Abstract
Pertussis toxin is an AB5 toxin comprised of protein subunits S1 through S5. The individual subunits are secreted by a Sec-dependent mechanism into the periplasm, where the toxin is assembled. The Ptl type IV secretion system mediates secretion of assembled toxin past the outer membrane. In this study, we examined the time course of protein expression, toxin assembly, and secretion as a function of the bacterial growth cycle. Logarithmic growth was observed after a 1-h lag phase. Secreted toxin was first observed at 3 h. Secretion continued throughout the logarithmic growth phase and decreased as the culture entered the stationary phase after about 24 h. On a per cell basis, toxin secretion occurred at a constant rate of 3 molecules/min/cell from 2 to 18 h. More of toxin subunits S1, S2, and S3 were produced than were secreted, resulting in periplasmic accumulation. Periplasmic S1, S2, and S3 were found to be soluble in the periplasm, as well as membrane associated. About one-half of the periplasmic S1, S2 and S3 subunits were incorporated into holotoxin. Secretion component PtlF was present at a low level at time zero, and the level increased between 2 and 24 h from 30 to 1,000 molecules per cell; however, the initial level of PtlF, 30 molecules per cell, supported maximal secretion. The accumulation of both periplasmic toxin and secretion components suggests that translation rates exceed the rate of secretion and that secretion, not toxin and Ptl complex assembly, is rate limiting.
Pertussis toxin is an AB5 toxin comprised of the products of five genes, S1 through S5 (25, 27). The A subunit of the toxin is the S1 polypeptide, while the pentameric B subunit is comprised of S2, S3, S4, and S5, assembled in a ratio of 1:1:2:1 (36). Pertussis toxin is secreted past the outer membrane of Bordetella pertussis by a type IV secretion system comprised of the products of the nine ptl (for “pertussis toxin liberation”) genes (8, 10, 42). The ptl genes are located immediately downstream of the pertussis toxin genes (42) and are transcribed from the same promoter (2, 18, 31).
It is not known how many secretion complexes are present in one bacterium during active secretion, nor is the stoichiometry of the proteins in the complex known. The structures of other type IV systems, such as the Agrobacterium tumefaciens virB system (11, 38, 40), which secretes a tumerogenic T-DNA and effector proteins into host plant cells, and the P-plasmid tra conjugation genes (23), have been more thoroughly studied than the Ptl system. The extensive similarity suggests that the Ptl proteins also form a large complex spanning both the inner and outer membranes. Nevertheless, the Ptl secretion system and the DNA transport systems have substantially different substrates and functions. The most intriguing difference is that the same basic machinery transports protein-coated DNA between the cytoplasm of two cells for the conjugation systems or a periplasmic protein complex across the outer membrane in the case of the Ptl system. Functionally, the DNA transport systems need to create a pore in three membranes, while the Ptl system only needs to create a pore in the bacterial outer membrane. However, comparing the two systems appears to be less problematic due to recent reports that suggest that the DNA transport systems also have substrates that are secreted from the periplasm past the outer membrane (29).
While structural studies of the conjugative type IV secretion systems have progressed rapidly, quantitative studies of DNA transfer between cells are very difficult. In contrast, B. pertussis secretes pertussis toxin directly into the supernatant during growth in vitro, and this substrate is easily quantified. Monoclonal antibodies to the toxin are available, which allows antigenic determination of protein concentrations, and the concentration of biologically active toxin can also be determined by examining its effect on susceptible cells. In this study, we examined the temporal relationship among expression of toxin subunits, Ptl proteins, and secretion of the assembled holotoxin following initiation of transcription of the ptx-ptl operon as a function of the bacterial growth cycle.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. pertussis strains used in the this study were BP338, a nalidixic acid-resistant derivative of Tohama I (41), and BPRA, a nalidixic acid- and streptomycin-resistant derivative of Tohama I containing a deletion of the ptx-ptl promoter and the genes encoding S1 through S5 (1). For routine propagation, the bacteria were grown on Bordet-Gengou (BG) agar (BD Biosciences, Sparks, Md.) containing nalidixic acid at a concentration of 30 μg/ml and streptomycin at a concentration of 100 μg/ml when appropriate.
Growth curve for de novo expression of S1 and PtlF proteins.
Modulation or suppression of the Bvg-regulated transcription of the ptx-ptl operon (26) was achieved by growth on BG agar plates containing 40 mM MgSO4. Strains were streaked onto modulating plates and incubated at 37°C for 72 h, and then they were restreaked onto modulating plates and incubated at 37°C for 24 h. Suspensions of modulated cells were made in 7 ml of Stainer-Scholte broth at an optical density at 600 nm (OD600) of 0.1 and overlaid onto BG plates containing appropriate antibiotics as previously described (10). The plates were incubated at 37°C, and 1-ml aliquots were harvested by centrifugation. Supernatant samples (for determination of secreted toxin) were filter sterilized to remove remaining bacteria. Cell pellets (for determination of cellular components) were washed in 1 ml of phosphate-buffered saline (pH 7.4) and suspended to an OD600 of 8 in phosphate-buffered saline to normalize the concentration of cells in each sample.
