and average distance from the platform. Each mouse was given 60 seconds to find the platform. If the mouse did not find the platform within 60 seconds, it was placed on it for 10 seconds and then returned to a holding cage.
1.2.6. Novel object recognition
A within-subjects, repeated-measures design was used to assess the effects of ciproxifan on object recognition behavior in a separate cohort of 11 APPTg2576 and 11 WT mice (6 females and 5 males per group). The mice in this cohort were 12–14 months old. A 60 cm × 60 cm × 60 cm gray wooden box with Plexiglas floor painted gray was used to test the mice. Four 50 ml polypropylene lids were anchored flush into the floor upside down 12 cm from each corner - these were used to secure objects to the floor. Six groups of three objects (glass or plastic) were used and each object had the threaded end (~2 cm in length) of a 50 ml polypropylene tube glued to its bottom. A digital camera mounted 6 feet above the apparatus was connected to a video monitor in an adjacent room and was used to observe object investigation.
Habituation
The mice were habituated by placing them in the testing room for 20 minutes with the overhead lights on. The mice were then placed in the apparatus without any objects for 15 minutes. Habituation was performed for three days.
Testing
The mice were placed in the testing room 20 minutes prior to testing. Two identical objects were placed in the apparatus, diagonal from one another. The mice were then placed into the apparatus for five minutes. Investigation of each object was measured continuously every minute. A mouse was considered to be exploring an object when it was within 2 cm of the object and oriented within 45 degrees of the object or had one paw on the object. Climbing or sitting on the object was not considered exploration. After five minutes, the mice were removed from the testing apparatus and placed in a holding cage for five minutes. During this time, the original objects were removed and replaced with one identical object and one novel object. After the five-minute delay, the mice were returned to the apparatus for three minutes. A record of exploration for each object was recorded each minute. The apparatus and objects were cleaned between trials with 75% ethanol/water.
1.2.7. Confirmation of plaque deposition in APPTg2576 mice
APPTg2576 and WT mice were sacrificed three months after the completion of drug and behavioral testing in order to determine if plaques were present in the brains of the APP Tg2576 mice. Plaque deposition was confirmed using thioflavin-S stain (Sigma, St. Louis MO). Mounted sections were rinsed with 0.1M PBS, stained with a 1% thioflain-S solution, and differentiated in 70% ethanol. Sections were allowed to dry overnight then dehydrated and coverslipped with DPX mountant (Fluka). Plaques were visualized using an FITC fluorescent filter on a Zeiss Axioskop 40 microscope.
1.2.8. Data analyses
In the locomotor testing, data were averaged across the five daily trials for each five-minute test bin. A three-way analysis of variance (ANOVA) was conducted using drug treatment and genotype as between-groups factors and time (i.e., five minute test bin) as a repeated-measure. For the swim maze data, a three-way ANOVA was used for the training data with drug treatment and genotype as between-groups factors and test day as a repeated-measure. Probe trial was analyzed using a two-way ANOVA with drug treatment and genotype as between-groups factors. The same analysis was performed on the object recognition data with the exception that drug treatment served as a within-group factor. All post-hoc testing was conducted using Tukey/Kramer significant difference (TSD) tests. Statistical differences for all tests were considered significant if p < .05. Statistical testing was performed using StatView 5.0 for the Mac (SAS Institute, Inc., Cary, North Carolina).
1.3 Results
1.3.1. Confirmation of amyloid plaques in APPTg2576 mice
The appearance of amyloid plaques was confirmed in the brain of each APP mouse after the completion of drug and behavioral testing (Figure 1). Numerous plaque deposits were observed in the dorsal hippocampus and cortex of the APPTg2576 mice. These plaques were not observed in the brains of WT mice. In the first cohort of APPTg2576 mice, statistical analyses did not indicate a difference in the number of hippocampal or cortical plaques between APPTg2576 mice previously exposed to four weeks of ciproxifan treatment versus those treated with saline.
Figure 1.

Amyloid plaques in APPTg2576 mouse brain as revealed through Thioflavin-S staining. Numerous plaque deposits were observed in the hippocampus and cortex of the APPTg2576 mice. The photomicrograph in B. shows a higher magnification image of the boxed image in panel A. Scale bar = 1 mm in A. and 0.1 mm in B.
