Abstract
Shp2 protein tyrosine phosphate (PTP) is a novel target for anticancer drug discovery. We identified estramustine phosphate as a Shp2 PTP inhibitor from the National Cancer Institute Approved Oncology Drug set. A focused structure-activity relationship study indicated that the 17- phosphate group is required for the Shp2 PTP inhibitor activity of estramustine phosphate. A search for estramustine phosphate analogs led to identification of two triperpenoids, enoxolone and celastrol, having Shp2 PTP inhibitor activity. With the previously reported PTP1B inhibitor trodusquemine, our study reveals steroids and triterpenoids with negatively charged phosphate, carboxylate, or sulfonate groups as novel pharmacophores of selective PTP inhibitors.
Keywords: Shp2, Protein tyrosine phosphatase, Estramustine phosphate, Enoxolone, Celastrol, Triterpenoids
Protein tyrosine phosphorylation is a major signaling mechanism that controls cellular activities pertinent to cancer initiation and progression. Protein tyrosine phosphorylation is coordinately regulated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). While many PTKs have well-demonstrated roles in human cancer and are validated targets of anticancer drugs, increasing evidence also shows that a number of PTPs, such as Shp2, PTP1B, Cdc25, and PRL-3, have oncogenic or cancer-promoting functions.1, 2 This has stimulated significant interest in developing PTP inhibitors as a new class of targeted therapy agents.2–4
Shp2 is a non-receptor PTP encoded by the PTPN11 gene.5 Shp2 is a positive regulator of growth factor-stimulated Src and Ras-Erk1/2 mitogen-activated protein (MAP) kinase pathways. Gain-of-function PTPN11 mutations that encode constitutively active Shp2 PTP are associated with leukemias.6 In particular, PTPN11 is the most frequently mutated gene in juvenile myelomonocytic leukemia (JMML), associating with ~35% of JMML cases.7 Leukemia-associated PTPN11 mutants have been established as oncogenes.6, 8 Although