Pertussis toxin production.
The amount of secreted toxin was determined by using filtered-sterilized culture supernatants. The amount of periplasmic pertussis toxin was determined as previously described (10, 42). Briefly, periplasmic toxin was released from the cell suspensions by shock treatment with lysozyme and EDTA.
Construction and purification of PtlF fusion protein.
To produce a standard in order to quantify PtlF expression antigenically, the maltose-binding protein portion of the PtlF fusion used to generate the polyclonal antibody (30) was replaced with a polyhistidine tag. The region of ptlF encoding amino acids 73 to 205 was amplified by PCR by using primers 5-F80 (GGCTCTAGAGACGGCTGGCAATTCAGCC) and 3-F80 (CAGAAGCTTACCCGGTCTGAACATGAGCC), which introduced an XbaI restriction site at the 5′ end and a HindIII restriction site at the 3′ end (30). The PCR product was cloned into the TA cloning vector pCR2.1 by using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.), cut out of the TA vector with XbaI and HindIII, and ligated into the pRSETB vector (Invitrogen) at the NheI and HindIII sites. The polyhistidine-tagged PtlF fusion protein was overexpressed in BL21[DE3] cells (Novagen, Madison, Wis.) and was purified by using an Ni-nitrilotriacetic acid spin kit (QIAGEN, Valencia, Calif.). Purification of the fusion protein was verified following sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) by Coomassie blue staining (Fig. 1A) and by Western blotting with anti-polyhistidine antibody (Sigma, St. Louis, Mo.) and with anti-PtlF antibody (30). The concentration of the purified protein was determined by the BCA protein assay (Pierce, Rockford, Ill.).
FIG. 1.
Standard curves for calculation of protein concentrations. (A) Purification of polyhistidine-tagged PtlF fusion protein. Lane 1, uninduced BL21[DE3](pAR334); lane 2, strain induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG); lane 3, wash; lane 4, eluant. The arrow indicates the position of purified PtlF fusion protein. (B to E) Standard curves obtained by densitometry. The volume (in pixels) was determined by densitometry of Western blots. (B) S1 standards (5, 10, 25, and 50 ng of purified pertussis toxin) with monoclonal antibody 3CX4; (C) S2 standard, purified pertussis toxin, with monoclonal antibody 11E6; (D) S3 standard, purified pertussis toxin, with monoclonal antibody 11E6; (E) PtlF standard, purified polyhistidine-tagged PtlF, with polyclonal antiserum.
Western blotting and densitometry.
Western blotting was performed essentially as previously described (43). Briefly, samples were mixed 1:1 with loading buffer. The samples were boiled for 10 min, and 20 μl was loaded into each well. For nonreducing gels, samples were mixed with loading buffer lacking β-mercaptoethanol. Purified pertussis toxin (List Biologicals, Inc., Campbell, Calif.) and a purified polyhistidine-tagged PtlF fragment (described above) were loaded as standards. A 24-h cell sample of wild-type strain BP338 and a 24-h cell sample of the pertussis toxin mutant BPRA were loaded on each gel as positive and negative controls. Proteins were separated by discontinuous SDS-PAGE performed with a Mini Protean II gel system (Bio-Rad, Richmond, Calif.) by using 12% acrylamide-Tris-glycine gels and transferred onto nitrocellulose membranes by using a Trans-Blot Cell wet blotting apparatus (Bio-Rad). The membranes were probed with a monoclonal anti-S1 antibody, either 3CX4 (17) or 1B7 (32), with a monoclonal antibody that recognizes both S2 and S3, 11E6 (34), or with a rat polyclonal anti-PtlF antibody (30). Samples probed with antibody 11E6 were electrophoresed in the absence of reducing agents, as previously described (33, 34). S2 and S3 migrate at different masses as determined by SDS-PAGE but exhibit about 70% amino acid identity. Monoclonal antibody 11E6 was raised against S2. This antibody reacts with both S2 and S3, although it reacts more strongly with S2 (34). Signals were detected by chemiluminescence by using a Dupont Western blot Renaissance kit (NEN Research Products, Boston, Mass.).
To quantify protein expression by Western blotting, a dilution series of purified protein was loaded onto each gel. For quantification of S1, 50, 25, 10, and 5 ng of purified pertussis toxin (List Biologicals, Inc.) were used. For quantification of S2 and S3, 75, 50, 25, and 10 ng of purified pertussis toxin were used due to the lower affinity of monoclonal antibody 11E6. For quantification of PtlF, 75, 50, 25, and 10 ng of purified polyhistidine-tagged PtlF were used. The signal strength (“volume” in Fig. 1) of the standards was determined by densitometry by using the program ImageQuant, version 5.1 (Molecular Dynamics), and the values were plotted against the concentrations of the standards. The best-fit line was calculated by using the linear regression function of Microsoft Excel (Fig. 1B through E), and the equation of the line was used to determine the protein concentrations in the unknown samples by using the sample signal strength. If necessary, a sample was diluted so that the amount of protein loaded for the sample fell within the linear range of the standards. Relative levels of protein expression were determined by comparing the unknowns to the 24-h sample as an internal standard.