1.3.2. Ciproxifan reduces elevated locomotor activity in APPTg2576 mice
The effects of ciproxifan (3.0 mg/kg) on locomotor activity were examined in the first cohort of APPTg2576 and WT mice. Mice received daily intraperitoneal injections of saline or ciproxifan beginning one week prior to testing and were injected 30 minutes prior to testing on each day of locomotor testing. Locomotor testing was conducted one week prior to swim maze testing, and was performed for one hour a day for five days. APPTg2576 mice treated with saline were significantly more active than WT mice treated with saline (Genotype × treatment × time interaction: F (11, 385) = 2.0, p < .03)(Figure 2). This effect was statistically significant over the first 25 minutes of testing (TSD test, p ≤ .05 for each comparison). Ciproxifan reversed this effect in that APPTg2576 mice treated with ciproxifan were significantly less active than the APPTg2576 mice treated with saline during the first 10 minutes of testing (TSD test, p ≤ .05 for each comparison).
Figure 2.

Locomotor testing in APPTg2576 and WT mice treated with 3.0 mg/kg of ciproxifan (Cpx) or saline. APPTg2576 mice treated with saline were significantly more active than the WT mice treated with saline during the first 25 minutes of testing (indicated by +) and significantly more active than the APPTg2576 mice treated with ciproxifan during the first 10 minutes of testing (indicated by *). All data represent means ± s.e.m.
1.3.3. Ciproxifan improves the performance of APPTg2576 mice in the swim maze task
One week after the start of locomotor testing, the swim maze task was performed for four trials a day over five consecutive days. Ciproxifan improved performance in APPTg2576 mice over the course of the training trials (Figure 3). There was a statistical interaction between ciproxifan treatment and APP genotype (Interaction term: F (1, 34) = 6.93, p < .01) on the time to find the platform measure. APPTg2576 mice treated with saline took significantly longer to find the hidden platform relative to WT mice treated with saline on the fourth day of training (Figure 3a) (TSD test, p ≤ .05 for each comparison). On the second, fourth, and fifth day of training, APPTg2576 mice treated with ciproxifan took less time to find the platform than APPTg2576 mice treated with saline (Figure 3a) (TSD test, p ≤ .05 for each comparison). During the training trials, there was also a treatment by genotype interaction (Interaction term: F (1, 34) = 4.46, p < .04) as well as an overall training day effect (F (4, 136) = 9.7, p < .0001) on the average distance from the platform measure (Figure 3b). On the second day of training, the APPTg2576 mice treated with saline swam farther away from the platform than the WT mice treated with saline (TSD test, p ≤ .05 for each comparison). On the last four days of training, APPTg2576 mice treated with saline also swam farther away from the platform when compared to APPTg2576 mice treated with ciproxifan (TSD test, p ≤ .05 for each comparison). There was a trend towards a significant treatment × genotype interaction for the distance travelled before finding the platform (Interaction term: F (1, 34) = 4.46, p = .11 (two-tailed))(Figure 3c).
Figure 3.
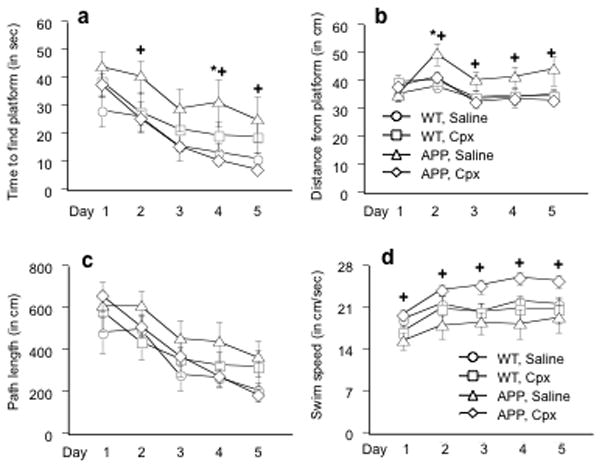
Hidden platform swim maze training in APPTg2576 and WT mice treated with 3.0 mg/kg of ciproxifan (Cpx) or saline. (a) APPTg2576 mice treated with saline took longer to find the escape platform in comparison to saline-treated WT mice on day 4 of training (indicated by *) and APPTg2576 mice treated with ciproxifan (3.0 mg/kg) on days 2, 4, & 5 of testing (indicated by +). (b) APPTg2576 mice swam a greater distance away from the platform in comparison to saline-treated WT mice on day 2 of training (indicated by *) and APPTg2576 mice treated with ciproxifan (3.0 mg/kg) on days 2–5 of testing (indicated by +). (c) There was a trend (p = .11) towards a treatment × genotype interaction on the path length measure during the hidden platform training trials. (d) The APPTg2576 mice treated with ciproxifan swam significantly faster than APPTg2576 mice treated with saline (indicated by +). All data represent means ± s.e.m.
