Abstract
The neural crest-derived cell population that colonizes the bowel (ENCDC) contains proliferating neural/glial progenitors. We tested the hypothesis that bone morphogenetic proteins (BMPs 2 and 4), which are known to promote enteric neuronal differentiation at the expense of proliferation, function similarly in gliogenesis. Enteric gliogenesis was analyzed in mice that overexpress the BMP antagonist, noggin, or BMP4 in the primordial ENS. Noggin-induced loss-of-function decreased, while BMP4-induced gain-of-function increased the glial density and glia/neuron ratio. When added to immunoisolated ENCDC, BMPs provoked nuclear translocation of phosphorylated SMAD proteins and enhanced both glial differentiation and expression of the neuregulin receptor ErbB3. ErbB3 transcripts were detected in E12 rat gut, before glial markers are expressed; moreover, expression of the ErbB3 ligand, glial growth factor 2 (GGF2) escalated rapidly after its first detection at E14. ErbB3-immunoreactive cells were located in the ENS of fetal and adult mice. GGF2 stimulated gliogenesis and proliferation and inhibited glial cell derived neurotrophic factor (GDNF)-promoted neurogenesis. Enhanced glial apoptosis occurred following GGF2 withdrawal; BMPs intensified this GGF2-dependence and reduced GGF2-stimulated proliferation. These observations support the hypotheses that BMPs are required for enteric gliogenesis and act by promoting responsiveness of ENCDC to ErbB3 ligands such as GGF2.
Keywords: development, BFABP, S100β, GFAP, HuC/D, GGF2, ErbB3, Sox10, noggin, enteric nervous system
Introduction
The enteric nervous system (ENS) is the largest and most complex division of the peripheral nervous system (PNS) (Furness, 2006; Gershon and Ratcliffe, 2006). Its component neurons are phenotypically diverse and form microcircuits that allow the ENS to regulate the behavior of the bowel independently of input from the brain and/or spinal cord (Gershon and Tack, 2007). Like the brain, the ENS lacks internal collagen and derives support for its neuronal elements from glia that resemble their counterparts in the central nervous system (CNS)(Gershon and Rothman, 1991). The ENS develops from precursors that migrate to the bowel from vagal, rostral truncal, and sacral levels of the neural crest (Burns and Le Douarin, 1998; Durbec et al., 1996; Heanue and Pachnis, 2007; Kapur, 2000; Le Douarin and Teillet, 1973; Le Douarin and Teillet, 1974; Pomeranz and Gershon, 1990; Pomeranz et al., 1991). Although many individual crest-derived cells are already determined before they reach the bowel (Henion and Weston, 1997; Pham et al., 1991; Reedy et al., 1998a; Reedy et al., 1998b), the enteric population of crest-derived cells (ENCDC) is multipotent (Natarajan et al., 1999). This population responds to growth factors and other components of the enteric microenvironment, the actions of which are responsible for the development of the unique properties of the ENS (Gershon and Ratcliffe, 2006; Heanue and Pachnis, 2007).
It is likely that the early ENCDC population contains precursors that are common both to neurons and to glia (Natarajan et al., 1999; Young et al., 2003; Young et al., 2005). Relatively little is known about signals that act on these uncommitted precursors to cause them to differentiate and give rise to divergent neuronal and glial cell lineages. Early ENCDC express both Phox2b and Sox10; however, ENCDC that commit to a neuronal lineage downregulate Sox10 expression while maintaining Phox2b (Pattyn et al., 1997; Young et al., 2003; Young et al., 1999). Conversely ENCDC committed to a glial lineage downregulate Phox2b expression while maintaining Sox10 (Bondurand et al., 2006; D'Autreaux et al., 2007; Deal et al., 2006; Kim et al., 2003; Paratore et al., 2001; Stanchina et al., 2006). Sox10, moreover, controls expression of ErbB3 (Britsch et al., 2001) and enteric glia are absent in mice that lack ErbB3 (Riethmacher et al., 1997), suggesting that this receptor is important in enteric gliogenesis. The neuregulin (Nrg) family of ligands signals through ErbB receptors (Birchmeier, 2009; Brinkmann et al., 2008). Nrgs are produced by mesenchymal cells and promote glial lineage commitment by primary neural crest cells in vitro at the expense of a neuronal fate (Shah and Anderson, 1997; Shah et al., 1994). Only one of the 4 types of Nrg1 isoform, glial growth factor 2 (GGF2; a type II isoform) is a secreted molecule (Marchionni et al., 1993); it promotes development of satellite cells, which are in a glial lineage, in developing sensory ganglia (Leimeroth et al., 2002).
During early fetal life, ENCDCs respond to and require stimulation by glial cell derived neurotrophic factor (GDNF), which promotes proliferation, differentiation, survival, and migration of enteric precursors (Asai et al., 2006; Chalazonitis et al., 1998a; Hao and Young, 2009; Hearn et al., 1998; Laranjeira and Pachnis, 2009; Young et al., 2001). The ability of GDNF to stimulate proliferation declines as a function of fetal age (Chalazonitis et al., 1998a) and is limited by the actions of BMP -2 and -4 (Chalazonitis et al., 2004). These BMPs oppose GDNF by enhancing differentiation at the expense of the continued proliferation of precursors. BMPs also induce the expression of certain growth factor receptors, such as TrkC, thereby promoting responsiveness to and dependence on their corresponding ligands, which in the case of TrkC is NT-3. This action of BMP signaling favors the development of some phenotypes of enteric neuron, such as NT-3/TrkC-dependent dopaminergic neurons, at the expense of others (Chalazonitis et al., 2004; Chalazonitis et al., 2008).
Although BMP-2 and -4 increase enteric neuronal differentiation, it is not clear whether or not they also enhance development of enteric glia. We therefore tested the hypothesis that BMPs stimulate uncommitted ENCDC to differentiate but do not determine whether they develop as neurons or glia. We postulated that this lineage choice instead depends on other factors, such as GDNF and GGF2, which promote, respectively, neuronal and glial development. Specifically, we proposed that a balance exists between Ret activation by GDNF, which favors the expansion of cells in a neuronal lineage, and ErbB3 activation by a Nrg such as GGF2, which expands the population in a glial lineage. Our observations support these hypotheses and indicate that BMPs enhance the responsiveness of ENCDC both to GDNF-induced neurogenesis and GGF2-induced gliogenesis. Portions of this work have previously been published in abstract form (Chalazonitis et al., 2009; Chalazonitis et al., 2007).
Materials and Methods
Animals
Pregnant female rats (Sprague-Dawley; Charles River laboratories, Waltham MA) were subjected to CO2 narcosis and killed by thoracotomy. The Animal Care and Use Committee of Columbia University approved these procedures. Gestational age was counted from the occurrence of a vaginal plug, which was considered E0. The entire gut, from stomach to rectum, was dissected as a single specimen under aseptic conditions. To isolate crest-derived cells, these specimens were pooled from 30–32 fetuses at E12, 20–25 fetuses at E14, and 15–20 fetuses at E16. Pregnant female mice (CD-1; Charles River) were euthanized as described above.
Transgenic mice
The neuron specific enolase (NSE) promoter was employed to overexpress noggin or BMP4 in the gut of transgenic animals (Chalazonitis et al., 2004; Gomes et al., 2003; Guha et al., 2004). An IRES-GFP sequence allows expression of the noggin transgene to be monitored by demonstrating the native fluorescence or immunoreactivity of GFP. GFP expression was not detectable in the gut of transgenic mice at E13, although it was observed to be present in the primordia of prevertebral sympathetic ganglia and chromaffin bodies. GFP was abundantly expressed in the gut by E16 and was maintained through P11, not only in the ENS, but also in scattered epithelial cells. By P0, GFP fluorescence was also exhibited by many cells in the subepithelial mesenchyme (Chalazonitis et al., 2008). These observations suggest that the onset of transgenic overexpression of noggin in the gut begins between E13 and E16 and persists into postnatal life. Although the NSE-BMP4 construct did not have an IRES-GFP sequence and thus was not specifically labeled, immunoblots at E16 revealed that the level of BMP4 expression was higher than that of WT animals (Gomes et al., 2003).
RT-PCR
Pairs of oligonucleotide primers for amplification of cDNAs encoding GGF2, ErbB3, p75NTR and β-Actin1 were designed from published rat cDNA sequences. The Gene bank accession number, the sequences of the primers used for PCR, the conditions of amplification and the expected sizes of the products are shown in Table 1. The identities of all PCR products were confirmed by sequence analysis. For this purpose, PCR products were subcloned into pGEM-T Easy vectors (Promega, Madison, WI) using the TA-cloning kit (Invitrogen Corporation, Carlsbad, CA). Inserts in two individual clones were sequenced by the dideoxynucleotide-chain termination method in the DNA Facility of Columbia University. The sequences of the PCR products obtained from brain and gut with the indicated primers were found to be identical to those of the appropriate regions of the GenBank sequences of the amplified cDNAs (Table 1).
Table 1.