To determine the amount of protein on a per cell basis, protein values were divided by the number of cells corresponding to the optical density of the culture at the time that the sample was collected. An OD600 of 1 corresponded to 3 × 109 bacteria per ml (39). Bacterial samples containing 10 μl of cells at an OD600 of 8 contained 2.4 × 108 bacteria. For protein expression and secretion rates, trend lines were plotted by using Excel, and equations were calculated by linear regression and were used to determine rates of change and x-intercept values.
CHO cell assay.
Chinese hamster ovary (CHO) cell assays were performed as previously described (10). Briefly, serial twofold dilutions of samples were made in Ham's F-12 medium containing 1% fetal bovine serum. Samples were added to CHO cell monolayers in 96-well plates and incubated for 48 h at 37°C with 5% CO2. Pertussis toxin causes CHO cells to lose contact inhibition and to produce a cluster-of-grapes morphology (14). The cell morphology in wells containing test samples was compared to that in wells containing twofold dilutions of purified pertussis toxin (List Biologicals, Inc.) and to that in control wells with no toxin. Each sample was assayed in duplicate, and there were four independent repeats of each experiment. The limit of detection for purified pertussis toxin was determined on each plate, and the last positive well of the test samples was assigned that value. In this study, the average limit of detection was 1 ng/ml. The standard error of the mean was graphed, and the Student t test was used to determine statistical significance.
Separation of membrane and soluble proteins.
B. pertussis BP338 or BPRA was grown in Stainer-Scholte broth on BG plates as described above. The cells in 10 ml of a 24-h culture were pelleted by centrifugation, washed two times in 10 ml of 4°C Tris-NaCl (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA), and suspended in 10 ml of Tris-NaCl containing 20 μl of protease inhibitor cocktail (catalog no. P 8465; Sigma). The cells were broken by sonication for 8 min in a sonicating water bath (Branson Ultrasonics Corp., Danbury, Conn.) in 2.5-ml aliquots. The sonicated cultures were centrifuged at 9,000 × g for 10 min to remove unbroken cells. Samples were then centrifuged at 100,000 × g for 1 h to separate the membrane and soluble fractions. The membranes were suspended in 1 ml of Tris-NaCl by sonication for 1 min and divided into 100-μl aliquots. The soluble fraction was precipitated with trichloroacetic acid at 20% saturation for 30 min on ice, suspended in 1 ml of Tris-NaCl, and divided into 100-μl aliquots. The 10×-concentrated aliquots of the soluble fraction and membrane fraction were stored at −80°C until they were used. The soluble and membrane fractions were examined by Western blotting for the presence of the membrane protein pertactin by using monoclonal antibody BB05 (5).
RESULTS
Bacterial growth and pertussis toxin secretion.
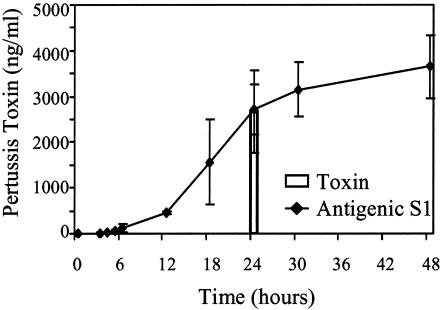
Bacterial growth and pertussis toxin secretion were monitored as B. pertussis was shifted from modulated conditions in the presence of MgSO4, in which virulence factor expression was suppressed, to conditions that permitted expression of the bacterial virulence factors, including pertussis toxin. A lag phase of about 1 h was observed, and this was followed by a period of logarithmic growth and then a gradual decrease in the growth rate; entry into the stationary phase began at about 24 h (Fig. 2). Pertussis toxin secretion (Fig. 3 and 4), as indicated by the presence of antigenic pertussis toxin subunit S1 (13 ng of toxin per ml) in the culture supernatant, was first observed at 3 h. The greatest increase in the amount of secreted pertussis toxin was observed between 12 and 24 h, and secretion tapered off during the stationary phase, so that the final concentration was 3,700 ng/ml at 48 h. The amount of biologically active toxin secreted at 24 h was determined to be 2,674 ± 892 ng/ml by using the CHO cell assay (Fig. 3). This value is consistent with the amount of pertussis toxin calculated from the amount of antigenic S1 recovered, 2,709 ± 548 ng/ml at 24 h, suggesting that all of the secreted S1 was incorporated into pertussis toxin holotoxin.
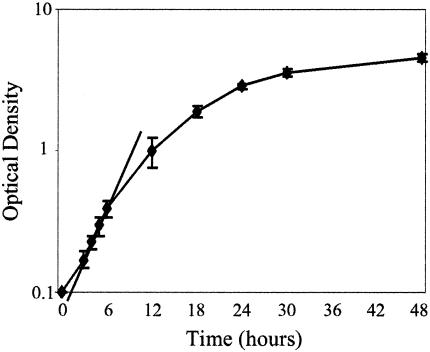
FIG. 2.
Growth curve for B. pertussis BP338. Optical density is plotted as a function of time in culture. The data are averages ± standard errors for four independent experiments. A linear trend line was calculated by using the early time points, and the time at which cultures entered the logarithmic growth phase was determined to be 1 h.