Ciproxifan increased swimming speed in APPTg2576 mice (Interaction term: F (1, 34) = 6.54, p < .02)(Figure 3d). Unlike the results for the other measures, swimming speed did not distinguish APPTg2576 mice treated with saline from WT mice treated with saline. For the swim speed measure, the only significant group difference found on each training trial was between the APPTg2576 mice treated with ciproxifan and APPTg2576 mice treated with saline (TSD test, p ≤ .05 for each comparison).
One hour after the final training session, all mice were placed in the pool for one minute without the platform. During this probe trial, the APPTg2576 mice treated with ciproxifan made as many crossings of the former platform site as did the WT mice treated with saline and made significantly more crossings than the APPTg2576 mice treated with saline (Interaction term: F (1, 34) = 4.68, p < .04, TSD test, p ≤ .05 for each comparison)(Figure 4a) When the amount of time spent in an area of the pool that included the former platform was measured, the same pattern of results emerged. APPTg2576 mice treated with ciproxifan spent as much time as each group of WT mice in the zone that formerly contained the platform. In contrast to each of these groups, APPTg2576 mice treated with saline spent significantly less time in this area (Interaction term: F (1, 34) = 6.45, p < .02, TSD test, p ≤ .05 for each comparison)(Figure 4b). Mice were also tested 48 hours after the last training trial. At this time point, significant genotype or drug treatment effects were not observed for the number of platform crossings or time spent in the former platform zone (Figure 4c & 4d).
Figure 4.

Hidden platform probe trials in APPTg2576 and WT mice treated with 3.0 mg/kg of ciproxifan (Cpx) or saline. (a) APPTg2576 mice treated with saline displayed fewer crossings of the former platform location one hour after the last training trial in comparison WT mice treated with saline (indicated by *) and APPTg2576 mice treated with ciproxifan (indicated by +). (b) APPTg2576 mice treated with saline spent less time near the former location of the platform one hour after the last training trial in comparison to all other groups (indicated by #). There were no statistically significant effects of treatment, genotype, or treatment × genotype on (c) the number of crossings or (d) time spent near the platform when the mice were tested 48 hours after the last training trial. All data represent means ± s.e.m.
Two days after the conclusion of the probe trial, a three-day swim maze test was performed using a visual cue to mark the location of the platform. Mice were provided with four trials per day for three days. The location of the platform was made visible by attaching a ball covered in aluminum foil 7 cm above the platform, and was changed on each day of testing. There was a significant treatment by genotype interaction on the time to find the platform (Treatment × genotype interaction: F (1, 33) = 7.05, p < .01)(Figure 5a). APPTg2576 mice treated with saline took significantly longer to find the platform on days 1 and 2 of training in comparison to the APPTg2576 mice treated with ciproxifan while WT mice treated with ciproxifan also took significantly longer to find the platform on day 2 of training when compared to APPTg2576 mice treated with ciproxifan (TSD test, p ≤ .05 for each comparison). There were no significant treatment or genotype effects on the average distance from the platform during each trial (Figure 5b), but there was a significant interaction between these two factors for the path length measure (Treatment x genotype interaction: F (1, 33) = 7.47, p < .01)(Figure 5c). APPTg2576 mice treated with saline swam farther before finding the platform than APPTg2576 mice treated with ciproxifan on days 2 and 3 of training, while WT mice treated with ciproxifan swam farther than APPTg2576 mice treated with ciproxifan on day 2 of training (TSD test, p ≤ .05 for each comparison). There were no significant treatment or genotype effects on swim speed during the visible platform trials (Figure 5d).
Figure 5.

Visible platform swim maze training in APPTg2576 and WT mice treated with 3.0 mg/kg of ciproxifan (Cpx) or saline. (a) APPTg2576 mice treated with saline took significantly longer to find the platform on the first two training days relative to the APPTg2576 mice treated with ciproxifan (indicated by +). (b) There were no significant treatment or genotype effects on the average distance from the platform during each trial. (c) Path length prior to finding the platform was greater for APPTg2576 mice treated with saline than APPTg2576 mice treated with ciproxifan on the last two training days (indicated by +). (d) There were no significant treatment or genotype effects on swim speed. All data represent means ± s.e.m.