PCR primers, conditions used for amplification, and expected sizes of PCR products
| Protein Genebank# |
Sense (5’-3’)/ Nucleotides |
Antisense (5’-3’)/ Nucleotides |
Cycles | Temperature °C (time s) /* Annealing Temp | Product (bp) |
|---|---|---|---|---|---|
| Rat GGF2/Nrg1 type II AF 194994 | GGTGGTGATCGAGGGA 424-439 | CGGTGAGTAGCGAGTC 698-683 | 40 | 94 (5 sec) 57* (10sec) 72 (12sec) | 275 Kringle domain |
| Rat ErbB3/Her3 U 29339.2 | TGTCGAAACTACAGCCG 1696-1712 | ACCGTCACCGCTATTAC 2081-2065 | 35 | 94 (30sec), 55* (45sec), 72 (45sec) | 386 |
| Rat p75NTR/Ngfr NM 012610 | GAGGGCACATACTCAGACGAAGCC 567-590 | GTCTATATGTTCAGGCTGGTAACC 1229-1206 | 30 | 94 (30sec), 60* (45sec), 72 (45sec) | 663 |
| Rat β-Actin1/Actb NM031144 | TGTTTGAGACCTTCAACAC 449-467 | CAGTAATCTCCTTCTGCATCC 1035-1015 | 40 | 94(5 sec), 58* (10 sec), 72(23 sec) | 586 |
Real time PCR
Real time PCR was used to quantify transcripts encoding GGF2 in the fetal and adult rat gut. Primers for GGF2 were chosen to amplify a PCR product that includes the Kringle domain, which is unique to GGF2 (Buonanno and Fischbach, 2001). The expression of GGF2 was normalized to that of β-actin1. Transcripts encoding β-actin1 and GGF2 were quantified by real time PCR (RT PCR) with a SYBR Green I kit (Roche Diagnostics Corporation, Indianapolis, IN) and Jumpstart Taq ReadyMix (Sigma, St. Louis, MO) using a LightCycler™2.0 instrument as previously described (Chalazonitis et al., 2008; D'Autreaux et al., 2007). Amplifications were carried out in a final volume of 20 µl that contained Taq DNA polymerase, reaction buffer, dNTPs in which dTTP is replaced by dUTP, SYBR Green I dye, and MgCl2. The final concentration of primers used for the amplification of cDNA encoding β-actin1 or GGF2 was 0.5 µM. The final concentration of MgCl2 was 4.0 mM. To this mixture were added 1 µl of either the serially diluted plasmid PGEM-T with the inserted PCR product DNA (standards) or the cDNA prepared from tissue. Measurements were obtained by referring to standard curves that were prepared by serially diluting plasmid DNA containing an insert of a PCR product that includes a portion of the sequence of GGF2 or β-actin1. The dilutions of β-actin1 and GGF2 plasmid DNA ranged from 1 ng to 100 fg in 5 series, each of which covered a 10-fold range. The standards and the cDNA from tissues were simultaneously subjected to RTPCR analysis in parallel capillary tubes. A first denaturation step for each round of PCR was carried out at 94°C for 10 minutes to activate the polymerase. The PCR reactions were carried out according to the programs in Table 1. The appearance of double stranded DNA was quantified by measuring the fluorescence of SYBR Green after each step of elongation. The ramp rate was 20°C/sec during the amplification program. A melting curve analysis with a ramp rate of 0.1°C/sec was carried out to verify that a single moiety had been amplified. Data were analyzed with computer assistance employing the LightCycler ™ software. Three independent experiments for GGF2 and 2 independent experiments for β-actin1 were carried out. The sequences of the products obtained from gut with the indicated primers (Table 1) were found to be identical to those of the appropriate regions of the GenBank sequence of Nrg1 type 2 amplified cDNAs.
Immunoselection
Crest-derived cells were immunoselected from the fetal rat gut with antibodies to the common neurotrophin receptor, p75NTR, at E12, E14 and E16 as described previously (Chalazonitis et al., 2004; Chalazonitis et al., 2008; Chalazonitis et al., 2001; Chalazonitis et al., 1998a; Chalazonitis et al., 1998b). Briefly, the bowel was dissociated with collagenase. The resulting suspension of single cells was exposed, first to antibodies to p75NTR (Table 2) and then to secondary antibodies coupled to magnetic beads (Miltenyi Biotec Inc Auburn, CA). The antibody decorated crest-derived cells were finally selected with a magnetic field. This procedure positively selects crest-derived cells, which are retained by the magnetic field, and negatively selects non-crest-derived cells, which pass through it. Both the monoclonal (MC192; donated by Dr. Robert A. Rush, Flinders University) and polyclonal antibodies to p75NTR (donated by Dr. Moses Chao; Skirball Inst. New York University) that were used for immunoselection react with the extracellular domains of mouse and rat p75NTR (Chandler et al., 1984; Huber and Chao, 1995).
Table 2.
Antibodies used to identify enteric glia and neurons
| Antigen/Species | Host | Dilution | Source | References |
|---|---|---|---|---|
| p75NTRextracellular domain/mouse | Rabbit polyclonal | 1:1000* | Moses Chao, Skirball Institute New York University, NY | Huber and Chao, 1995; Chalazonitis et al., 2008 |
| 1:2000 | ||||
| p75NTR/rat PC12 membranes | Mouse monoclonal clone MC192 | 20 µg/ml* | Robert A. Rush, Flinders University, Adelaide, Australia | Chandler et al., 1984; Chalazonitis et al., 2001 |
| BFABP recombinant protein/mouse | Rabbit polyclonal | 1:170 | Thomas Müller, Max Delbrück Center for Molecular Med., Berlin | Kurtz et al., 1994; Young et al., 2003 |
| S100 (A and B) cow brain | Rabbit polyclonal | 1:1000 | DAKO Glostrup Denmark Cat# Z 0311 | Young et al., 2003 |
| S100 β purified recombinant protein/Human | Rabbit polyclonal | 1:2000 (whole mount) | Cat# A 5110 | |
| 1:400 (cultures) | ||||
| GFAP purified/bovine | Rabbit polyclonal | 1:1000 (cultures) | Chemicon/Millipore Temecula, CA Cat# AB5804 | Jungling et al., 2003 |
| GFAP purified glial filament/human | Mouse monoclonal clone G-A-5 | 1:150 (cultures) | Chemicon/Millipore Cat#MAB3402 | Yang et al., 2005 |
| GFAP purified | Chicken IgY Polyclonal | 1:1000 (sections) | Neuromics, Edina MN, Cat# CH22102 | Liu et al. 2009 |
| PH-3 peptide (aa7–20) of P Histone-3/Human | Rabbit polyclonal | 1:200 (cultures) | Upstate, cell signal. solutions/Millipore Lake Placid, NY Cat#06-570 | Bondurand et al., 2003 |
| ErbB3 p160 (17aa peptide in last 50 aa of C-terminal domain)/Human | Mouse monoclonal Clone G4 | 1:50 (cultures) | Santa Cruz Biotechnology, Inc. Santa Cruz, CA Cat# SC-7390 | Ritch et al., 2003 |
| ErbB3 (19aa peptide of N-terminal domain/Human | Rabbit polyclonal | 1:50 (sections) | Abnova, Taiwan Corp. Taipei, ROC | |
| Sox10, epitope first 65 aa/mouse or rat | Mouse monoclonal Clone 20B7 | 1:10 | David J. Anderson, California Institute of Technology HHMI, CA | Lo et al., 2002 |
| pSmad1,5,8 peptide residues around P Ser 463/465 and P Ser426/428/Human | Rabbit polyclonal | 1:100 | Cell Signaling Technology, Inc. Beverly, MA Cat#9511 | Ahn et al., 2001 |
| HuC/HuD, RNA binding protein of the Elav family/Human | Mouse monoclonal biotinylated clone 16A11 | 1:50 | Molecular Probes, Eugene, OR Cat#A21272 | Wakamatsu et al., 2001 |
| PGP 9.5 purified from brain/Human | Rabbit polyclonal | 1:800 | Biogenesis Ltd, Poole,UK Cat#7863-0504 | Wilkinson et al., 1989 |
Dilution used for immunoselection.
Tissue culture
Crest-derived cells were plated at a density of 1.2 × 105 cells/ml onto 12 mm diameter glass coverslips (RESY No. 1001, Germany) that were coated with poly-D-lysine, rat tail collagen and laminin, as described previously (Chalazonitis et al., 1998b). Cultures were maintained in a defined medium (Basic Brazeau; BBM) (Ziller et al., 1983). At the time of plating, the medium was supplemented with 20% horse serum (JRH, Lenexa, KA) to promote adherence of cells to the substrate. After 18 hrs, this medium was changed to serum-free BBM. When cultures were maintained for longer than 4 days, they were fed with fresh medium on the 4th day. In all experiments, the factors to be studied, which included BMPs -2 and -4 (human recombinant; Wyeth, Cambridge MA and R&D systems Berkeley CA), GGF2 (Acorda Therapeutics, Hawthorne, NY), and GDNF (rat recombinant; R&D Systems), were added to the medium at the time of plating or of medium change and were present throughout the culture period. Control cultures were supplemented with the vehicle in which the growth factors were diluted (0.5 % bovine serum albumin in BBM). At least 4 replicate cultures were studied for each condition in each experiment.
Immunocytochemistry
Immunocytochemical detection of markers was used to identify neurons and glia differentiating in vitro (Chalazonitis et al., 2004; Chalazonitis et al., 2001). Cultures were fixed for 1hr (or 15 min to demonstrate the mitotic marker phospho-histone-3) with 4% formaldehyde (freshly prepared from paraformaldehyde) in 0.1M PBS and permeabilized with 0.1%Triton X. The cultures were then processed and examined as whole mounts. Fixed cultures were incubated with primary antibodies (Table 2) at room temperature overnight (or for 48 hours at 4°C to demonstrate ErbB3). Bound antibodies were detected with species-specific secondary antibodies labeled with Alexa 488, Alexa 594 (Molecular Probes, Eugene, OR), or biotin (Kirkegaard & Perry, Gaithersburg, MD). Biotinylated antibodies were visualized with streptavidin coupled to Alexa 594 (Molecular Probes). Controls included immunostaining in the absence of primary antibodies. DNA was stained with bizbenzamide (1 µg/ml; Sigma, St Louis, MO) to facilitate counting nuclei to determine the total number of cells/culture. Terminal deoxynucleotidyl transferase (TdT)-mediated biotinylated UTP nick end labeling (TUNEL method; TACS™ Apoptosis Detection Kit; R&D Systems, Minneapolis, MN) was used to identify cells undergoing apoptosis (Chalazonitis et al., 2004). When used to identify apoptotic glia, GFAP immunoreactivity was demonstrated prior to application of the TUNEL method.
Gut was dissected from E17 and adult CD-1 mice and fixed with 4% formaldehyde (freshly prepared from paraformaldehyde) in 0.1 M phosphate buffer for 4 hours. After rinsing, the gut was immersed in 30% sucrose overnight followed by immersion in embedding medium (Neg50, Richard-Allan Scientific, Kalamazoo, MI) and frozen in blocks. Sections (10–12µ thick) were cut in a cryostat-microtome and dried on Superfrost slides (Fischer Scientific, UK). Immunocytochemistry for neuronal and glial markers (see Table 2) followed procedures similar to those used for cultured cells, except that the Triton X concentration was 0.3%.
Laminar preparations of gut wall
Bowel was removed from NSE-Nog and NSE-BMP4 mice and their WT littermates and dissected to obtain laminar preparations of the wall of the small intestine (duodenum and ileum) (Chalazonitis et al., 2004; Chalazonitis et al., 2008). Layers containing the submucosal and myenteric plexuses were analyzed separately. These preparations were fixed as described above, processed, and examined as whole mounts. Immunoreactivity of S100β (Table 2) was used to identify glia. Primary antibodies were applied overnight at room temperature. Secondary antibodies were labeled with horseradish peroxidase (HRP). Endogenous peroxidase activity was blocked with 0.3% H2O2 in PBS. Peroxidase activity was visualized with H2O2 and 3, 3’-diaminobenzidine with or without nickel intensification (Vector laboratories, Burlingame, CA). To simultaneously demonstrate glia and neurons, the neurons were stained first for 1 hour at 37°C with cuprolinic blue (0.5% in 0.05 mM Na acetate buffer, pH5.6, containing 1.0 mM MgCl2) (Chalazonitis et al., 2004; Karaosmanoglu et al., 1996; Phillips et al., 2004).