Shp2 mutants are detected infrequently in solid tumors, the wildtype Shp2 is activated frequently in cancer cells by growth factor receptor oncogenes such as epidermal growth factor receptor (EGFR) and ErbB2 and is required for malignant phenotypes caused by these oncogenes.9, 10 These findings point to Shp2 PTP as a target for novel anticancer drug discovery.2, 9, 11–13 Moreover, Shp2 also limited STAT1 activation by interferon γ in response to viral infection.14, 15 Inhibition of Shp2, therefore, has the potential of increasing antiviral activity of interferon γ. We recently reviewed the development of Shp2 inhibitors.2 Other compounds have since been reported with µM activity including those in a paper that describes an inhibitor-Shp2 co-crystal structure.16 However, there is still a need for improved inhibitors combining good potency, cell permeability, and in vivo activity.
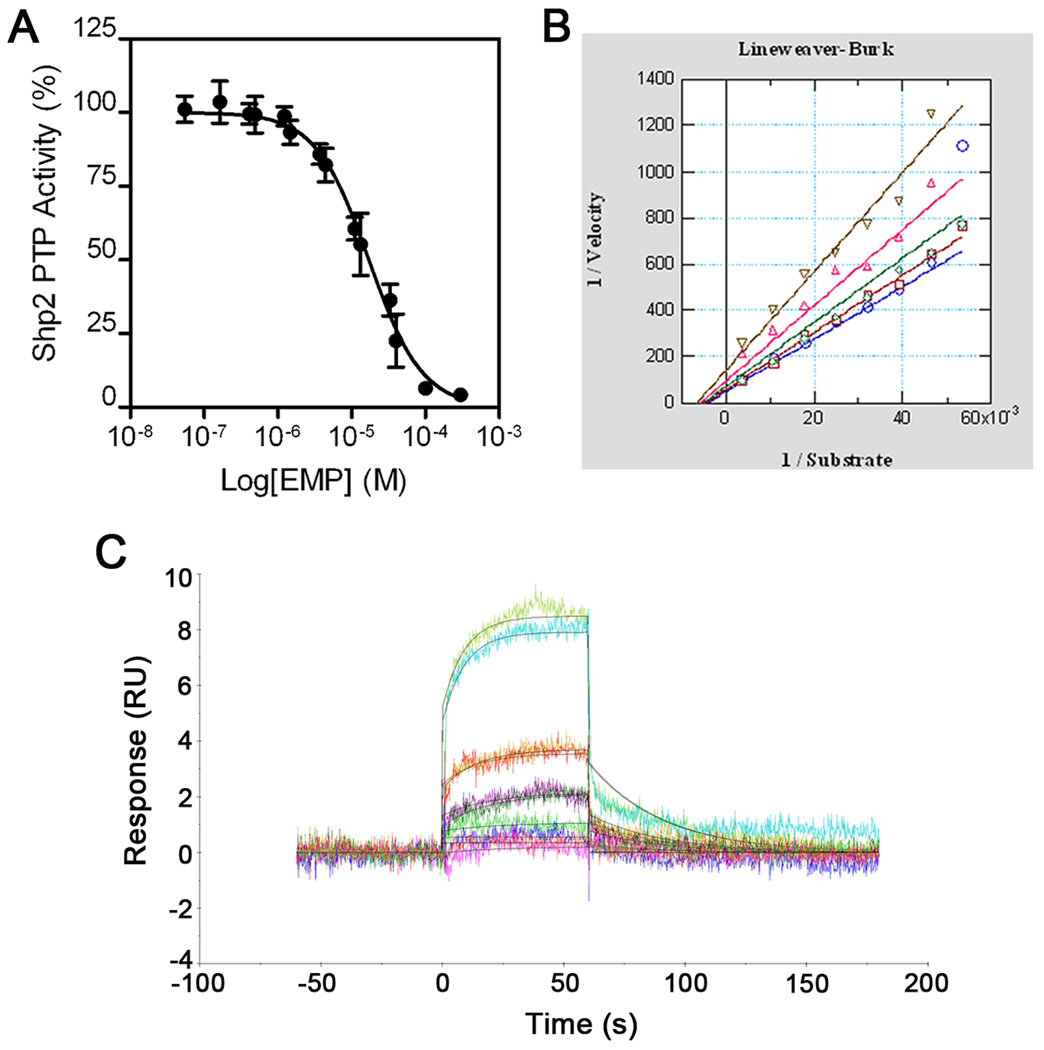
In a continuing effort to identify new Shp2 PTP inhibitors, we screened a small molecule library comprising the National Cancer Institute (NCI) Approved Oncology Drug set (89 compounds) and the NIH Clinical Collection (450 compounds). After further evaluation of initial hits, estramustine phosphate (Fig. 1) was verified as a Shp2 PTP inhibitor. Estramustine phosphate is a chemotherapy agent used to treat prostate cancer. As shown in Fig. 2A and Table I, estramustine phosphate inhibited the Shp2 PTP activity with an IC50 of 17.1 ± 9.2 µM. In an enzyme kinetic assay using 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP, Invitrogen) as the substrate (see Supplementary Information), inhibition by estramustine phosphate was best fitted with a mixed inhibition kinetics (Kis: 22.8 µM, Kii: 10.8 µM, Fig. 2B). Surface Plasmon resonance (SPR) binding assay illustrated a 1:1 stoichiometric binding kinetics of estramustine phosphate to Shp2 with a kinetic constant (KD) of 8.4 µM and the association and dissociation rate constants of ka = 2.2 × 103/Ms and kd = 0.020/s (Fig. 2C).