1.3.4. Ciproxifan improves the performance of APPTg2576 mice in the novel object recognition task
Novel object recognition was tested in a separate cohort of APPTg2576 and WT mice. The effects of ciproxifan were assessed using a repeated-measures, within-subjects design. The order of drug treatments was counter-balanced across subjects within each genotype group. Ciproxifan improved object recognition in APPTg2576 mice (Interaction term: F (1, 20) = 11.8, p < .003) (Figure 6a). APPTg2576 mice spent significantly less time exploring a novel object relative to a familiar one in comparison to all other groups, including the APPTg2576 mice treated with ciproxifan (TSD test, p ≤ .05 for each comparison). The preference of this latter group of mice for the novel object did not differ from the WT mice treated with saline. Neither genotype or drug treatment altered the total time spent exploring both objects during testing (Figure 6b).
Figure 6.

Novel object recognition in APPTg2576 and WT mice treated with 3.0 mg/kg of ciproxifan (Cpx) or saline. (a) APPTg2576 mice treated with saline spent less time exploring a novel object relative to the time spent exploring a familiar one when compared to all other groups, including the APPTg2576 mice treated with ciproxifan (indicated by #). (b) The total time spent exploring both objects during the test trial did not differ between groups. All data represent means ± s.e.m.
1.4. Discussion
These experiments are the first to demonstrate that an H3 antagonist can alleviate hyperactivity and memory impairment in a transgenic mouse model of Alzheimer’s disease. The capacity for ciproxifan to improve cognitive and behavioral outcomes across multiple tests opens the door for the pursuit of H3 antagonism as a therapeutic strategy in the treatment of Alzheimer’s disease. Obviously, more work is needed to discern the types of behavioral deficits in APPTg2576 mice and other transgenic models of Alzheimer’s disease that are sensitive to H3 antagonists as well as identifying the types of H3 antagonists that are effective in these models and the mechanism by which they work.
Relative to reports on learning and memory, relatively few studies have described changes in locomotor activity in APPTg2576 mice. At least two studies (Golub et al., 2008; Tabuchi et al., 2009) have shown that APPTg2576 mice are more active than WT controls, with hyperactivity emerging as early as four months of age and persisting through 15 months of age (Golub et al., 2008). It is not clear what neurobiological mechanism accounts for elevated locomotor activity in APPTg2576 mice, although the reported loss of synapses in the hippocampus of APPTg2576 mice (Dong, Martin, Chambers, & Csernansky, 2007) could possibly lead to dysregulation of downstream targets, such as the nucleus accumbens, that modulate locomotion (e.g., David & Abraini, 2001; White, Whitaker, & White, 2006), akin to the hyperlocomotion observed in animals with overt hippocampal damage (Bardgett, Jacobs, Jackson, & Csernansky, 1997; Bardgett, Baum, O’Connell, Lee, & Hon, 2006). If true, then the ability of ciproxifan to mitigate the elevated locomotor activity seen in APPTg2576 mice may be related to the ability of H3 antagonists to enhance neurotransmitter release (Bacciottini et al., 2002) or electrophysiological activity (Hajos et al., 2008) in the hippocampus, or induce immediate early gene expression in brain regions, such as the nucleus accumbens (Southam, Cilia, Gartlon, Woolley, Lacroix et al., 2009) or motor cortex (Bonaventure et al., 2007), that directly regulate locomotor behavior. Another possibility is that ciproxifan disrupts dopaminergic activity related to hyperactivity since H3 antagonists have been shown decrease locomotor responses to dopamine agonists, such as apomorphine, cocaine, and methamphetamine (Clapham & Kilpatrick, 1994; Morisset et al., 2002; Fox et al., 2005; Ligneau et al., 2007a; although see Brabant et al., 2009; Ferrada et al., 2008), although enhancement of psychostimulant effects by H3 antagonists has also been reported (Bardgett, Points, Roflow, Blankenship, & Griffith, 2009; Bardgett et al., 2010; Brabant, Alleva, Grisar, Quertemont, Lakaye, et al., 2009). Finally, it would be helpful to determine the effects of additional doses of ciproxifan on the locomotor and cognitive deficits seen in APPTg2576 mice. However, it should be noted that the dose of ciproxifan (3.0 mg/kg) chosen for study binds nearly 90% of H3 receptors (Le, Gruner, Mathiasen, Marino, & Schaffhauser, 2008) – a higher dose would likely effect increased antagonism of other receptors (alpha2 adrenergic, 5-HT3) with known affinity for ciproxifan (Esbenshade, Krueger, Miller, Kang, & Denny, et al., 2003).