FIG. 3.
Accumulation of pertussis toxin in supernatant. The amount of secreted pertussis toxin as a function of time in culture was determined by assessing the amount of S1 in filter-sterilized culture supernatant by densitometry of Western blots with monoclonal antibody 3CX4 (Antigenic S1). Samples taken at 24 h were also assessed by the CHO cell activity assay (Toxin). The data are averages ± standard errors for four independent experiments.
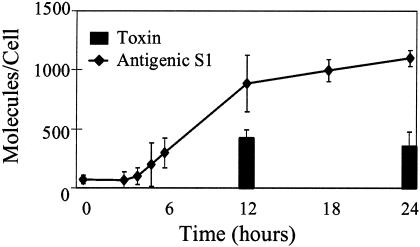
FIG. 4.
Toxin secretion on a per cell basis. The amount of secreted S1 on a per cell basis was determined as a function of time in culture. The data are averages ± standard errors for four to seven independent experiments. The trend line was plotted by linear regression for time points corresponding to 3 to 18 h in culture and was found to have a slope of 173 molecules/cell/h and an x intercept of 2 h.
As shown in Fig. 2, bacterial growth occurred, and a different number of cells contributed to pertussis toxin production at each time point. To determine the amount of S1 produced on a per cell basis, the molar amount of S1 was determined and divided by the number of bacteria present in the culture for each time point. S1 accumulation in the supernatant was fairly linear (Fig. 4) between 2 and 18 h. The rate of pertussis toxin secretion over this 16-h period was 173 molecules per cell per h (R2 = 0.9902) or about 3 molecules of pertussis toxin secreted per cell per min.
The presence of extracellular pertussis toxin represents the end of a multistep process involving transcription, translation, assembly, and secretion. We wanted to examine the accumulation of toxin intermediates and components of the secretion complex to begin to dissect the steps of this complex process.
Characterization of periplasmic S1 and holotoxin.
Previous studies have shown that both pertussis toxin subunit S1 and assembled pertussis toxin accumulate in the bacteria (10, 42). All of the cell-associated S1 migrated in SDS gels at the mass of the processed peptide lacking the secretion signal sequence (12), which is consistent with periplasmic accumulation but not cytoplasmic accumulation. The pool of periplasmic toxin could represent secretion precursors. Alternatively, intracellular toxin could accumulate because secretion of pertussis toxin is rate limiting. We examined accumulation of cell-associated toxin as a function of the growth cycle. In contrast to secreted S1, the amount of which increased by 3 h in culture (Fig. 4), cell-associated S1 (Fig. 5) did not accumulate until after 4 h in culture.
FIG. 5.
Periplasmic S1 and periplasmic toxin. The amount of periplasmic antigenic S1 was determined by densitometry by using monoclonal antibody 3CX4 and was expressed on a per cell basis as a function of time in culture. The amount of S1 assembled into pertussis toxin (Toxin) was determined by the CHO cell assay. The data are averages ± standard errors for four independent experiments. The concentration of toxin detected by the CHO cell assay at 24 h was significantly different from the concentration of S1 detected by densitometry (P < 0.0001).
The periplasmic pool of antigenic S1 could consist of unassembled S1 subunits or S1 incorporated into pertussis toxin. CHO cell assays were performed to determine the amount of periplasmic S1 incorporated into holotoxin. Very little periplasmic S1 was detected before 6 h in the culture, and samples obtained at 12 and 24 h were selected for characterization (Fig. 5). The amount of S1 incorporated into pertussis toxin, as detected by the CHO cell assay, was about one-half of the total amount of antigenic S1. The active toxin levels were 421 ± 69 molecules per cell at 12 h and 353 ± 124 molecules per cell at 24 h. These values were not significantly different from each other but were significantly less than the concentration of antigenic S1 at 24 h. The accumulation of a pool of periplasmic toxin subunits and assembled toxin after secretion was initiated suggests that pertussis toxin secretion, not assembly, is rate limiting.
Much of the periplasmic S1 has been reported to be associated with the bacterial outer membrane (12). Cell fractionation was performed with the 24-h samples to determine the relative amounts of membrane and soluble (cytoplasmic and periplasmic) proteins (Fig. 6A). In addition to S1, the cell fractions were examined for the presence of pertactin (4) and the secretion component PtlF (16), two known membrane proteins. Pertactin (Fig. 6B) and PtlF (Fig. 6C) were found to localize in the membrane fraction. In contrast, equivalent amounts of antigenic S1 were detected in the soluble and membrane fractions (Fig. 6A, compare lanes 3 and 4), as determined by densitometry, suggesting that one half of the S1 is soluble and the other half is membrane associated. Holotoxin is soluble and accounted for one-half of the cell-associated antigenicity (Fig. 5), suggesting that all of the soluble S1 is assembled into holotoxin. The remaining S1 is membrane bound, which is consistent with the suggestion that S1 has a membrane-associated intermediate prior to incorporation into soluble holotoxin (12).
FIG. 6.
