Summary
Children with severe asthma have a premature loss of lung function during the adolescent years that is associated with increased symptom frequency and greater allergic sensitization during childhood.
Keywords: Severe asthma, children, lung function, lung growth, airway remodeling
To the Editor
Severe asthma in children is heterogeneous disorder associated with extreme morbidity despite treatment with high doses of inhaled corticosteroids (ICS). We previously reported the phenotypic features of children with mild-to-moderate and severe asthma (mean age, 10 years) enrolled in the National Heart, Lung and Blood Institute’s (NHLBI) Severe Asthma Research Program (SARP) at Emory University (1). Similar to adults with severe asthma (2), children with severe asthma were characterized by baseline airflow limitation that was not completely reversed with bronchodilation (1). However, the magnitude of airflow limitation was significantly less in children with severe asthma (FEV1: 81% predicted, FEV1/FVC: 0.74) as compared to adults (FEV1: 62% predicted, FEV1/FVC: 0.65) (2). These findings raise important questions about the stability of the severe asthma phenotype in children and the critical developmental time frame during which loss of lung function occurs. While the answers to these questions are not entirely clear, longitudinal studies have shown that children with more severe, frequent symptoms have ongoing airflow limitation and more severe asthma throughout adulthood (3–5). Using longitudinal follow-up data from children previously characterized in the NHLBI SARP, we report that children with severe asthma have a premature loss of lung function during the adolescent years that is associated with an increased frequency of wheezing and asthma symptoms and greater allergic sensitization during childhood.
Children with mild-to-moderate (n = 12, 67% male) and severe (n = 28, 57% male) asthma previously characterized in the NHLBI SARP at Emory University between 2004–2007 were re-evaluated between 2008–2010. At the follow-up visit, children repeated questionnaires and underwent severity classification assignment and spirometry with maximal bronchodilator reversibility testing with up to 8 inhalations of albuterol sulfate (90 μg/inhalation) as previously described (1). The differences between the initial and follow-up spirometric data were calculated and divided by the number of years between study visits. Multivariate logistic regression models were used to determine the association between characterization variables and changes in lung function.
The mean age at the initial evaluation and follow-up visit was 11 ± 3 and 14 ± 3 years, respectively, with a mean visit interval of 3 ± 2 years. There were no changes in subject severity classification between the initial characterization visit and the follow-up assessment. Demographic features were also similar to our previous report (1). Children with severe asthma had a higher frequency of daily symptoms (46 vs. 8%, p = 0.030) and hospitalization within the previous year (32 vs. 0%, p = 0.037). This was despite higher daily doses of ICS (744 ± 328 vs. 365 ± 280 μg fluticasone/day, p < 0.001) and a higher frequency of controller medication use (montelukast: 100% vs. 75%, p = 0.029; long-acting beta agonists: 93 vs. 17%, p < 0.001). Lung function percent predicted values also remained significantly lower in children with severe versus mild-to-moderate asthma at the follow-up assessment (FEV1: 78 ± 20 vs. 102 ± 15%, p = 0.001; FVC: 96 ± 19 vs. 105 ± 18%, p = 0.168; FEV1/FVC: 82 ± 13 vs. 97 ± 9%, p < 0.001).
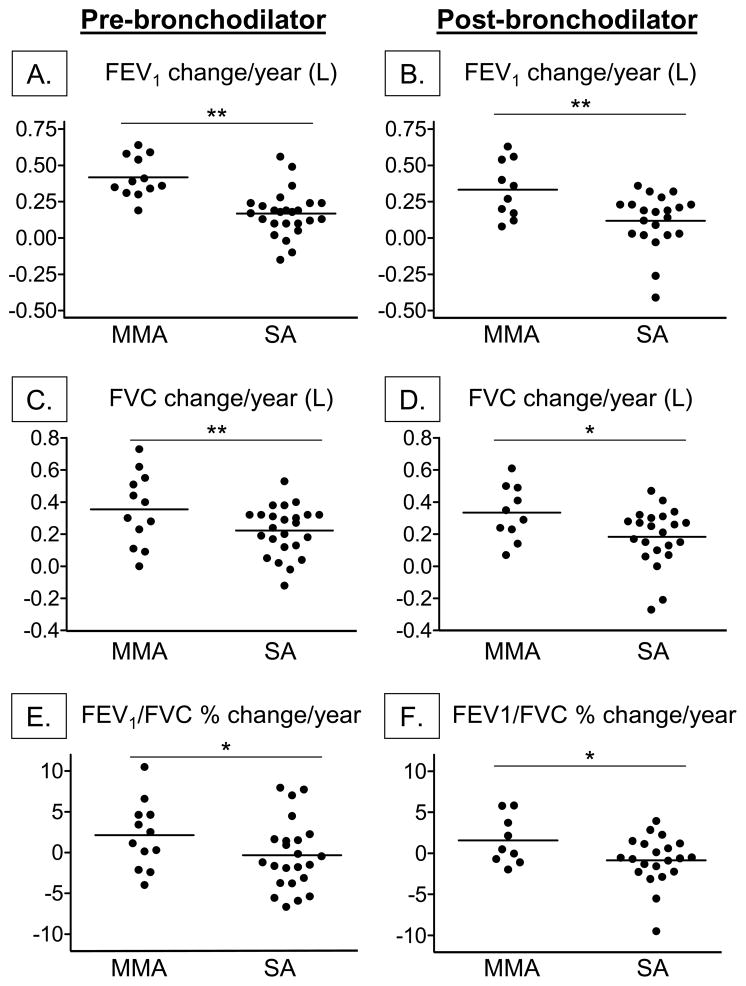
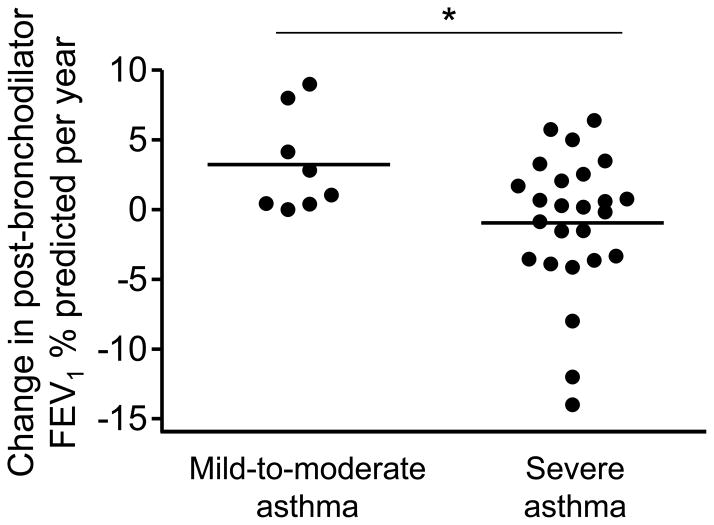