Ciproxifan improved the performance of the APPTg2576 mice in two well-characterized memory tasks: the swim maze and the object recognition test. These tasks were chosen because APPTg2576 mice demonstrate clear deficits in each one (Hsiao et al., 1996; Westerman, Cooper-Blacketer, Mariash, Kotilinek, Kawarabayash, et al., 2002; Taglialatela et al., 2009). Previous studies have found that ciproxifan and other H3 antagonists, such as ABT-239, GSK189254, GSK207040, and JNJ-10181457, enhance social memory (Fox et al., 2003; 2005), reversal learning (Gallici et al., 2009), and object recognition (Medhurst et al., 2007; Southam et al., 2009) in normal rats, improve social memory (Fox et al., 2005) and swim maze learning and memory in aged rats (Medhurst et al., 2007), and reverse the debilitating effects of scopolamine in a two-choice, swim maze-based spatial discrimination task (Fox et al., 2005), a passive avoidance task (Medhurst et al., 2007), and a delayed non-matching to sample task (Gallici et al., 2009). However, no studies have reported the effects of an H3 antagonist on the performance of APPTg2576 mice in any of these tasks. The ability of ciproxifan to improve performance in the swim maze and object recognition tasks may be related to the H3-mediated neurotransmitter release thought to contribute to the cognitive-enhancing effects of H3 antagonists seen in other models. For example, H3 antagonists have been shown to elevate acetylcholine and dopamine in the prefrontal cortex (Fox et al., 2005; Ligneau et al., 2007b; Gallici et al., 2009), acetylcholine, dopamine, and norepinephrine in the anterior cingulate cortex (Medhurst et al., 2007; Southam et al., 2009), and acetylcholine in the hippocampus (Fox et al., 2005). Given these findings, it would be of interest to determine if H3 antagonists such as ciproxifan elevate neurotransmitter release in a comparable manner in APPTg2576 mice.
An alternative explanation for the ability of ciproxifan to improve performance in APPTg2576 mice relates to the effects of H3 antagonists on Aβ toxicity. Using a cell culture model, it has been reported that the H3 antagonist, clobenpropit, protects cells from injury produced by Aβ42 toxicity via a glutamate receptor-mediated mechanism (Fu, Dai, He, Hu, Fan, et al., 2010). Given the limited time course of ciproxifan treatment, it is unlikely that the behavioral changes produced by ciproxifan in the present study were related to H3 receptor-mediated modification of Aβ’s progressive toxic effects in APPTg2576 mice. It will be important for future studies to test whether longer-term treatment with H3 antagonists can modify these and other pathophysiological processes (e.g. synapse loss; Dong et al., 2007) observed in the APP mouse model.
The interpretation that ciproxifan enhances task performance in APPTg2576 mice due to exclusive effects on learning or memory merits caution for several reasons. First, the poor performance of the APPTg2576 mice on the visible platform task may suggest that these mice harbor sensory or perceptual deficits. King and Arendash (2002) reported more significant deficits in APPTg2576 mice in the visible version of the swim maze relative to the hidden version, whereas Westerman et al., (2002) found differences between APPTg2576 and WT mice only in the hidden platform task. In the latter study, mice were tested in the visible platform version first, whereas in the former study and present one, mice were tested in the visible platform task after the hidden platform testing was performed, perhaps suggesting that the sequence of tests significantly alters performance. While the visible platform task has been intended to identify more basic sensorimotor deficits in mice, King and Arendash (2002) nicely argued that this task may still rely on intact learning processes by citing research showing that: 1) visual acuity is not always associated with performance in the task (Lindner, Plone, Shallert, & Emerich, 1997), 2) lesions to “cognitive” brain regions, such as the frontal cortex, impair performance in the visible, but not the hidden, platform task (de Bruin, Sanchez-Santed, Heinsbroek, Donker, & Postmes, 1994), and 3) performance in the visible platform task correlates well with performance on other cognitive tasks (King & Arendash, 2002). It is noteworthy that, like its effect in the hidden version of the swim maze task, ciproxifan improved performance in visible platform version of the task in APP mice. It is possible that ciproxifan improved attention to visual cues in APPTg2576 mice, since ciproxifan has been shown to improve visual attention in other studies (Ligneau et al., 1998).