Immunostained cultures, sections, or laminar preparations of bowel wall were analyzed and photographed with a Leica DMRXA2 microscope equipped with dichroic mirror/filter assemblies that permitted no-cross detection of bisbenzimide, Alexa 488 and Alexa 594 fluorescence. Images were captured with a cooled CCD camera (Retiga Exi) mounted on the microscope and analyzed with Improvision software (Lexington, MA).
Quantitation of glia and neurons
Neurons, glia, and total numbers of cells developing in culture were identified as described above and quantified. Both the absolute numbers of each cell type and their proportion of the total number of cells in each culture were thus ascertained. When the quantitative data for identified cell types are presented, they were normalized, most often as a percent of vehicle to facilitate comparison of different experimental conditions and of repeated experiments. When total numbers of cells changed, data were often shown both as a proportion of total cells and as a percent of vehicle. When necessary data were normalized to the numbers of a particular type of cell; These methods have been previously published (Chalazonitis et al., 2004; Chalazonitis et al., 2001). Similarly, the packing densities of glia and neurons, identified in laminar preparations as described above, were determined and expressed as a function of tissue area (mm2). Individual measurements (n) were defined as the sum of glia or neurons counted, using a 40X objective, in 10 contiguous, non-overlapping fields covering an area of 1.254 mm2. The packing densities of neurons and glia in the small intestines of NSE-Nog and NSE-BMP4 mice at ages P26–34 were compared to those measured in their WT littermates. For NSE-Nog, small intestines were obtained from 9 heterozygotes (NSE-Nog/+), 5 homozygotes (NSE-Nog/Nog), and 5 WT littermates. For NSE-BMP4 mice, 4 heterozygotes and 4 WT littermates were studied.
Statistical analyses
Data are expressed as means ± standard errors and were compared using ANOVA (Prism 4.0b for the Macintosh computer; GraphPad Software, Inc.). Post hoc analysis was carried out with the Tukey-Kramer test for multiple comparisons. The Bonferroni test was employed when only 3 groups were compared. An unpaired t-test was carried out to compare one group with another.
Results
Transgenic overexpression of noggin under control of the NSE-promoter (NSE-Nog) impedes development of enteric glia
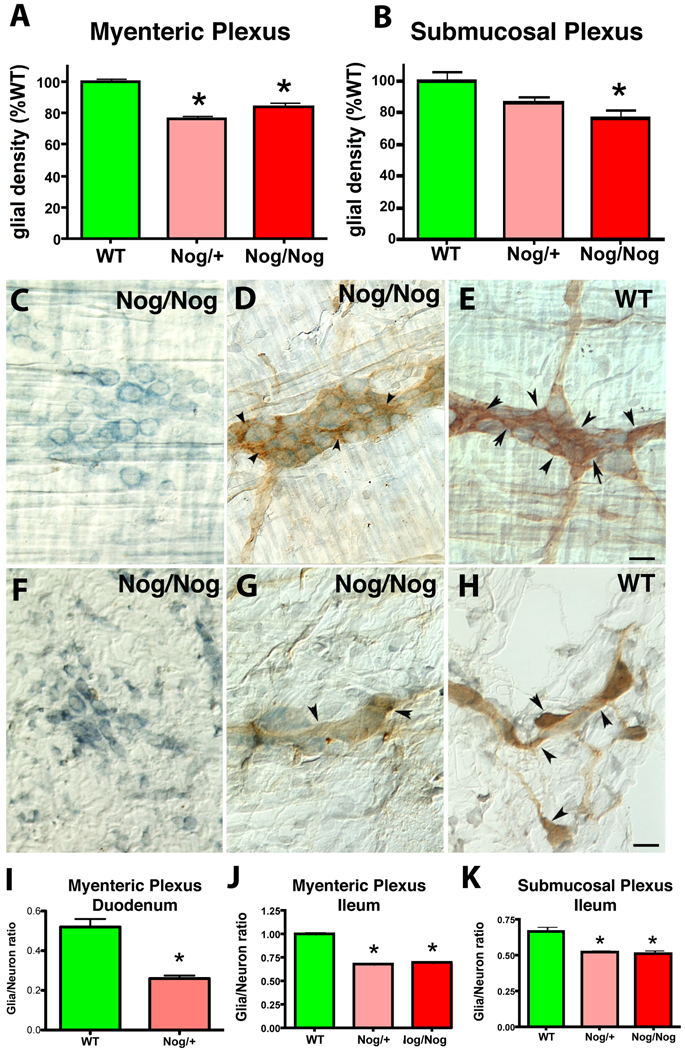
The packing densities of glia in the duodenum and ileum of NSE-Nog/+ and NSE-Nog/Nog mice were compared to that in WT littermates (Fig. 1A, B). Laminar preparations containing, respectively, the myenteric (Fig.1C–E) and submucosal plexuses (Fig. 1F–H), were examined immunocytochemically as whole mounts using S100β as a glial marker (Fig. 1D, E, G, H). The glial packing density was significantly higher in WT than in either NSE-Nog/+ (p < 0.05) or NSE-Nog/Nog mice (p < 0.001) (Fig. 1A, B). No difference, however, was observed between the NSE-Nog/+ and NSE-Nog/Nog animals. Because neuron numbers are greater in both types of transgenic mouse than in WT animals (Chalazonitis et al., 2004; Chalazonitis et al., 2008), the packing densities of neurons, demonstrated with cuprolinic blue (Fig. 1C–H), in the two plexuses were also determined and used to normalize the densities of enteric glia. The glia/neuron ratio in myenteric ganglia of wild-type mice was found to be significantly higher (p < 0.0001) in the ileum (1.00 ± 0.02; n = 42) than in the duodenum (0.52 ± 0.02; n = 32); therefore, the myenteric glia/neuron ratios in both the duodenum (Fig. 1I) and ileum (Fig. 1J) in WT mice were compared to those in the same regions of NSE-Nog/+ animals. In both the duodenum and the ileum, the myenteric glia/neuron ratios were significantly higher in WT than in NSE-Nog/+ mice (p < 0.001). This was also true of the submucosal (Fig. 1K; p < 0.01) glia/neuron ratios although the submucosal plexus was analyzed only in the ileum. The glia/neuron ratios in NSE-Nog/+ and NSE-Nog/Nog mice (examined in the ileum) did not differ significantly from one another in either plexus, but both were lower than those of WT littermates. These data suggest that BMP signaling promotes development of glia, which are thus reduced both in absolute numbers and relative to neuron numbers when BMP signaling is antagonized by the NSE-directed overexpression of noggin.
Fig. 1. Over-expression of noggin in the developing mouse ENS reduces glial density and the glia to neuron ratio.
S100β immunoreactivity in whole mounts of laminar preparations of bowel served as a glial marker. A. Glial density in the ileal myenteric plexus of WT and transgenic mice. The numbers of measurements were WT (n = 57), heterozygotes (Nog/+; n = 37), homozygotes (Nog/Nog, n = 34). * p < 0.05 Nog/+ and p < 0.001 Nog/Nog. B. Glial density in the submucosal plexus of WT and transgenic mice. The numbers of measurements were WT (n = 49), Nog/+ (n = 34), Nog/Nog (n = 29). * p < 0.05. C–E. Neurons and glia (arrowheads) in the myenteric plexus of the ileum of homozygous noggin overexpressing (Nog/Nog) and WT mice. Scale bar = 30µm. Neurons were identified by staining with cuprolinic blue without (C, F) or together with simultaneous co-immunostaining for S100β (D, E). F–H Submucosal plexus. F. Cuprolinic blue staining in a Nog/Nog mouse. (G, H) Cuprolinic blue and S100β immunoreactivity are demonstrated in Nog/Nog (G) and WT (H) littermates. I. The ratios of glia to neurons in the duodenal myenteric plexus. The numbers of measurements were WT (n = 16), Nog/+ (n =33); * p < 0.0001. J. The ratios of glia to neurons in the ileal myenteric plexus. The numbers of measurements were in WT (n = 42), in Nog/+ (n = 27), and in Nog/Nog (n = 15); * p < 0.01 compared to WT. K. The ratios of glia to neurons in the ileal submucosal plexus. The numbers of measurements were WT (n = 34), Nog /+ (n = 24), and Nog/Nog (n = 18); * p < 0.01 compared to WT.
Transgenic overexpression of BMP4 enhances development of enteric glia
The effects of transgenic overexpression of BMP4 were determined to test the hypothesis, suggested by the overexpression of noggin, that BMP signaling promotes enteric glial development. Laminar preparations of the duodenal wall were examined using immunocytochemical detection of S100 as a glial marker (Fig. 2A, B). The packing densities of enteric glia and the glia to neuron ratios were measured in the myenteric plexus of WT and NSE-BMP4/+ mice after immunostaining glia with antibodies to S100β and light counterstaining neurons with cuprolinic blue (Fig. 2A inset). Only heterozygous NSE-BMP4/+ mice were studied because sufficient numbers of surviving homozygotes could not be obtained. Both the glial packing density (Fig. 2C; p < 0.0005) and the glia/neuron ratio (Fig. 2D; p <0.0001) in NSE-BMP4/+ animals were significantly greater than those of WT littermates. These observations are consistent with those obtained from studies of NSE-Nog mice and together they suggest that BMP signaling promotes enteric glial development in vivo.
Fig. 2. The density of glial cells and the glia to neuron ratio are increased in myenteric plexus of mice over-expressing BMP4.
The immunoreactivity of S100β was used to identify enteric glial cells in whole mounts of laminar preparations of bowel containing the myenteric plexus. A. WT mouse. Inset: A high magnification image of the cell body of an S100β-immunostained glial cell body (arrow). Cuprolinic blue counterstaining. This is the type of preparation used to quantify glia; note that glial processes wrap around the light blue non-immunostained neurons. B. Heterozygous NSE-BMP4 (BMP4/+) mouse. The ganglia and connectives of the myenteric plexus are made visible by the brown S100β immunoreactivity of enteric glia. The clear lacunae in the glial network are the non-immunostained neurons. The bars = 60 µm. C. The glial density (glia/mm2 plexus) was quantified (see Fig. 2A inset) and expressed as % of WT. D. The glial density was normalized to that of neurons (marked by staining with cuprolinic blue [see Fig. 1 and 2A inset, above]) and expressed as a glia/neuron ratio. Both the glial density and the glia/neuron ratio were significantly greater in BMP4/+ than in WT mice. * p < 0.001, n = 12.