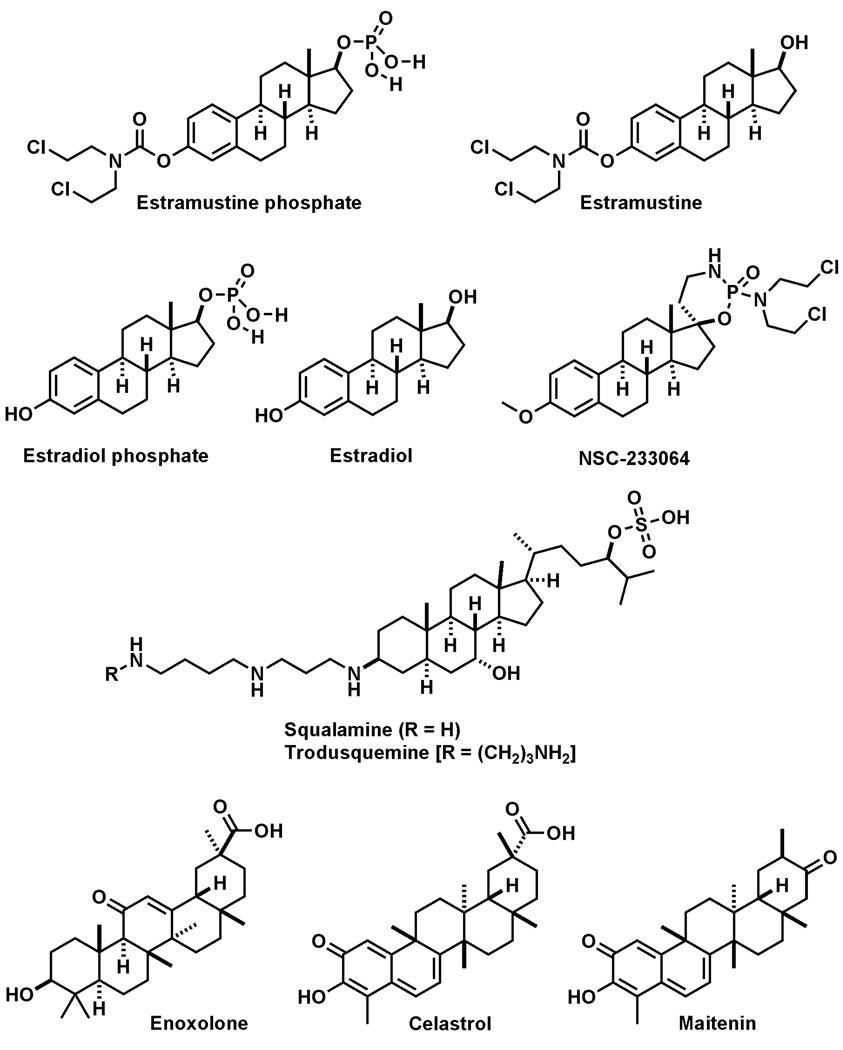
Fig. 1.
Chemical structures of compounds reported in this letter.
Fig. 2.
Inhibition and binding of estramustine phosphate to Shp2. (A) IC50 curve of Shp2 PTP inhibition by estramustine phosphate (EMP). (B) Inhibitor kinetics analysis of EMP on the Shp2 PTP. (C) Surface plasmon resonance assay of EMP binding to Shp2. A representative sensorgram and the associated curve fit are shown.
Table 1.
Shp2 PTP inhibitory activity of Estramustine phosphate analogs
| Compound | IC50 (µM) |
|---|---|
| Estramustine phosphate | 17.10 ± 9.21 |
| Estramustine | >300 |
| Estradiol phosphate | 19.9 ± 10.3 |
| Estradiol | >300 |
| NSC233064 | >300 |
| Enoxolone | 9.6 ± 3.2 |
| Celastrol | 3.3 ± 0.6 |
| Maitenin | 61.6 ± 24.2 |
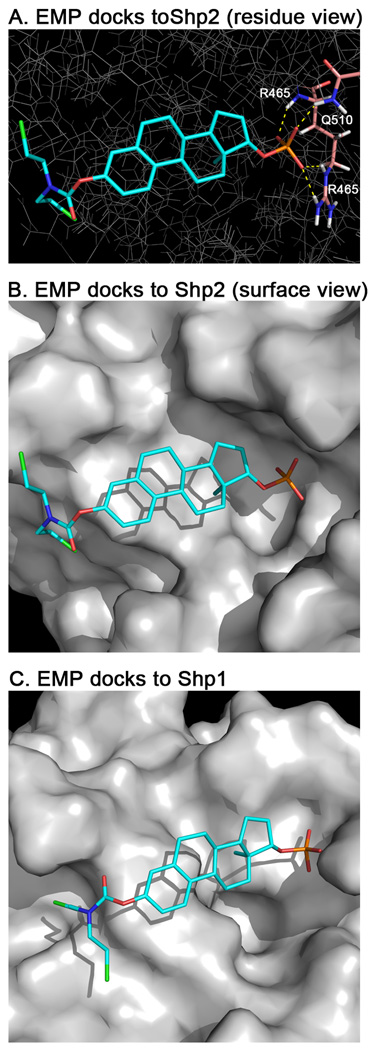
To explore the structural components of estramustine phosphate necessary for Shp2 inhibition, we analyzed the Shp2 inhibitory activity of estramustine, estradiol phosphate, estradiol, and NSC233064 (Fig. 1). Whereas estramustine, estradiol, and NSC23306417 did not inhibit the Shp2 PTP activity at the highest concentration tested (300 µM), estradiol phosphate inhibited the Shp2 PTP activity with a potency (IC50:19.9 ± 10 µM) similar to that of estramustine phosphate (Table 1). These data indicated that the 17-phosphate group of estramustine phosphate is necessary for the Shp2 PTP inhibitor activity while the nitrogen mustard group is dispensable. Consistent with these experimental data, computer docking of estramustine phosphate to the Shp2 PTP domain (PDB: 3B7O that has an accessible catalytic site) suggested that the aryl phosphate group could insert deep into the catalytic pocket of Shp2 and form three hydrogen bonds with the backbone and side chain of Arg-465 and one hydrogen bond with the side chain of Asn-510 in the catalytic pocket (Fig. 3). On the other hand, the nitrogen mustard group points to the open space in the catalytic pocket (Fig. 3)
Fig. 3.