It is also possible that general changes in anxiety or activity account for the effects of ciproxifan in the swim maze task. Some studies have provided evidence that anxiety is increased in some transgenic models of AD (España, Giménez-Llort, Valero, Miñano, Rábano, et al., 2010; Le Cudennec, Faure, Ly, & Delatour, 2008), although APPTg2576 mice may actually show an anxiolytic phenotype (Gil-Bea, Aisa, Schliebs, & Ramírez, 2007; Lalonde, Lewis, Strazielle, Kim, & Fukuchi, 2003). Other work has shown that stress can induce greater memory impairment in APPTg2576 mice (Dong, Goico, Martin, Csernansky, Bertchume, et al., 2004; Dong, Yuede, Yoo, Martin, Deal, et al., 2008), raising the possibility that ciproxifan improves memory in APPTg2576 mice by simply reducing the stress associated with the swim maze task. These explanations are unlikely, however, since H3 agonists are known to elicit anxiolytic effects in animals (Yokoyama, Yamauchi, Oyama, Okuma, Onozawa 2009) while there are no reports of such effects after treatment with H3 antagonists - although H3 knockout mice demonstrate signs of reduced anxiety (Rizk, Curley, Robertson, & Raber 2004). While a significant influence of H3 receptors over the anxiety produced by water exposure in the swim maze is unlikely, the possible contribution of the hyperactivity observed in the APPTg2576 mice to the performance deficit observed in these mice is more difficult to refute. As proposed by King and Arendash (2002) and Lalonde et al., (2003), greater locomotor activity in APPTg2576 mice may represent a core deficit in inhibitory control that could be expected to affect the attentional processing required to complete the swim maze task. This idea merits further investigation.
Another aspect of the data that deserves consideration is the increased swim speed observed after ciproxifan treatment in the hidden version of the swim maze. Such an increase fits with other characterizations of ciproxifan as a mild stimulant drug, since earlier studies found that ciproxifan induces less slow-wave EEG activity and promotes greater wakefulness in rats and cats (Ligneau et al., 1998; Fox et al., 2003). To our knowledge, this is the first report of ciproxifan increasing swim speed in the swim maze, although no such effect has been seen with another H3 antagonist, GSK189254 (Medhurst et al., 2007). While increases in swim speed could explain differences in the latency to find the platform or the distance from the platform measures, it should be noted that unlike the latter data sets, the swim speed of the APPTg2576 mice treated with saline did not differ from WT mice treated with saline during the last four days of hidden platform training. These distinct patterns of data suggest that group differences in swim speed do not account completely for the significant differences found on the other measures of training performance.
APPTg2576 mice treated with ciproxifan spent more time near the former platform location relative to APPTg2576 mice treated with saline when tested one hour after the last training trial. This same difference was not observed two days after the last training trial, nor was there a genotype effect observed during this latter trial. These data would seem to suggest that ciproxifan can at least enhance short-term memory processes in APPTg2576 mice. The idea that ciproxifan improves shorter-term memory also is buttressed by the finding that ciproxifan alleviated object recognition deficits in APPTg2576 mice when a five minute delay was used. Overall, the results point to the likelihood that ciproxifan acts as a mild stimulant drug that provides short-term alleviation for the cognitive deficits and hyperactivity observed in APPTg2576 mice.
1.4.1. Conclusions
Current palliative treatments for Alzheimer’s disease include acetylcholinesterase inhibitors and NMDA antagonists, yet these treatments possess only limited efficacy and significant side effects. Receptor binding data has shown that H3 receptor densities are preserved in the brains of people with Alzheimer’s despite disease progression (Medhurst et al., 2007), indicating that the target for H3 antagonists remains a viable one throughout the disease process. Therefore, antagonism of H3 receptors may represent an alternative pathway to cognitive enhancement and remediation of other behavioral symptoms in Alzheimer’s disease, or at least an approach that can be coupled with current treatments (Bembenek, Keith, Letavic, Apodaca, Barbier, et al., 2008).