BMPs promote enteric glial differentiation in vitro
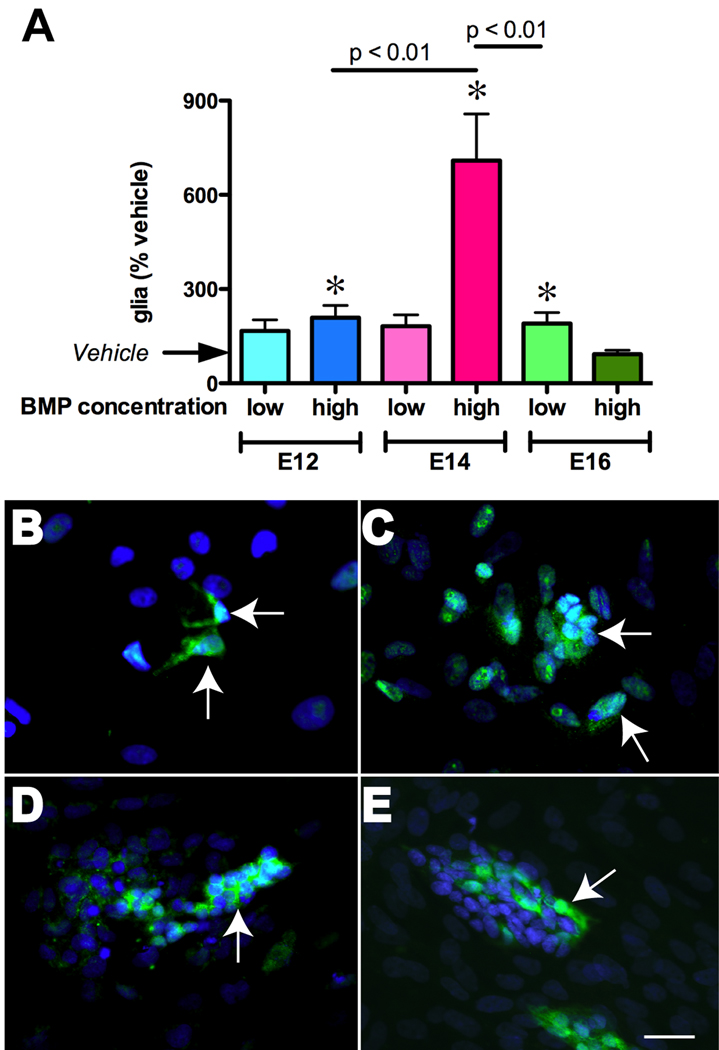
To determine whether the effects of BMPs on glial precursors are direct or indirect and to define when, during development, gliogenesis is BMP-dependent, ENCDC were isolated from fetal rat gut at E12, E14, and E16 and exposed to BMP-2 or 4 in vitro. Immunoselection with antibodies to p75NTR was used to obtain ENCDC and immunocytochemistry for BFABP, S100β, and GFAP was employed to identify cells developing in a glial lineage. BFABP appears before S100β and GFAP during ENS development (Young et al., 2003) and thus was used to detect cells committing early (E12) to a glial lineage (see Supplemental Fig. 1), while GFAP (Fig. 3B–D) and S100β (Fig. 3E) were used to detect more mature glia at later ages. Since effects of low and high concentrations of BMPs on neuronal differentiation differ in vitro (Chalazonitis et al., 2004), we examined the effects on gliogenesis of five days of continuous exposure of ENCDC to both low (0.5–1 ng/ml) and high (20 ng/ml) concentrations of BMP4. At both E12 and E14 the high, but not the low concentration of BMP4 significantly increased numbers of glia (Fig. 3A; E12: p < 0.05; n = 18; E14: p < 0.001; n = 18). The effects of BMP4 were significantly greater in cells isolated at E14 than in those isolated at E12 (p < 0.01). In contrast, at E16, only the low dose of BMP4 altered gliogenesis (p < 0.05; n = 36); moreover, the effect was significantly smaller than at E14 (p < 0.01). These observations are consistent with a direct effect of BMP signaling on ENCDC to promote glial development but the efficacy of BMP-promoted gliogenesis is developmentally regulated. It is to be noted that concomitant to the differentiating effect on glia, high BMP treatment also decreased total cells to 72.5±19% that of vehicle-treated cultures, an effect previously reported (Chalazonitis et al., 2004).
Fig. 3. BMPs promote gliogenesis in vitro.
ENCDC were immunoisolated from rat fetal bowel with antibodies to p75NTR at E12, E14, and E16. Cultures were treated for 5 days in the presence of vehicle, low (0.5–1 ng/ml) or high (20 ng/ml) concentrations of BMP-2 or -4. A. The numbers of cells in a glial lineage were quantified immunocytochemically. BFAPB was used as the glial marker at E12, while S100β and GFAP served as glial markers at E14 and E16. Because no differences were found in glial numbers at E14 or E16 in counts of preparations immunostained with antibodies to S100β and GFAP, data obtained with these two markers was pooled. Data at each age were normalized to the numbers of cells expressing glial markers in vehicle-treated cultures. * p < 0.05 vs. vehicle. The high concentration of BMPs was most effective at E14. BMP-stimulated gliogenesis declined between E14 and E16. B–D. Illustrations of glia (arrows), marked with GFAP immunoreactivity (green), developing from crest-derived cells immunoselected at E14. DNA was stained with bisbenzimide (blue). The conditions were vehicle (B), low BMP4 (C), and high BMP4 (D). Note that increasing concentrations of BMP promote aggregation of cells as well as gliogenesis. E. Illustration of glia developing from crest-derived cells immunoselected at E16. Glia are now marked with S100β-immunoreactivity (green) and have been cultured in the presence of low BMP2. The bar = 30 µm.
BMP receptor activation leads to the phosphorylation and nuclear translocation of receptor-activated Smad proteins (ten Dijke et al., 2002). The nuclear translocation of phosphorylated Smad1/5/8 (pSmad) was thus investigated immunocytochemically to determine whether differentiated enteric glia respond directly to BMP4 stimulation. ENCDC were immunoselected at E16 and cultured for 5 days to accumulate a sufficient number of glial cells to examine. A low concentration of BMP4 was first added to the medium (3 days) to enhance glial differentiation and to promote ErbB3 expression, which is necessary for development of enteric glia (Riethmacher et al., 1997). GGF2 was then applied to maximize glial numbers (last 2 days). Following this treatment, cultures were always starved for 6 hours in a medium that lacked GFF2, insulin, basic fibroblast growth factor, and epidermal growth factor in order to minimize possible interference with Smad translocation evoked by ongoing activity in the ERK pathway (Kretzschmar et al., 1997) prior to provoking them with a test concentration of BMP. Translocation was thus studied in the absence of GGF2. Cultures were exposed for 1 hr either to vehicle (Fig4B, C) or a high concentration of BMP4 (20 ng/ml) to ensure that BMP receptors would be saturated (Chalazonitis et al., 2004). (Fig 4D,E) or BMP-4 + noggin (100 ng/ml) (Fig4 F.G). BMP4 exposure significantly (almost 20-fold) increased the nuclear translocation of phosphorylated Smad1/5/8 in GFAP-immunoreactive cells (Fig. 4A). This increase was specific in that it was abolished by noggin (100 ng/ml). These data support the idea that enteric glia are BMP-responsive.
Fig. 4. BMP4 induces phosphorylation and nuclear translocation of Smad 1/5/8 in cultured enteric glia.
The ability of BMP4 to induced the phosphorylation and nuclear translocation of Smad proteins was studied in glia developing in vitro from ENCDC immunoisolated from E16 rat gut with antibodies to p75NTR. Cultures were starved for 6 hrs prior to the test exposure of 1 hr to vehicle, BMP4 (20 ng/ml) or BMP4 plus noggin (100 ng/ml). Smad phosphorylation and nuclear translocation were detected with antibodies that recognize the phosphorylated forms of the receptor activated Smads 1, 5, and 8 and glia were simultaneously identified with antibodies to GFAP. A. The proportions of GFAP-immunoreactive glia in which the nuclear translocation of phosphorylated Smad were detected were quantified in cultures treated with vehicle, BMP4, or BMP4 + noggin. Because of the magnitude of the effect of BMP4, the ordinate is displayed as a logarithmic scale. The translocation of pSmad 1/5/8 was significantly greater (p < 0.001) in cultures exposed to BMP4 than in those exposed only to vehicle. (n = 4 cultures each). Noggin blocked the BMP4-induced nuclear translocation of pSmads. These effects are illustrated for cultures exposed to vehicle (B, C), BMP4 (D, E), and BMP4 + noggin (F, G). The images at the left (B, D, F) show the filamentous green immunofluorescence of GFAP and blue fluorescence of DNA stained with bisbenzimide (nuclei). The images at the right show the same fields with merged images in which the red immunofluorescence of pSmad 1/5/8 is superimposed on that of GFAP. The starred arrows point to GFAP-immunoreactive cells in which translocation occurred; the filled arrows point to GFAP-immunoreactive cells in which translocation has not occurred; the non-filled arrows point to cells that lack GFAP immunoreactivity but in which translocation occurred. The arrow with a tail points to a dividing GFAP-immunoreactive glial cell in which nuclear translocation occurred. The bar = 50µm.