Localization of cellular S1. (A) Amounts of S1 from various cell fractions determined by Western blotting by using monoclonal antibody 3CX4. The lanes contained 50 ng of purified pertussis toxin (Purified PTX), BP338 whole cells (Whole Cells), the BP338 soluble fraction (Soluble), the BP338 membrane fraction (Membranes), and BPRA (pertussis toxin mutant) whole cells (ΔPTX) as a negative control. (B) BP338 whole cells and soluble and membrane fractions probed with anti-pertactin antibody BB05. PRN, pertactin. (C) BP338 whole cells and soluble and membrane fractions probed with anti-PtlF antibody.
Characterization of B-subunit protein expression.
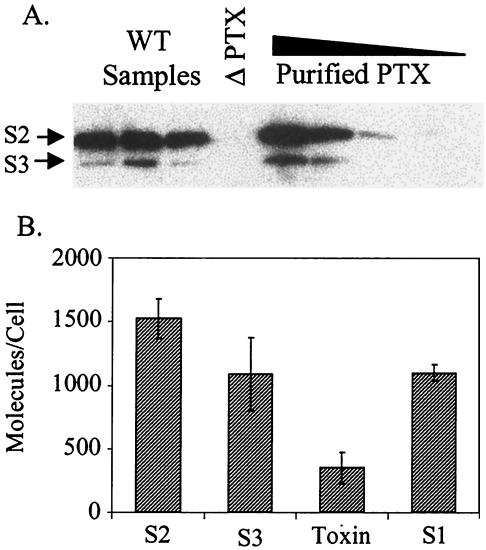
The periplasmic A-subunit protein (S1) was found in excess of folded toxin in the cells (Fig. 5). To determine whether this was also true for proteins in the B oligomer, we quantified expression of S2 and S3 at 24 h (Fig. 7) using monoclonal antibody 11E6, which recognizes both S2 and S3 under nonreducing conditions (33, 34). The levels of these proteins, 1,524 ± 156 molecules per cell for S2 and 1,088 ± 286 molecules per cell for S3, correspond to slightly more than a twofold excess over the amount incorporated into active toxin, which is similar to the amount of S1 detected under reducing conditions. In cell fractionation studies we detected S2 and S3 in both the membrane and soluble fractions (Fig. 8).
FIG. 7.
Periplasmic S2 and S3 and pertussis toxin. (A) Western blotting with monoclonal antibody 11E6. The lanes contained three individual 24-h samples of BP338 (WT Samples), a 24-h sample of pertussis toxin mutant BPRA (ΔPTX), and 75, 50, 25, and 10 ng of purified pertussis toxin (Purified PTX) (from left to right). (B) Amounts of antigenic S2 and S3 per cell compared to the amounts of active holotoxin and antigenic S1 at 24 h.
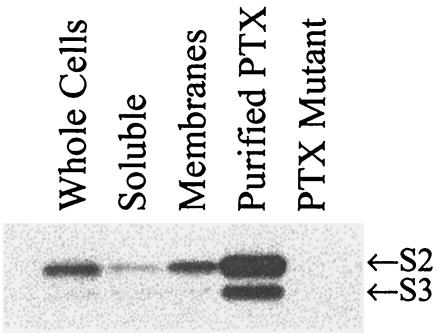
FIG. 8.
Localization of cellular S2 and S3. The amounts of S2 and S3 from various cell fractions were determined by Western blotting by using monoclonal antibody 11E6. The lanes contained BP338 whole cells (Whole Cells), the BP338 soluble fraction (Soluble), the BP338 membrane fraction (Membranes), 50 ng of purified pertussis toxin (Purified PTX), and BPRA (pertussis toxin mutant) whole cells (PTX Mutant).
Expression of PtlF protein.
The amount of cell-associated PtlF was determined by Western blotting (Fig. 9A). Cells grown under modulating conditions (time zero) were found to have about 30 molecules of PtlF per cell, and this level did not change by the 3-h time point, when secreted pertussis toxin was detected. From 3 to 24 h, a linear increase in the amount of PtlF was observed (R2 = 0.9697). Accumulation stopped after 24 h, and there were 1,072 molecules per cell at 24 h and 1,078 molecules per cell at 48 h. The amount of PtlF was less than the total amount of S1 (cell associated and secreted) at all time points, and the ratio of S1 to PtlF was 4:1 at 24 h (Fig. 9B). The rate of pertussis toxin secretion on a per cell basis was constant throughout the logarithmic phase (Fig. 4) despite a 30-fold increase in PtlF expression, suggesting that the basal level of PtlF protein expression (30 molecules per cell) is sufficient for maximum pertussis toxin secretion.
FIG. 9.
Accumulation of PtlF. (A) Amount of PtlF expressed on a per cell basis determined as a function of time in culture. The data are averages ± standard errors for four independent experiments, and the trend line was determined by linear regression for time points corresponding to 3 to 24 h in culture. (B) Comparison of PtlF and total S1 expressed on a per cell basis as a function of time in culture. The total amount of S1 was calculated by determining the amount of secreted S1 plus the amount of periplasmic S1, and the standard error was calculated by error propagation.