Acknowledgments
This work was supported by National Center for Research Resources Grant 2P20 RR16481 and National Institute of Mental Health Grant R15 MH076788 to M.E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacciottini L, Passani MB, Giovannelli L, Cangioli I, Mannaioni PF, Schunack W, Blandina P. Endogenous histamine in the medial septum-diagonal band complex increases the release of acetylcholine from the hippocampus: a dual-probe microdialysis study in the freely moving rat. European Journal of Neuroscience. 2002;15:1669–1680. doi: 10.1046/j.1460-9568.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Baum KT, O’Connell SM, Lee NM, Hon JC. Effects of risperidone on locomotor activity and spatial memory in rats with hippocampal damage. Neuropharmacology. 2006;51:1156–1162. doi: 10.1016/j.neuropharm.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Jacobs PS, Jackson JL, Csernansky JG. Kainic acid lesions enhance locomotor responses to novelty, saline, amphetamine, and MK-801. Behavioural Brain Research. 1997;84:47–55. doi: 10.1016/s0166-4328(96)00132-5. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Points M, Kleier J, Blankenship M, Griffith MS. The H3 antagonist, ciproxifan, alleviates the memory impairment but enhances the motor effects of MK-801 (Dizocilpine) in rats. Neuropharmacology. 2010;59:492–502. doi: 10.1016/j.neuropharm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Points M, Roflow J, Blankenship M, Griffith MS. Effects of the H3 receptor antagonist, thioperamide, on behavioral alterations induced by systemic MK-801 administration. Psychopharmacology. 2009;205:589–597. doi: 10.1007/s00213-009-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bembenek SD, Keith JM, Letavic MA, Apodaca R, Barbier AJ, Dvorak L, Aluisio L, Miller KL, Lovenberg TW, Carruthers NI. Lead identification of acetylcholinesterase inhibitors-histamine H3 receptor antagonists from molecular modeling. Bioorganic & Medicinal Chemistry. 2008;16:2968–2973. doi: 10.1016/j.bmc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Bernaerts P, Lamberty Y, Tirelli E. Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice. Behavioural Brain Research. 2004;154:211–219. doi: 10.1016/j.bbr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Berry SD, Seager MA. Hippocampal theta oscillations and classical conditioning. Neurobiology of Learning and Memory. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Letavic M, Dugovic C, Wilson S, Aluisio L, Pudiak C, Lord B, Mazur C, Kamme F, Nishino S, Carruthers N, Lovenberg T. Histamine H3 receptor antagonists: from target identification to drug leads. Biochemical Pharmacology. 2007;73:1084–1096. doi: 10.1016/j.bcp.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Grisar T, Quertemont E, Lakaye B, Ohtsu H, Lin JS, Jatlow P, Picciotto MR, Tirelli E. Effects of the H3 receptor inverse agonist thioperamide on cocaine-induced locomotion in mice: role of the histaminergic system and potential pharmacokinetic interactions. Psychopharmacology. 2009;202:673–687. doi: 10.1007/s00213-008-1345-y. [DOI] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Histamine H3 receptors modulate the release of [3H]-acetylcholine from slices of rat entorhinal cortex: Evidence for the possible existence of H3 receptor subtypes. British Journal of Pharmacology. 1992;107:919–923. doi: 10.1111/j.1476-5381.1992.tb13386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham J, Kilpatrick GJ. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. European Journal of Pharmacology. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Differential modulation of the D1-like- and D2-like dopamine receptor-induced locomotor responses by group II metabotropic glutamate receptors in the rat nucleus accumbens. Neuropharmacology. 2001;41:454–463. doi: 10.1016/s0028-3908(01)00082-x. [DOI] [PubMed] [Google Scholar]
- de Bruin J, Sanchez-Santed F, Heinsbroek R, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Research. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;27:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. Journal of Comparative Neurology. 2007;10:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Molecular Interventions. 2006;6:77–88. doi: 10.1124/mi.6.2.5. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Krueger KM, Miller TR, Kang CH, Denny LI, Witte DG, Yao BB, Fox GB, Faghih R, Bennani YL, Williams M, Hancock AA. Two novel and selective nonimidazole histamine H3 receptor antagonists A-304121 and A-317920: I. In vitro pharmacological effects. Journal of Pharmacological and Experimental Therapeutics. 2003;305:887–896. doi: 10.1124/jpet.102.047183. [DOI] [PubMed] [Google Scholar]
- España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biological Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Ferrada C, Ferré S, Casadó V, Cortés A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Radek RJ, Lewis AM, Bitner RS, Esbenshade TA, Faghih R, Bennani YL, Williams M, Yao BB, Decker MW, Hancock AA. Two novel and selective nonimidazole H3 receptor antagonists A-304121 and A-317920: II. In vivo behavioral and neurophysiological characterization. Journal of Pharmacology and Experimental Therapeutics. 2003;305:897–908. doi: 10.1124/jpet.102.047241. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley M, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA. Pharmacological properties of ABT-239 [4-2-{2-[2R-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-ylbenzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. Journal of Pharmacology and Experimental Therapeutics. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Fu Q, Dai H, He P, Hu W, Fan Y, Zhang W, Chen Z. The H3 receptor antagonist clobenpropit protects against Abeta42-induced neurotoxicity in differentiated rat PC12 cells. Pharmazie. 2010;65:257–260. [PubMed] [Google Scholar]