ErbB3 is expressed in the fetal bowel
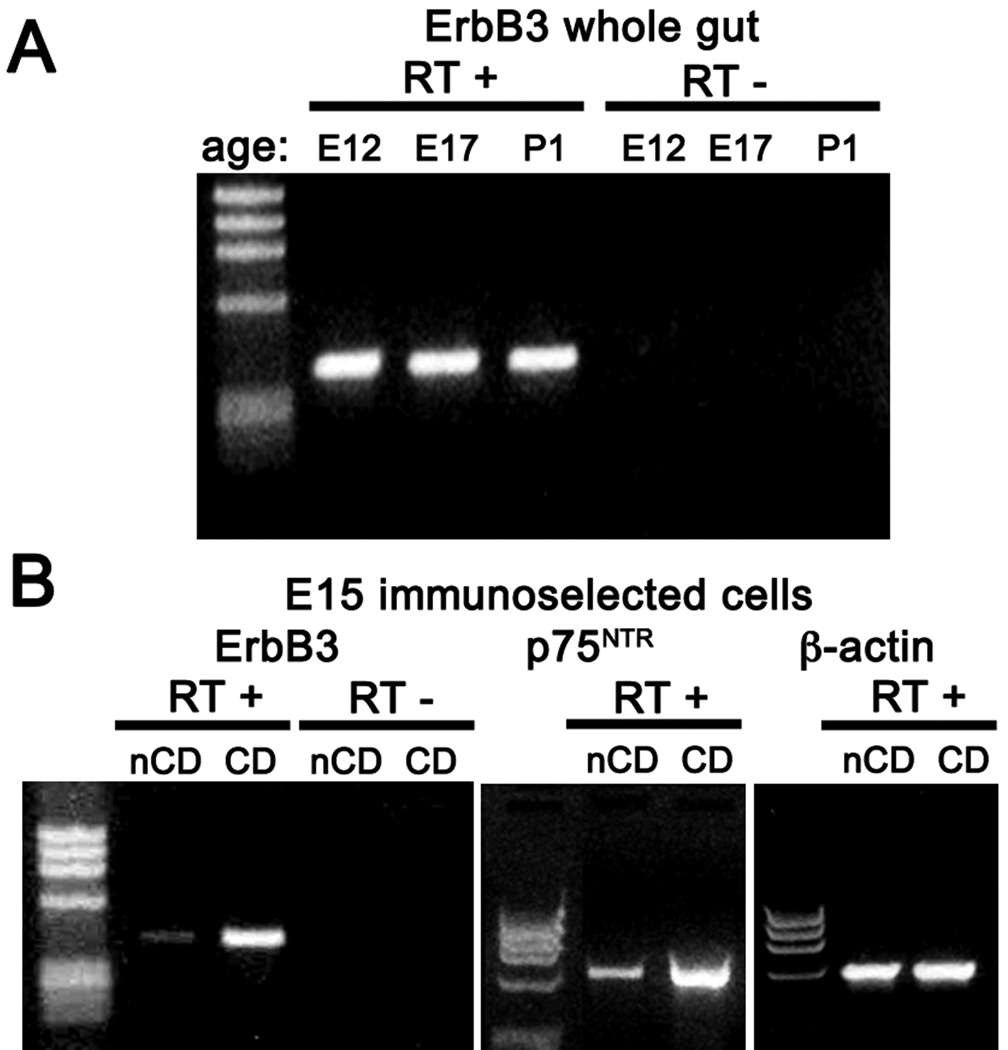
RT-PCR was used to determine whether transcripts encoding ErbB3 are present in developing fetal rat gut and isolated ENCDC. RNA was extracted from the bowel at E12, E17, and P1. At E12, neuronal development is well underway but glia cannot yet be recognized (Rothman and Gershon, 1982; Young et al., 2003); by E17, both early and late-born neurons have appeared and glial development has begun. By P1, the ENS has matured sufficiently well to enable the gut to function well enough to support oral feeding. Transcripts encoding ErbB3 were detected in the whole gut as early as E12 and continued to be expressed through P1 (Fig. 5A). Transcripts encoding ErbB3 were also detected in E15 ENCDC isolated by immunoselection with antibodies to p75NTR (Fig. 5B) and in the fraction of negatively selected cells from dissociated bowel that were not bound to beads coated with antibodies to p75NTR (Fig. 5B); however, because p75NTR transcripts were detected in this fraction, it must have contained some ENCDC (Fig. 5B, middle panel). The abundance of transcripts encoding ErbB3 in isolated fractions paralleled the abundance of transcripts encoding p75NTR. These data are thus consistent with the idea that ENCDC express ErbB3, although they do not exclude the possibility that ErbB3 might also be expressed by some bowel wall cells that are not of crest origin.
Fig. 5. Transcripts encoding ErbB3 can be detected in rat fetal bowel and immunoisolated ENCDC.
RNA was extracted from the whole bowel at E12, E17, and P1 and from ENCDC immunoselected from the gut at E15 with antibodies to p75NTR. RT-PCR was used to analyze expression of ErbB3. A. Transcripts encoding ErbB3 were detected in the whole gut at each age examined. Note that no products of amplification appeared in controls when RT was omitted (RT−). B. Transcripts encoding ErbB3 were found primarily in immunoisolated ENCDC (CD), although some can also be found in negatively immunoselected non-crest-derived cells (nCD). Despite the negative selection, the nCD preparation contained some residual transcripts encoding p75NTR and thus was slightly contaminated with ENCDC.
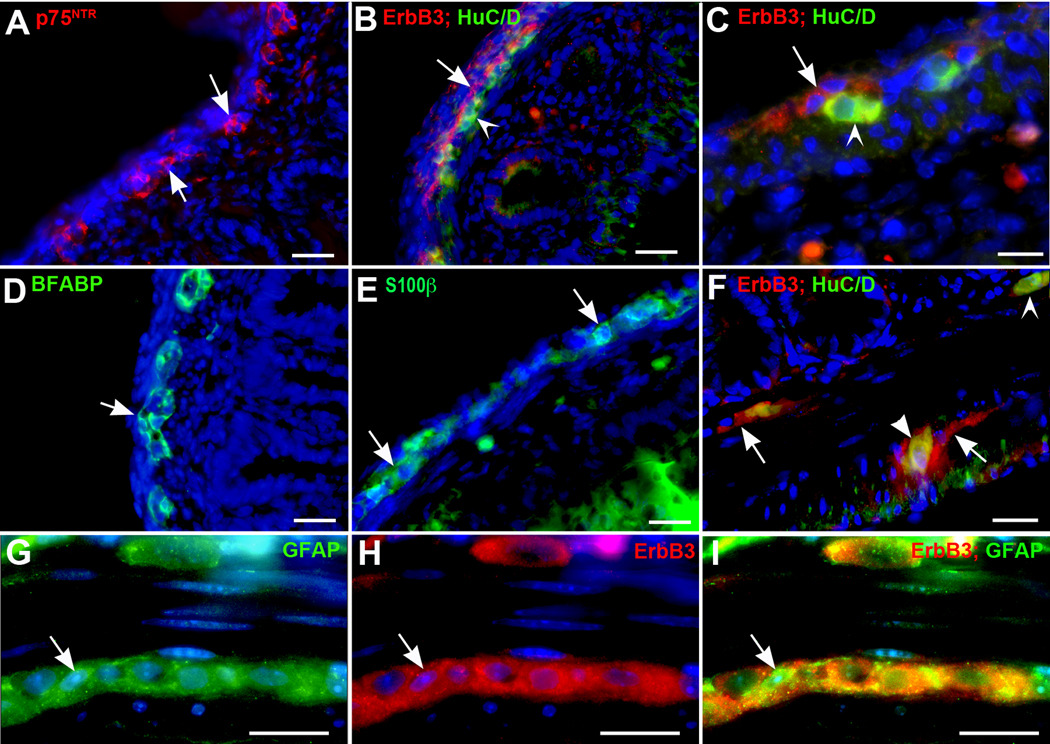
Immunocytochemistry was used to locate cells that express ErbB3 in the mouse fetal and adult gut wall. These experiments were carried out in conjunction with appropriate antibodies to locate ENCDC, neurons and glia. Double label immunocytochemistry with antibodies to ErbB3 and cell type-specific markers could not be carried out in fetal bowel because antibodies raised in different species were not available. The immunoreactivity of p75NTR outlined the region in the outer bowel mesenchyme in which development of primordial myenteric ganglia occurs (Fig; 6A). This is the same region that also contained ErbB3-immunoreactive cells, HuC/D-immunoreactive neurons (Fig. 6B, C), and both BFABP-(Fig. 6D) and S100β-immunoreactive glia (Fig. 6E). The ErbB3-immunoreactive cells were found to be adjacent to developing neurons, with which they partially overlapped, but the cells expressing ErbB3 immunoreactivity were concentrated in a band of the fetal bowel that was just serosal to most of the developing neurons (Fig. 6B, C). The location of ErbB3-immunoreactive cells in relation to those of neurons and glia was also investigated in the adult bowel to determine whether the association of ErbB3 with the primordial myenteric plexus was maintained as the ENS matured. Chicken antibodies to GFAP were found to react with adult enteric glia (Fig. 6G,I), although they did not do so when applied to section of fetal gut. These antibodies made it possible to determine directly whether ErbB3 is expressed in glia. In the adult bowel, ErbB3 immunoreactivity was exclusively located within the ganglia of the myenteric and submucosal plexuses (Fig. 6F). Coincident localization of ErbB3 with GFAP immunoreactivity implies that glia express ErbB3 (Fig 6 G,H,I). A subset of enteric neurons was also found to be ErbB3-immunoreactive (Fig. 6F, I), although most ErbB3 expression in enteric ganglia was in non-neuronal cells (Fig. 6F). The association of ErbB3-immunoreactive cells with the developing and mature ENS and their expression in mature enteric glia and a subset of neurons is consistent with the ideas that ErbB3-mediated signaling contributes to gliogenesis and may also be involved in the development and/or function of a subset of enteric neurons.
Fig. 6. ErbB3 immunoreactivity is restricted in the gut wall to developing and mature elements of the ENS.
ErbB3 was located immunocytochemically in sections of E17 and mature mouse intestine in relation to cells of neural crest origin (p75NTR-immunoreactive), neurons (HuC/D-immunoreactive), and glia (marked in fetal gut with antibodies to BFABP and S100β and in adult bowel with antibodies to GFAP). Nuclei were counterstained with bisbenzimide. A. E17. p75NTR immunoreactivity (red). ENCDC have begun to form clusters (arrows) that will develop into the ganglia of the myenteric plexus. B. E17. ErbB3 immunoreactivity (red) and HuC/D immunofluorescence (green) are demonstrated. The ErbB3-immunoreactive cells are found in a band (arrow) that is adjacent to the neurons (arrowhead) and concentrated on their serosal side. (Compare with panel A). C. At higher magnification, the ErbB3-immunoreactive cells (red; arrow) can be identified serosal to neurons (green; arrow head). Partial overlapping of cells occurs (yellow fluorescence). D. E17. BFABP (green) immunoreactivity is demonstrated to determine the location of cells developing in a glial lineage. These cells are found in the region of the developing ganglia (arrow; compare with panel A and B). E. E17. Glia are demonstrated with antibodies to S100β. Again, the cells are located in the region of developing myenteric ganglia (arrows). Some cells (green) can be seen internal to the circular muscle in the submucosa. F. Adult. Monoclonal antibodies to HuC/D (green immunofluorescence) were combined with rabbit antibodies to ErbB3 (red immunofluorescence) for double label immunocytochemistry. ErbB3-immunoreactive cells can be found in both the myenteric and submucosal plexuses (arrows) that lack HuC/D immunoreactivity. ErbB3 and HuC/D immunoreactivities are also coincident in some neurons (arrowhead, myenteric plexus), whereas others display HuC/D but lack ErbB3 immunoreactivity (submucosal plexus; concave arrowhead). G–I. Adult. Chicken antibodies to GFAP (G; green immunofluorescence) were combined with rabbit polyclonal antibodies to ErbB3 (H; red immunofluorescence) for double label immunocytochemistry (I; merge). Coincident immunoreactivity is found in GFAP-immunoreactive glia (arrow). Some neurons also show ErbB3 immunoreactivity. Bars A, B, D, and E = 50 µm; C, F–I = 25 µm).