Docking of estramustine phosphate (EMP) to Shp2 and Shp1. EMP was docked into the catalytic site of Shp2 and Shp1 using GLIDE 5.6. (A) Stick representation of the lowest energy binding pose of EMP in Shp2. Potential hydrogen bonds are indicated as dashed-yellow lines showing interactions between the phosphate moiety and residues R465 (3 bonds) and Q510 (1 bond). (B) Water accessible surface showing the binding pocket. The binding pose of EMP fits into the deep pocket where the phosphate moiety can interact with the positive electrostatics of the region. (C) Water accessible surface of Shp1 with electrostatics. As with (B) the binding pose of EMP in Shp1 is shown. This pocket is much shallower and that may contribute to EMP’s inferior inhibition of Shp1 compared to Shp2.
We searched for available analogs of estramustine phosphate, specifically looking for steroids or triterpenoids containing a negatively charged phosphate, carboxylate, or sulfonate group. The aminosterol squalamine18, 19 as well as triterpenoids enoxolone (glycyrrhetic acid)20 and celastrol21 (Fig. 1) fit these criteria. Interestingly, trodusquemine (MSI-1436), a derivative of squalamine with one additional animopropyl unit (Fig. 1), has been reported as a PTP1B inhibitor (IC50: 1 µM).22 Pure squalamine or trodusquemine were not available to us for testing of their potencies against the Shp2 PTP. However, an ethanol extract of the squalamine-enriched shark liver product Squalamax (Nu-gen Nutrition) consistently displayed Shp2 PTP inhibitor activity. Enoxolone and celastrol inhibited Shp2 with IC50 of 9.6 ± 3.2 µM and 3.3 ± 0.6 µM, respectively. Maitenin (Fig. 1), a celastrol analog lacking the carboxylate group, was 18-fold less potent than celastrol in Shp2 inhibition (Table I), suggesting that the carboxylate group contributes to the inhibitor activity.
To determine if estramustine phosphate, estradiol phosphate, enoxolone, and celastrol selectively inhibit the Shp2 PTP activity or if they also inhibit other PTPs, we measured the activity of these compounds on three other PTPs, Shp1, PTP1B, and HePTP. The results are presented in Table 2. All four compounds had various degrees of selectivity against Shp1, PTP1B, and HePTP. Comparing with Shp2 (PDB: 3B7O), the Shp1 PTP catalytic pocket in the crystal structure (PDB: 1GWZ) appears to be more shallow (Fig. 3B and C), which may explain why Shp1 is less sensitive to these compounds. HePTP had the least sensitivity to all four compounds. Comparison of the surface electrostatic potential between Shp2, Shp1, PTP1B, and HePTP PTP domains23, 24 showed that the HePTP has a surface charge distribution quite different from that of Shp2, Shp1, and PTP1B. Whereas Shp2, Shp1 and PTP1B have positively-charged surfaces, this is not the case in HePTP.23 This divergence in the surface charge distribution may contribute to the resistance of HePTP to the phosphate and carboxylate inhibitors analyzed in this study. These data indicate that these compounds do not non-specifically inhibit PTPs.
Table 2.