The average yearly change in FEV1 and FVC (in liters) and FEV1/FVC (%) for each group is shown in Figure 1. Although FEV1 and FVC increased in both groups with age, the average yearly increase in FEV1 and FVC was significantly less in children with severe asthma. These findings were apparent before (Figure 1A, 1C) and after maximal bronchodilation (Figure 1B, 1D) and persisted after age-appropriate lung function reference equations (that account for variations in height during the adolescent growth spurt) were applied to the data (6). Similarly, the FEV1/FVC percent predicted remained lower in children with severe asthma (Figure 1E) and even worsened in a subset (Figure 1F). Of further note, post-bronchodilator FEV1 percent predicted values declined in 46% of the children with severe asthma between the follow-up interval and 29% of these children had post-bronchodilator FEV1 declines of more than 1% per year (Figure 2). Using multivariate models, race, gender, and healthcare utilization history (e.g., prior hospitalizations, intubations and emergency room visits) were not associated with significant declines in FEV1 in the severe asthma group. However, daily asthma symptoms including coughing or wheezing (odds ratio [OR], 3.66; 95% CI, 1.29–10.34, p = 0.015) and the number of aeroallergen skin prick responses (OR, 1.29; 95% CI, 1.05–1.58, p = 0.011) at the initial evaluation were strong predictors of post-bronchodilator FEV1 declines of more than 1% per year.
Figure 1.
Calculated change per year in baseline and post-bronchodilator FEV1 (shown in liters; A, B), FVC (shown in liters; B, C), and FEV1/FVC (shown as percent predicted; E, F). Lines represent the mean. MMA = mild-to-moderate asthma; SA = severe asthma. *p < 0.05; **p ≤ 0.01
Figure 2.
Calculated yearly change in post-bronchodilator FEV1 percent predicted values in children with mild-to-moderate and severe asthma. Lines represent the mean. *p < 0.05
These results support the hypothesis that progressive airflow limitation is a feature of severe asthma in children. Although progressive airflow limitation was previously observed in a subset of children with mild-to-moderate asthma enrolled in the Childhood Asthma Management Research Program, not all children in that subset had clinical evidence of more severe disease. Rather, the average post-bronchodilator FEV1 in that subgroup was 108% at baseline and 96% at the completion of the study (7, 8). However, further analysis revealed significantly lower lung function, greater allergic sensitization and increased asthma morbidity in children with persistent versus remitting disease (9), similar to what we report here. Indeed, our sample of children with severe asthma was characterized by a high degree of morbidity and substantial airflow limitation that persisted throughout the follow-up interval, although the magnitude of airflow limitation in these children was significantly less than what is commonly seen in adults with severe asthma (2). The fact that airflow limitation persisted and even worsened in many children with severe asthma despite high doses of ICS and other asthma controller medications is intriguing and raises important questions about the corticosteroid sensitivity of this sample. Whether this loss of lung function represents a reduction in lung growth velocity or progression of airway remodeling is also unclear and warrants further study.
Sincerely,
Anne M. Fitzpatrick, Ph.D., M.S.C.R.
Emory University School of Medicine, Department of Pediatrics, Atlanta, Georgia
W. Gerald Teague, M.D.
University of Virginia School of Medicine, Department of Pediatrics, Charlottesville, Virginia
Acknowledgments
This work was supported by National Institute of Health grants RO1HL069170 and RO1NR012021 and was supported in part by the Center for Developmental Lung Biology, Children’s Healthcare of Atlanta, and PHS grants UL1 RR025008, KL2 RR025009 and TL1 RR025010 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources
Abbreviations
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- NHLBI
National Heart, Lung and Blood Institute
- SARP
Severe Asthma Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe R, Carlin JB, Oswald H, Olinsky A, Phelan PD, Robertson CF. Association between allergy and asthma from childhood to middle adulthood in an Australian cohort study. Am J Respir Crit Care Med. 2000;162:2177–81. doi: 10.1164/ajrccm.162.6.9812019. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–8. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 5.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 7.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004;170:234–41. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 8.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118:1040–7. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125:359–66. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]