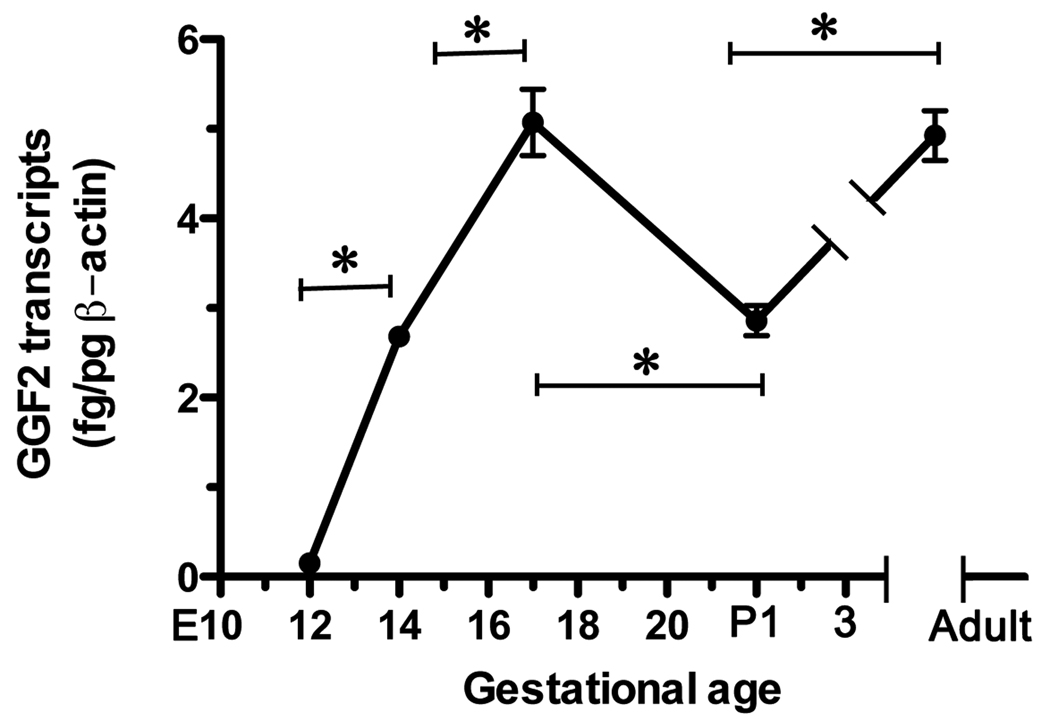
Enteric GGF2 expression follows that of ErbB3
Since, GGF2, is potentially a gliogenic ligand for ErbB3 in the developing rat gut, we used quantitative RT-PCR to define expression of GGF2 in the fetal bowel as a function of gestational age (Fig. 7). Transcripts encoding GGF2 were detectable in the fetal bowel at E12, the earliest time examined. The abundance of such transcripts at E12 was low but increased significantly between E12 and E14 and continued to increase significantly through E17, when GGF2 expression peaked. The abundance of transcripts encoding GGF2 decreased significantly perinatally, but increased again in the adult bowel to a level that was equivalent to that at E17. Because ErbB3 is already expressed in the E12 gut, cells with the potential to respond to GGF2 are present in the bowel during the period (E12–E17) when GGF2 expression is maximal.
Fig. 7. The enteric abundance of transcripts encoding GGF2 is developmentally regulated.
Real-time PCR was used to quantify transcripts encoding GGF2 in the fetal and postnatal rat bowel as a function of age. Transcripts were detected as early as E12 and increased significantly through E17. Following a perinatal decline in abundance, transcripts encoding GGF2 increased again reaching levels in the adult gut that were comparable to those achieved at E17. * p < 0.001.
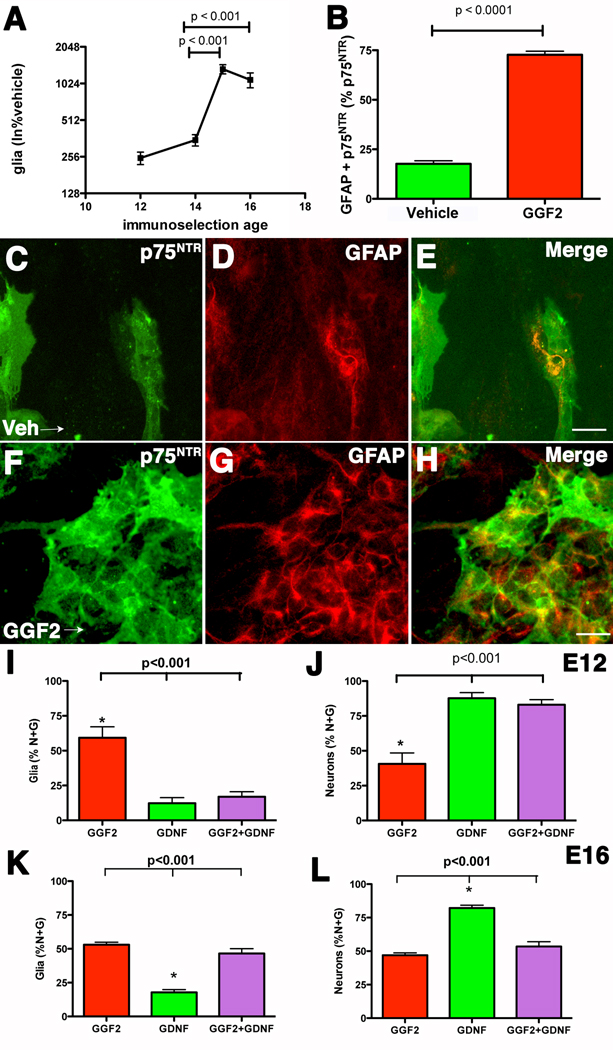
GGF2 stimulates enteric gliogenesis in vitro
GGF2 was added to isolated ENCDC to test the hypothesis that GGF2 promotes the development/survival of enteric glia. ENCDC were isolated from the fetal rat gut at E12, E14, E15, and E16 by immunoselection with antibodies to p75NTR. BFABP, S100β, and GFAP were used as glial markers. GGF2 (100ng/ml) increased the numbers of enteric glial cells differentiating in vitro in an age-dependent fashion. Although GGF2 significantly increased gliogenesis in ENCDC isolated at E12 (250 ± 29 % vehicle; p < 0.01) and E14 (351 ±37% vehicle; p < 0.001), the magnitude of the effect increased exponentially between E14 and E15. No further increase was seen between E15 and E16, but at both ages GGF2-promoted gliogenesis was significantly greater than at E12 or E14 (Fig 8A). At E16, moreover, GGF2 significantly increased the proportion of p75NTR-immunoreactive cells that coincidentally expressed GFAP (Fig. 8B–H). This latter observation suggests that GGF2 favors gliogenesis at the expense of neurogenesis because p75NTR immunoreactivity is maintained when ENCDC differentiate into glia but not when they develop as neurons (Young et al., 2003). By comparison at E16, GGF2 increased the total number of non-glial cells in ENCDC cultures to 158 ± 19% vehicle, the number of p75NTR-immunoreactive cells to 207 ± 36% vehicle, but the number of glia (GFAP-immunoreactive) to 4824 ± 675 % vehicle. The proportion of p75NTR-immunoreactive cells in the cultures that were identifiable as glia (GFAP-immunoreactive), moreover, increased in the presence of GGF2 to 436 ± 11% vehicle (Fig. 8B). The GGF2-induced increase in glia thus transcends whatever increase GGF2 might also provoke in other types of cell.
Fig. 8. GGF2 promotes gliogenesis in vitro and inhibits GDNF-driven neurogenesis.
A. GGF2 enhances gliogenesis when added to ENCDC in vitro. The efficacy of GGF2 increases as a function of age in ENCDC isolated from rat fetal gut at E12–E16. BFABP, detected immunocytochemically, was used as a glia marker at E12 while antibodies to S100β and GFAP were employed to identify glia at E14–16. B. ENCDC were isolated from the fetal bowel at E16 and cultured for 5 days in the presence of either vehicle or GGF2. GGF-2 significantly increases the proportion of ENCDC developing as glia. Antibodies to p75NTR were used to identify ENCDC and antibodies to GFAP were used to detect glia. C–H. Illustrations showing p75NTR and GFAP immunoreactivities in cultures of ENCDC exposed either to vehicle (C–E) or to GGF2 (F–H). The markers = 20 µm. C. Vehicle exposure, p75NTR immunoreactivity (green fluorescence). D. Vehicle exposure, GFAP immunoreactivity (red fluorescence). E. Vehicle exposure, merged image. F. GGF2 exposure, p75NTR immunoreactivity. G. GGF2 exposure, GFAP immunoreactivity. H. GGF2 exposure, merged image. I–J. The effects on gliogenesis and neurogenesis of GGF2 and GDNF, applied individually or in combination to ENCDC immunoselected at E12. Antibodies to BFABP were used to detect glia while neurons were detected with antibodies to HuC/D or PGP9.5. I. Glia, as a proportion of the total number of cells in each culture developing as neurons plus glia. J. Neurons, as a proportion of the total number of cells in each culture developing as neurons plus glia. K–L. The effects on gliogenesis and neurogenesis of GGF2 and GDNF, applied individually or in combination to ENCDC immunoselected at E16. K. Glia as a proportion of the total number of neurons plus glia. L. Neurons as a proportion of the total number of neurons plus glia.