- Galici R, Boggs JD, Aluisio L, Fraser IC, Bonaventure P, Lord B, Lovenberg TW. JNJ-10181457, a selective non-imidazole histamine H3 receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology. 2009;56:1131–1137. doi: 10.1016/j.neuropharm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, Aisa B, Schliebs R, Ramírez MJ. Increase of locomotor activity underlying the behavioral disinhibition in tg2576 mice. Behavioral Neuroscience. 2007;121:340–344. doi: 10.1037/0735-7044.121.2.340. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Mercer M, Gordon MN, Morgan DG, Mayer LP, Hoyer PB. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiology of Aging. 2008;29:1512–1523. doi: 10.1016/j.neurobiolaging.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Siok CJ, Hoffmann WE, Li S, Kocsis B. Modulation of hippocampal theta oscillation by histamine H3 receptors. Journal of Pharmacology and Experimental Therapeutics. 2008;324:391–398. doi: 10.1124/jpet.107.130070. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckmanm C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiology & Behavior. 2002;75:627–642. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Lewis TL, Strazielle C, Kim H, Fukuchi K. Transgenic mice expressing the betaAPP695SWE mutation: effects on exploratory activity, anxiety, and motor coordination. Brain Research. 2003;977:38–45. doi: 10.1016/s0006-8993(03)02694-5. [DOI] [PubMed] [Google Scholar]
- Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H. Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists. Journal of Pharmacology and Experimental Therapeutics. 2008;325:902–909. doi: 10.1124/jpet.107.135343. [DOI] [PubMed] [Google Scholar]
- Le Cudennec C, Faure A, Ly M, Delatour B. One-year longitudinal evaluation of sensorimotor functions in APP751SL transgenic mice. Genes Brain & Behavior. 2008;7(supplement 1):83–91. doi: 10.1111/j.1601-183X.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, Robert P, Parmentier R, Anaclet C, Lin JS, Burban A, Arrang JM, Schwartz JC. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochemical Pharmacology. 2007a;73:1215–1224. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. Journal of Pharmacology and Experimental Therapeutics. 1998;287:658–666. [PubMed] [Google Scholar]
- Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, Lecomte JM, Parmentier R, Anaclet C, Lin JS, Bertaina-Anglade V, la Rochelle CD, d’Aniello F, Rouleau A, Gbahou F, Arrang JM, Ganellin CR, Stark H, Schunack W, Schwartz JC. BF2.649 [1-{3-[3-4-Chlorophenylpropoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. Journal of Pharmacology and Experimental Therapeutics. 2007b;320:365–375. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- Lindner M, Plone M, Schallert T, Emerich D. Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Cognitive Brain Research. 1997;5:329–333. doi: 10.1016/s0926-6410(97)00006-2. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, Cilia J, Cluderay JE, Crook B, Davis JB, Davis RK, Davis RP, Dawson LA, Foley AG, Gartlon J, Gonzalez MI, Heslop T, Hirst WD, Jennings C, Jones DN, Lacroix LP, Martyn A, Ociepka S, Ray A, Regan CM, Roberts JC, Schogger J, Southam E, Stean TO, Trail BK, Upton N, Wadsworth G, Wald JA, White T, Witherington J, Woolley ML, Worby A, Wilson DM. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. Journal of Pharmacology and Experimental Therapeutics. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. [DOI] [PubMed] [Google Scholar]
- Morisset S, Pilon C, Tardivel-Lacombe J, Weinstein D, Rostene W, Betancur C, Sokoloff P, Schwartz JC, Arrang JM. Acute and chronic effects of methamphetamine on tele-methylhistamine levels in mouse brain: selective involvement of the D(2) and not D(3) receptor. Journal of Pharmacological & Experimental Therapeutics. 2002;300:621–628. doi: 10.1124/jpet.300.2.621. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor−/− mice. European Journal of Neuroscience. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- Southam E, Cilia J, Gartlon JE, Woolley ML, Lacroix LP, Jennings CA, Cluderay JE, Reavill C, Rourke C, Wilson DM, Dawson LA, Medhurst AD, Jones DN. Preclinical investigations into the antipsychotic potential of the novel histamine H3 receptor antagonist GSK207040. Psychopharmacology. 2009;201:483–494. doi: 10.1007/s00213-008-1310-9. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, Kase Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer’s disease. Journal of Ethnopharmacology. 2009;122:157–162. doi: 10.1016/j.jep.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behavioural Brain Research. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. Journal of Neuroscience. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, Whitaker C, White W. Amphetamine-induced hyperlocomotion in rats: Hippocampal modulation of the nucleus accumbens. Hippocampus. 2006;16:596–603. doi: 10.1002/hipo.20189. [DOI] [PubMed] [Google Scholar]
- Yokoyama F, Yamauchi M, Oyama M, Okuma K, Onozawa K, Nagayama T, Shinei R, Ishikawa M, Sato Y, Kakui N. Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: a comparative study with antidepressants and benzodiazepine anxiolytic. Psychopharmacology. 2009;205:177–187. doi: 10.1007/s00213-009-1528-1. [DOI] [PubMed] [Google Scholar]