DISCUSSION
Type IV secretion systems of gram-negative bacteria mediate the transfer of complexes of biomolecules across membranes for at least two diverse processes, conjugation and protein secretion. The assembly and structure of the type IV conjugation systems have been extensively studied (3, 6, 11, 19, 38); however, the studies have been performed largely in the absence of the target recipient cells, and the results likely are results for the inactive, closed secretion complex. The pertussis toxin secretion system of B. pertussis has nine proteins, PtlA to PtlI, which is fewer than the conjugation systems have. Much is known about the secretion substrate, pertussis toxin, and the dynamics of the secretion process. The steps in pertussis toxin secretion (Fig. 10) include transcription and translation of the ptx-ptl operon, Sec-mediated secretion of the subunits into the periplasm, proteolytic removal of the signal peptides, intramolecular disulfide bond formation, periplasmic assembly of the toxin subunits and the secretion apparatus, and finally toxin secretion. We were able to examine several of these steps in this study.
FIG. 10.
Model of pertussis toxin assembly and secretion. In step 1, toxin subunits are transported through the inner membrane via Sec. In step 2, S1 and other subunits associate with the outer membrane and disulfide bonds are formed. In step 3, the B subunit assembles and associates with the A subunit (S1), forming holotoxin. In step 4, holotoxin is secreted through the outer membrane via the Ptl secretion system.
The ptx-ptl operon (Fig. 10) is large, about 13 kb. Ribosome binding sites have not been detected for the downstream genes of the ptx-ptl operon, suggesting that translation must initiate at S1 and continue down the mRNA and that PtlH is the final protein to be translated. A predicted stem-loop structure located between the ptx structural gene and the ptl secretion gene has been proposed to act as a translational terminator (31) and could play a role in differentially regulating levels of the pertussis toxin and the secretion proteins. Our data indicate that both PtlF and pertussis toxin S1 accumulate at linear rates (Fig. 9B), although PtlF accumulates at a lower rate. These results suggest that translation of the ptl genes may be attenuated with respect to translation of the pertussis toxin structural genes.
It has been suggested that membrane-associated S1 may constitute a nucleation site for assembly of the holotoxin on the periplasmic face of the outer membrane (12), as shown in Fig. 10, step 3. We found that the soluble pool of periplasmic S1 was equivalent to the pool of soluble, assembled pertussis toxin, suggesting that all of the unassembled S1 was associated with the membrane fraction. Similarly, membrane-associated and soluble pools of the B-subunits S2 and S3 were found. The C-terminal portion of S1 has been proposed to mediate association with the membrane (12); however, the C-terminal portion of S1 is required for association with the B pentamer (20), and assembly into holotoxin would promote disassociation of S1 from the membrane. In previous studies workers have identified a domain on S1 (amino acids 55 to 57), distal from the C terminus, which is required for secretion of pertussis toxin (9). This region is thought to mediate recognition of pertussis toxin by the Ptl secretion complex; however, it is currently not known how the Ptl secretion system can discriminate among the secretion substrate, assembled pertussis toxin, and unassembled subunits.
By analogy with the VirB system, PtlF is thought to form the actual secretion pore in the outer membrane (6). As few as 30 molecules of PtlF per cell were present when pertussis toxin secretion was first observed. The closest homolog of this protein, VirB9, forms multimers (19), and PtlF is also thought to multimerize, but the number of molecules per secretion complex is not known for either protein. The secretion pores of the type IV secretion systems can be compared to the secretion pores of filamentous phage. The filamentous phage particle is a protein-coated DNA molecule, as are the substrates secreted by many of the type IV systems. The phage is secreted through a pore in the outer membrane composed of multimerized pIV (24). The structure of the pIV secretion pore of filamentous phage f1 has been characterized, and the pIV protein forms tetradecamers (28). The stoichiometry of PtlF in the Ptl secretion complex is unknown, but if by analogy with pIV 14 PtlF subunits are incorporated into each pertussis toxin secretion complex, then maximal pertussis toxin secretion (3 molecules of toxin per cell per min) can occur with only two secretion complexes per cell. In contrast, at 24 h, each cell expressed about 1,070 molecules of PtlF, enough for 76 secretion complexes containing 14 molecules of PtlF. However, the rate of pertussis toxin secretion per cell was the same at 3 h, when 30 molecules of PtlF were present, as at 18 h, when hundreds of molecules of PtlF were present, suggesting that a pool of inactive PtlF had accumulated, similar to the pool observed for the pertussis toxin structural subunits S1, S2, and S3. It is likely that the subunits in biologically active secretion complexes fractionate in different compartments than inactive protein, confounding analysis of the secretion process. Our studies suggest that secretion should be examined early in the growth cycle before inactive complexes have accumulated in the cells. Functional complex assembly may be limited to only certain sites on the membrane. Polar localization would limit the number of active secretion complexes to two per cell. Polar localization has been observed for some extracellular bacterial complexes (35), such as flagella, pili, and the IcsA complex, which mediates intracellular spreading in Shigella. Several of the VirB proteins, VirB8, VirB9, and VirB10, localize to a few discrete foci in the membranes of bacterial cells (22), and about one-half of these foci exhibit polar localization; however, it has not been established whether all of these foci represent active secretion complexes. The A. tumefaciens VirD4 protein, which is required for transfer of cytoplasmic substrates via the VirB secretion complex, exhibits polar localization (21), suggesting that secretion of cytoplasmic substrates may be restricted to the polar VirB complexes. It remains to be determined whether secretion from the VirB and Ptl secretion complexes is limited to polar sites.