The effects of GGF2 were then compared with those of GDNF, which has previously been shown to promote neurogenesis in cultures of ENCDC (Chalazonitis et al., 2004; Chalazonitis et al., 1998a). GDNF was applied to cultures by itself or in combination with GGF2. At E12, GGF2 weakly promoted gliogenesis and GDNF co-treatment inhibited GGF2-promoted gliogenesis (Fig. 8I). In contrast, GDNF promoted neurogenesis and GGF2 was unable to oppose the neuron-promoting action of GDNF (Fig. 8J). At E16, however, GGF2 strongly stimulated gliogenesis and GDNF co-treatment no longer blocked the GGF2-promoted gliogenesis (Fig. 8K). At E16 GDNF still promoted neurogenesis, but at this age GGF2 co-treatment inhibited the neuron-promoting effect of GDNF (Fig. 8L). These observations suggest that GGF2 and GDNF exert opposing effects on the divergence of enteric glia and neurons, with GGF2 favoring gliogenesis and GDNF favoring neurogenesis. These effects are age-dependent. At E16, the pro-glial effects of GGF2 predominate over GDNF-promoted neurogenesis whereas at E12 the pro-neuronal effects of GDNF predominate.
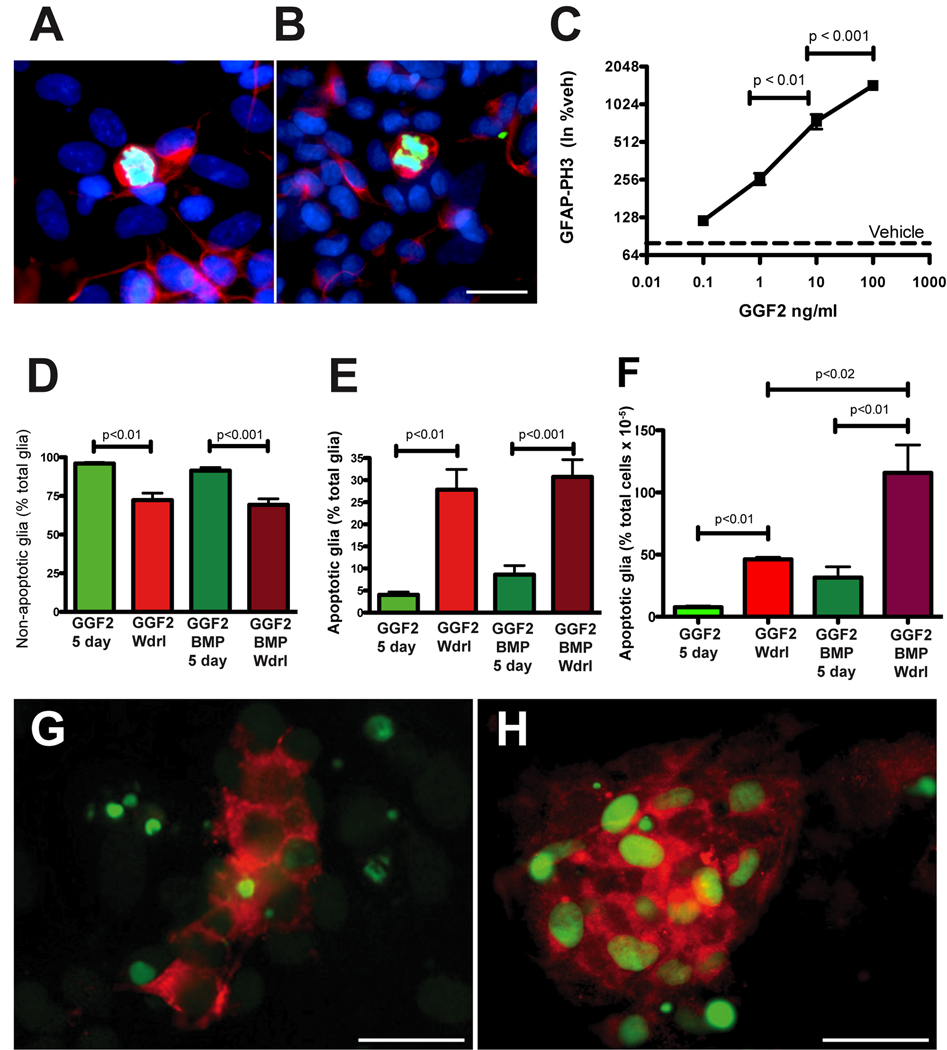
GGF-2 promotes glial proliferation and GGF2-dependency
To determine whether GGF2 stimulates the proliferation of committed glial cells in vitro, ENCDC were isolated from rat fetal gut at E15 when GGF2-promoted gliogenesis is maximal (see Fig 8A) and were examined immunocytochemically for the proliferation marker phosphohistone 3 (PH3) and for GFAP. Exposure to GGF2 for 5 days significantly increased the proportion of cells doubly labeled with antibodies to PH3 and GFAP (Fig. 9A–C) in a concentration-dependent manner (Fig. 9C). To determine whether GGF2 also promoted survival of enteric glia, ENCDC were isolated at E15 and allowed to develop for 5 days in the continuous presence of GGF2 (100 ng/ml) or vehicle. Far fewer cells acquired GFAP immunoreactivity in the vehicle-treated cultures, than in those exposed to GGF2; moreover, the proportion of glia undergoing apoptosis was significantly higher in vehicle- than in GGF2-treated cultures (17.6% ± 3.7% vs. 4.04% ± 0.6%; p < 0.02). To study the effects of GGF2 withdrawal, ENCDC were exposed for 3 days to GGF2 and then were deprived of it for the final 2 days of the 5-day culture period. Non-apoptotic glia (cytoplasmic red fluorescence, Fig. 9D) and those displaying apoptosis (Fig. 9E, F), which was assessed by the TUNEL method (nuclear green fluorescence), were then quantified. Withdrawal from GGF2 significantly decreased the number of non-apoptotic glia (Fig. 9D) and significantly increased the number of apoptotic glial cells (Fig. 9E,H). Because BMP signaling increases the dependency of some ENCDCs on growth factors (Chalazonitis et al., 2004), the effects of BMP4 on the GGF2-dependence of glial cells were examined. Addition of BMP4 for the first 3 days of culture enhanced the effects of GGF2 withdrawal; two days after withdrawing GGF2 the number of non-apoptotic glia declined significantly (Fig. 9D) and the number of apoptotic glia increased (Fig. 9E,H). In contrast cultures maintained with GGF2 for the last 2 days exhibited low numbers of apoptotic glia (Fig 9E,F,G). Although prior exposure to BMP4 did not alter the percentage of GFAP-immunoreactive cells undergoing apoptosis, upon GGF-2 withdrawal. (Fig. 9E), it significantly increased the overall number of apoptotic glia (TUNEL+/GFAP+) expressed as a proportion of total cells in each culture (Fig. 9 F–H). These observations suggest that GGF2 stimulates proliferation and enhances the survival of enteric glia developing in vitro; moreover, glia become GGF2-dependent and this dependence is enhanced by prior exposure to BMPs.
Fig. 9. GGF2 stimulates enteric glial proliferation and GGF2-dependency.
ENCDC were isolated from fetal rat gut at E15 and glial development was assayed in vitro using GFAP immunoreactivity (red fluorescence) as a glial marker. PH3 immunoreactivity (green fluorescence) was used to identify proliferating cells, and bisbenzimide (blue fluorescence) was used to counterstain DNA. A, B. Cells were exposed to GGF2 (100 ng/ml) for 5 days. GFAP-immunoreactive cytoplasm surrounds the PH3- immunoreactive chromosomes of dividing glia. In the merged image, the color of the chromosomes is aqua because they have been counterstained with bisbenzimide. The bar = 10 µm. C. The graph shows that the ability of GGF2 to increase the proliferation of GFAP-immunoreactive glia is concentration-dependent. D. Significantly more non-apoptotic glia are found in cultures exposed to GGF2 (100 ng/ml) continuously for 5 days than in cultures in which GGF2 was withdrawn for the final 2/5 days of incubation. The co-addition of BMP2 or 4 (20 ng/ml) for the first 3 days of culture did not alter the effect of GGF2 withdrawal on the proportion of non-apoptotic glial cells found in the cultures. E–H. The TUNEL method (green fluorescence in panels G and H) was used to assess apoptosis and coincidence with GFAP immunoreactivity (red fluorescence in panels G and H) to identify apoptotic glia. Significantly fewer apoptotic glial cells (E) are found in cultures exposed to GGF2 (100 ng/ml) continuously for 5 days than in cultures in which GGF2 was withdrawn for the final 2/5 days of incubation. F. The co-addition of BMP-2 or 4 (20 ng/ml) for the first 3 days of culture increased the effect of GGF2 withdrawal on the apoptosis of glial cells found in the cultures. This effect was masked (E) when apoptosis was expressed as a % of cells expressing GFAP, but is evident (F) when apoptotic glia were quantified as a proportion of total cells. G. Culture exposed to GGF2 (100 ng/ml) + BMP-2 for the first 3/5 days and then maintained with GGF2 for the last 2/5 days. H. Same as in (G) except that GGF2 is withdrawn for the final 2/5 days of incubation. The bar (G, H) = 50 µm.