Selectivity of Shp2 inhibitors
| Compound | IC50 (µM) |
|||
|---|---|---|---|---|
| Shp2 | Shp1 | PTP1B | HePTP | |
| Estramustine phosphate | 17.1 ± 9.2 | 40.4 ± 14.0 | 62.4 ± 7.3 | 152.7 ± 63.2 |
| Estradiol phosphate | 19.9 ± 10.3 | >300 | >300 | >300 |
| Enoxolone | 9.6 ± 3.2 | 65.4 ± 28.3 | 45.8 ± 37.1 | >300 |
| Celastrol | 3.3 ± 0.6 | 27.8 ± 1.7 | 13.2 ± 1.5 | 75.7 ± 8.9 |
Triterpenoids constitute a large number of natural products,25, 26 including those with carboxyl groups as well as various esters27 that in principle can be hydrolyzed in vivo to the free aryl carboxylic acid.11 Many of these triterpernoids are biologically active compounds that include anticancer and antiviral activities.26 However, their mechanisms of action are largely undefined. Our study reveals the previously unknown activity of enoxolone and celastrol as selective PTP inhibitors. Moreover, our findings also point to a rich natural source for discovery of lead compounds of novel PTP inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants P01CA118210, R01CA077467, and P30CA076292.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ostman A, Hellberg C, Bohmer FD. Nat. Rev. Cancer. 2006;6:307. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 2.Scott LM, Lawrence HR, Sebti SM, Lawrence NJ, Wu J. Curr. Pharm. Des. 2010;16:1843. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutros R, Lobjois V, Ducommun B. Nat. Rev. Cancer. 2007;7:495. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 4.Vintonyak VV, Antonchick AP, Rauh D, Waldmann H. Curr. Opin. Chem. Biol. 2009;13:272. doi: 10.1016/j.cbpa.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Neel BG, Gu H, Pao L. Trends Biochem. Sci. 2003;28:284. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 6.Chan G, Kalaitzidis D, Neel BG. Cancer Metastasis Rev. 2008;27:179. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hahlen K, Hasle H, Licht JD, Gelb BD. Nat. Genet. 2003;34:148. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 8.Chan RJ, Feng G-S. Blood. 2007;109:862. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan Y, Counelis GJ, O'Rourke DM. Exp. Cell Res. 2009;315:2343. doi: 10.1016/j.yexcr.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Agazie YM. J. Biol. Chem. 2009;284:12226. doi: 10.1074/jbc.M900020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Pernazza D, Scott LM, Lawrence HR, Ren Y, Luo Y, Wu X, Sung SS, Guida WC, Sebti SM, Lawrence NJ, Wu J. Biochem. Pharmacol. 2010;80:801. doi: 10.1016/j.bcp.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Mol. Pharmacol. 2006;70:562. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence HR, Pireddu R, Chen L, Luo Y, Sung SS, Szymanski AM, Yip ML, Guida WC, Sebti SM, Wu J, Lawrence NJ. J. Med. Chem. 2008;51:4948. doi: 10.1021/jm8002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron M, Davignon JL. J. Immunol. 2008;181:5530. doi: 10.4049/jimmunol.181.8.5530. [DOI] [PubMed] [Google Scholar]
- 15.You M, Yu DH, Feng GS. Mol. Cell. Biol. 1999;19:2416. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, He Y, Liu S, Yu Z, Jiang ZX, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM, Wang L, Chan RJ, Zhang ZY. J. Med. Chem. 2010;53:2482. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster EL, Blickenstaff RT. Steroids. 1974;24:737. doi: 10.1016/0039-128x(74)90025-7. [DOI] [PubMed] [Google Scholar]
- 18.Rao MN, Shinnar AE, Noecker LA, Chao TL, Feibush B, Snyder B, Sharkansky I, Sarkahian A, Zhang X, Jones SR, Kinney WA, Zasloff M. J. Nat. Prod. 2000;63:631. doi: 10.1021/np990514f. [DOI] [PubMed] [Google Scholar]
- 19.Sills AK, Jr, Williams JI, Tyler BM, Epstein DS, Sipos EP, Davis JD, McLane MP, Pitchford S, Cheshire K, Gannon FH, Kinney WA, Chao TL, Donowitz M, Laterra J, Zasloff M, Brem H. Cancer Res. 1998;58:2784. [PubMed] [Google Scholar]
- 20.Hibasami H, Iwase H, Yoshioka K, Takahashi H. Int. J. Mol. Med. 2006;17:215. [PubMed] [Google Scholar]
- 21.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Cancer Res. 2006;66:4758. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 22.Lantz KA, Hart SG, Planey SL, Roitman MF, Ruiz-White IA, Wolfe HR, McLane MP. Obesity (Silver Spring) 2010;18:1516. doi: 10.1038/oby.2009.444. [DOI] [PubMed] [Google Scholar]
- 23.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Muller S, Knapp S. Cell. 2009;136:352. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Cell. 2004;117:699. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Connolly JD, Hill RA. Nat. Prod. Rep. 2003;20:640. doi: 10.1039/b204068a. [DOI] [PubMed] [Google Scholar]
- 26.Liby KT, Yore MM, Sporn MB. Nat. Rev. Cancer. 2007;7:357. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz S, Cusk R. Bioorg. Med. Chem. 2010;18:7458. doi: 10.1016/j.bmc.2010.08.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.