B. pertussis invests a great deal of resources to produce and secrete pertussis toxin; however, our studies suggest that it is a surprisingly inefficient process. The maximum secretion rate was only 3 molecules per min. Furthermore, over the time course characterized in this study, about 12% of the assembled pertussis toxin detected by the CHO cell assay was retained in the periplasm, and a vast excess of the secretion component PtlF accumulated. Secretion, not assembly of pertussis toxin, appears to be the rate-limiting step.
In contrast to pertussis toxin secretion, Vibrio cholerae secretes over 95% of its folded cholera toxin into the supernatant (15). Cholera toxin is secreted by the type II secretion system also used for secretion by the cholera toxin-encoding filamentous phage CTXφ (7), and a type II system for secretion of heat-labile enterotoxin has recently been identified in enterotoxigenic E. coli (37). Unlike V. cholerae and enterotoxigenic E. coli, B. pertussis has no reservoir other than the human host, and to maintain itself as a species it must be capable of persisting in the presence of an immune response. Pertussis toxin inhibits the ability of the host to generate an immune response. It is likely that improperly assembled pertussis toxin could elicit toxin-neutralizing antibodies and defeat the objective of pertussis toxin. It may be that the Ptl secretion system has evolved to ensure release of properly assembled toxin at the expense of efficiency.
While the process of pertussis toxin secretion may be inefficient, the amount of toxin secreted by B. pertussis is substantial, 3 μg/ml in 24 h. For comparison, the total amount of cholera toxin secreted in 24 h (0.36 μg/ml) (7) and the total amount of heat-labile toxin secreted in 24 h (0.19 μg/ml) (37) were 10-fold lower. Only 2.5 μg of pertussis toxin is needed to kill susceptible strains of mice (13), and it is likely that a higher rate of secretion would be counterproductive to the organism's survival.
Acknowledgments
We thank Trevor Stenson for preparation of supernatants containing the 1B7 and 11E6 monoclonal antibodies and the National Cell Culture Center for purification of these antibodies. We also thank Paula Mobberley-Schuman for her technical assistance.
This work was supported by grant RO1 AI23695 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Antoine, R., and C. Locht. 1990. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect. Immun. 58:1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, S. M., A. Masi, D. F. Liu, B. K. Novitsky, and R. A. Deich. 1995. Pertussis toxin export genes are regulated by the ptx promoter and may be required for efficient translation of ptx mRNA in Bordetella pertussis. Infect. Immun. 63:3920-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, C., D. O'Callaghan, and E. Lanka. 2002. Bacterial secrets of secretion: EuroConference on the Biology of Type IV Secretion Processes. Mol. Microbiol. 43:1359-1365. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, M. J., Z. M. Li, J. L. Cowell, M. E. Bisher, A. C. Steven, P. Novotny, and C. R. Manclark. 1988. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect. Immun. 56:3189-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles, I. G., J. L. Li, M. Roberts, K. Beesley, M. Romanos, D. J. Pickard, M. Francis, D. Campbell, G. Dougan, M. J. Brennan, et al. 1991. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur. J. Immunol. 21:1147-1153. [DOI] [PubMed] [Google Scholar]
- 6.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell, T. D., D. J. Metzger, M. Wang, M. G. Jobling, and R. K. Holmes. 1995. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infect. Immun. 63:4091-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covacci, A., and R. Rappuoli. 1993. Pertussis toxin export requires accessory genes located downstream from the pertussis toxin operon. Mol. Microbiol. 8:429-434. [DOI] [PubMed] [Google Scholar]
- 9.Craig-Mylius, K. A., T. H. Stenson, and A. A. Weiss. 2000. Mutations in the S1 subunit of pertussis toxin that affect secretion. Infect. Immun. 68:1276-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig-Mylius, K. A., and A. A. Weiss. 1999. Mutants in the ptlA-H genes of Bordetella pertussis are deficient for pertussis toxin secretion. FEMS Microbiol. Lett. 179:479-484. [DOI] [PubMed] [Google Scholar]
- 11.Ding, Z., Z. Zhao, S. J. Jakubowski, A. Krishnamohan, W. Margolin, and P. J. Christie. 2002. A novel cytology-based, two-hybrid screen for bacteria applied to protein-protein interaction studies of a type IV secretion system. J. Bacteriol. 184:5572-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farizo, K. M., S. Fiddner, A. M. Cheung, and D. L. Burns. 2002. Membrane localization of the S1 subunit of pertussis toxin in Bordetella pertussis and implications for pertussis toxin secretion. Infect. Immun. 70:1193-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, J. F., S. B. Call, P. D. Fillmore, T. Watanabe, N. D. Meeker, and C. Teuscher. 2003. Analysis of the role of Bphs/Hrh1 in the genetic control of responsiveness to pertussis toxin. Infect. Immun. 71:1281-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst, T. R., and J. Holmgren. 1987. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 84:7418-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, F. D., and D. L. Burns. 1994. Detection and subcellular localization of three Ptl proteins involved in the secretion of pertussis toxin from Bordetella pertussis. J. Bacteriol. 176:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenimer, J. G., K. J. Kim, P. G. Probst, C. R. Manclark, D. G. Burstyn, and J. L. Cowell. 1989. Monoclonal antibodies to pertussis toxin: utilization as probes of toxin function. Hybridoma 8:37-51. [DOI] [PubMed] [Google Scholar]
- 18.Kotob, S. I., S. Z. Hausman, and D. L. Burns. 1995. Localization of the promoter for the ptl genes of Bordetella pertussis, which encode proteins essential for secretion of pertussis toxin. Infect. Immun. 63:3227-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krueger, K. M., and J. T. Barbieri. 1994. Assignment of functional domains involved in ADP-ribosylation and B-oligomer binding within the carboxyl terminus of the S1 subunit of pertussis toxin. Infect. Immun. 62:2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar, R. B., and A. Das. 2002. Polar location and functional domains of the Agrobacterium tumefaciens DNA transfer protein VirD4. Mol. Microbiol. 43:1523-1532. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, R. B., Y. H. Xie, and A. Das. 2000. Subcellular localization of the Agrobacterium tumefaciens T-DNA transport pore proteins: VirB8 is essential for the assembly of the transport pore. Mol. Microbiol. 36:608-617. [DOI] [PubMed] [Google Scholar]
- 23.Lessl, M., D. Balzer, W. Pansegrau, and E. Lanka. 1992. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J. Biol. Chem. 267:20471-20480. [PubMed] [Google Scholar]
- 24.Linderoth, N. A., M. N. Simon, and M. Russel. 1997. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science 278:1635-1638. [DOI] [PubMed] [Google Scholar]
- 25.Locht, C., and J. M. Keith. 1986. Pertussis toxin gene: nucleotide sequence and genetic organization. Science 232:1258-1264. [DOI] [PubMed] [Google Scholar]
- 26.Melton, A. R., and A. A. Weiss. 1989. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol. 171:6206-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicosia, A., M. Perugini, C. Franzini, M. C. Casagli, M. G. Borri, G. Antoni, M. Almoni, P. Neri, G. Ratti, and R. Rappuoli. 1986. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc. Natl. Acad. Sci. USA 83:4631-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opalka, N., R. Beckmann, N. Boisset, M. N. Simon, M. Russel, and S. A. Darst. 2003. Structure of the filamentous phage pIV multimer by cryo-electron microscopy. J. Mol. Biol. 325:461-470. [DOI] [PubMed] [Google Scholar]
- 29.Pantoja, M., L. Chen, Y. Chen, and E. W. Nester. 2002. Agrobacterium type IV secretion is a two-step process in which export substrates associate with the virulence protein VirJ in the periplasm. Mol. Microbiol. 45:1325-1335. [DOI] [PubMed] [Google Scholar]
- 30.Rambow-Larsen, A. A., and A. A. Weiss. 2002. The PtlE protein of Bordetella pertussis has peptidoglycanase activity required for Ptl-mediated pertussis toxin secretion. J. Bacteriol. 184:2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci, S., R. Rappuoli, and V. Scarlato. 1996. The pertussis toxin liberation genes of Bordetella pertussis are transcriptionally linked to the pertussis toxin operon. Infect. Immun. 64:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato, H., A. Ito, J. Chiba, and Y. Sato. 1984. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect. Immun. 46:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato, H., and Y. Sato. 1990. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect. Immun. 58:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato, H., Y. Sato, A. Ito, and I. Ohishi. 1987. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect. Immun. 55:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro, L., H. H. McAdams, and R. Losick. 2002. Generating and exploiting polarity in bacteria. Science 298:1942-1946. [DOI] [PubMed] [Google Scholar]
- 36.Tamura, M., K. Nogimori, S. Murai, M. Yajima, K. Ito, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 37.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorstenson, Y. R., G. A. Kuldau, and P. C. Zambryski. 1993. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J. Bacteriol. 175:5233-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, K. E., and A. A. Weiss. 1994. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect. Immun. 62:3817-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, D. V., O. Draper, J. R. Zupan, and P. C. Zambryski. 2002. Peptide linkage mapping of the Agrobacterium tumefaciens vir-encoded type IV secretion system reveals protein subassemblies. Proc. Natl. Acad. Sci. USA 99:11493-11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss, A. A., F. D. Johnson, and D. L. Burns. 1993. Molecular characterization of an operon required for pertussis toxin secretion. Proc. Natl. Acad. Sci. USA 90:2970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss, A. A., P. S. Mobberley, R. C. Fernandez, and C. M. Mink. 1999. Characterization of human bactericidal antibodies to Bordetella pertussis. Infect. Immun. 67:1424-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]