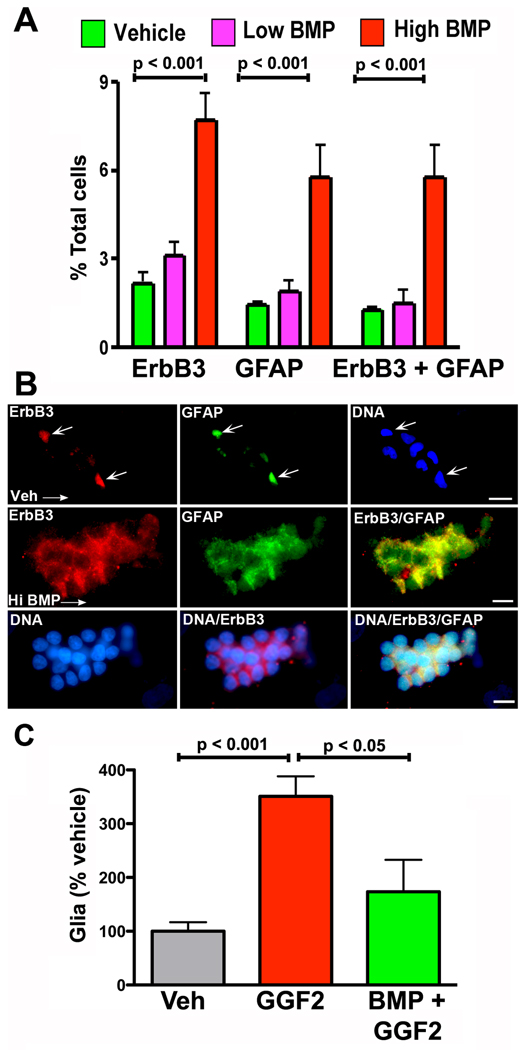
BMP signaling promotes ErbB3 expression
Experiments described above demonstrate that BMP and GGF2 signaling both stimulate enteric gliogenesis. Since BMP signaling can induce expression of growth factor receptors by ENCDCs (Chalazonitis et al., 2004), it is possible that increased expression of ErbB3 mediates the gliogenic response to BMPs. To test this idea, ENCDC were isolated from fetal rat gut at E14 by immunoselection with antibodies to p75NTR, cultured for 5 days with vehicle or in the continuous presence of BMP4 (0.5 ng/ml or 20 ng/ml) and ErbB3 was detected immunocytochemically. The high, but not the low concentration of BMP4 significantly increased the proportion of cells in the cultures that expressed ErbB3 immunoreactivity (Fig. 10A). BMP4 treatment also increased the proportion of cells expressing GFAP immunoreactivity, which co-localized with that of ErbB3 (Fig. 10B). BMP4 thus enhanced the percentage of cells undergoing glial differentiation. This effect was seen in experiments in which ENCDC were immunoisolated at E14 and cultured for 5 days in the presence of vehicle or GGF2 (100 ng/ml), with or without BMP4 (20 ng/ml). When BMP4 and GGF2 were combined, however, cells were exposed to BMP4 only for the first 3 days. GGF2 alone significantly increased the numbers of GFAP-expressing glial cells (p < 0.001 vs. vehicle; Fig 10C) but co-treatment with BMP4 prevented this increase (p < 0.05). The inhibitory effects of BMP4 did not reflect decreased glial survival; the number of glial cells undergoing apoptosis in cultures exposed to BMP4 plus GGF2 (8.6 ± 2%) was not significantly different from that in cultures exposed only to GGF2 (4.0 ± 0.6%) although the proportion of apoptotic glia in each was significantly less than that in cultures exposed to vehicle (17.6 ± 3.7%; p < 0.001). The inhibitory effects of BMP4 on GGF2-driven expansion of the glial population, therefore, can be explained most parsimoniously as a reflection of the ability of BMP4 to promote exit of ENCDC from the cell cycle. Although the BMP-driven exit of GGF2-stimulated glia from the cell cycle has not been demonstrated directly, such an action would be analogous to the previously demonstrated effects of BMPs on development of neurons from isolated ENCDC (Chalazonitis et al., 2004).
Fig. 10. Exposure to BMPs promote the in vitro differentiation of cells that express ErbB3.
ENCDC were isolated from fetal rat gut at E14. A. Exposure to a high (20 ng/ml), but not a low (0.5 ng/ml) concentration of BMP4 significantly enhanced expression of ErbB3. In the same cultures, the development of cells expressing the glial marker, GFAP, and cells expressing both ErbB3 and GFAP was also significantly increased. B. Cultures exposed to vehicle (top 3 panels) or a high concentration of BMP4 (lower 6 panels). In cultures exposed only to vehicle (Veh), few cells show coincidence of ErbB3 and GFAP immunoreactivities (arrows); nuclear fluorescence of DNA, counterstained with bisbenzimide, reveals the presence of neighboring cells in the culture that show little or no ErbB3 or GFAP immunoreactivities. The bar = 10 µm. In cultures treated with a high concentration of BMP4 (Hi BMP), however, many cells express the immunoreactivities of ErbB3 (red) and GFAP (green), which are for the most part coincident (yellow when ErbB3 and GFAP immunofluorescence is merged). In the lower set of panels DNA is counterstained with bisbenzimide (blue) and images are illustrated in which DNA fluorescence is merged with ErbB3 immunofluorescence, and both the immunofluorescence of ErbB3 and GFAP. These cultures also illustrate the tendency of ENCDC to aggregate following exposure to BMPs. The marker = 50 µm. C. GGF2 promotes the proliferation of ENCDC that are able to give rise to glia. This GGF2-driven proliferation is significantly inhibited by co-exposure with high concentration of BMP4.
Pre-exposure and simultaneous exposure to BMPs exert different effects on GGF2-driven gliogenesis
Because the simultaneous exposure of ENCDC to both GGF2 and BMP4 resulted in the development of significantly fewer glial cells than exposure to GGF2 alone, the effect of pre-exposure to BMP4 was investigated. BMP4 promotes expression of ErbB3; therefore, a pre-exposure to BMP might, by increasing ErbB3 expression in ENCDC, provide a larger number of potential targets primed to respond to subsequent GGF2 exposure. ENCDC were immunoisolated from fetal rat gut at E16 and exposed to BMP4 (1 ng/ml) or to vehicle for 3 days. The cultured cells were then washed, exposed for 2 additional days to GGF2 (100 ng/ml), and examined for GFAP and the transcription factor Sox10 immunoreactivity. Pretreatment with BMP4 significantly increased the proportion of cells expressing GFAP immunoreactivity. Measured as fold-enhancement over vehicle pretreatment, the BMP4-induced increment in GGF2-stimulated GFAP expression was 9.7 ± 0.9 (p < 0.0001). Pre-exposure to BMP4 also increased the proportion of cells doubly labeled with antibodies to Sox10 and GFAP (see supplemental Fig. 2). Because Sox10 is expressed in uncommitted ENCDC and retained in glia, the proportion of immunoisolated ENCDC in which Sox10 and GFAP immunoreactivities are coincident reflects the degree to which the ENCDC with a gliogenic potential have become committed to a glial lineage. This proportion was 50.0% ± 3.4% in cultures that were pre-exposed only to vehicle prior to incubation with GGF2 but 80.0% ± 5.2% in cultures that were pre-exposed to BMPs (p < 0.003). Pre-exposure to BMPs thus increases the gliogenic efficacy of subsequent exposure to GGF2.
Discussion
We tested the hypothesis that growth factors secreted by the enteric mesenchyme regulate the differentiation of uncommitted neural/glial precursors in the ENCDC population that colonizes the bowel. Specifically, we proposed that BMPs induce ENCDC to become committed and acquire responsiveness to the neurogenic effects of GDNF or to the gliogenic actions of GGF2. A consequence of this hypothesis is that BMPs would be essential not only for development of the neuronal component of the ENS, as previously established (Chalazonitis et al., 2004; Chalazonitis et al., 2008), but for the glial component as well. In fact, mice that overexpress noggin under the control of the NSE promoter, which interferes with BMP2- or BMP4-mediated responses in the primordial ENS (Chalazonitis et al., 2004; Chalazonitis et al., 2009), were found to have fewer glia and a depressed glia/neuron ratio, whereas, conversely, mice that overexpress BMP4 had more glia and an enhanced glia/neuron ratio. These observations confirm that BMP signaling is important in enteric gliogenesis. Interference with BMP signaling distorts the glia/neuron ratio in favor of neurons, suggesting that BMP signaling may be even more important in the development of enteric glia than in enteric neurogenesis. BMP signaling in neural crest stem cells had previously been reported to promote neurogenesis rather than gliogenesis (Shah and Anderson, 1997). ENCDC, however, have already migrated to the gut and thus are not identical to premigratory neural crest stem cells. Crest-derived cells that migrate to the gut are subjected to a variety of microenvironmental influences as they traverse their migratory pathway to and within the bowel. ENCDC thus express a number of genes, such as ret (Pachnis et al., 1993), that their premigratory ancestors in the crest do not; moreover, premigratory crest cells express genes, such as slug/snail (Sauka-Spengler and Bronner-Fraser, 2008), which ENCDC do not express. Secreted factors, such as the BMPs, which are important in the dorsalization of the neural tube to form the neural crest (Liem et al., 1995) and the segmentation of the enteric mesenchyme (Roberts, 2000), can thus be utilized for a variety of purposes at different times and locations in an embryo.
Treatment of cultured ENCDC with BMP2 or BMP4 demonstrated that BMP signaling directly promotes the development of enteric glia and does so in a concentration-dependent and developmentally regulated manner; ENCDC were maximally sensitive to BMP-promoted gliogenesis when immunoisolated from E14 rat gut. That cultured ENCDC respond directly to BMPs was also evident in the ability of BMPs to induce nuclear translocation of phosphorylated SMAD proteins (Chen et al., 2004). BMP signaling thus enhances formation of both neurons and glia during ENS development, suggesting that BMP signaling is by itself insufficient to account for the ultimate divergence of enteric neuronal and glial lineages. We therefore tested the idea that BMP enhances glial development by promoting expression of ErbB3 by ENCDC and thus the responsiveness of cells to GGF2.
Isolated ENCDC were found to express transcripts encoding ErbB3 and also to exhibit ErbB3 immunoreactivity. This observation is consistent with the idea that ErbB3 signaling is involved in gliogenesis. ErbB3-immunoreactive cells were confined to the region of the developing myenteric plexus. This region was identified immunocytochemically with antibodies to markers of ENCDC (p75NTR), neurons (HuC/D), and glia (BFABP, and S100β). Interestingly, the location of ErbB3-immunoreactive cells was adjacent to and partially overlapping with that of neurons; however, ErbB3-immunoreactive cells were strikingly concentrated on the serosal side of the neurons. The association of ErbB3 immunoreactivity within the ENS (both myenteric and submucosal plexuses) persisted in the adult gut, where it was possible to determine that ErbB3 immunoreactivity was coincident with that of GFAP and thus was present in enteric glia. ErbB3 immunoreactivity was also found in a subset of HuC/D-immunoreactive neurons. The serosa-facing orientation of ErbB3 immunoreactive cells, however, was lost in the ganglia of the mature intestine. It is possible that the serosa-facing orientation of ErbB3-immunoreactive cells in the fetal mouse gut reflects its expression in cells that have not yet become committed to neuronal or glia lineages. The E17 mouse gut contains many dividing ENCDC that are presumably uncommitted (Chalazonitis et al., 2008; Pham et al., 1991). The recent observation that neurogenesis in adult gut occurs in specialized sites that are located serosal to neurons in adult intestine (Liu et al., 2009) is consistent with the idea that ENCDC precursors persist serosal to mature neurons and glia. These observations thus are consistent with the idea that ErbB3-mediated signaling participates in gliogenesis, but they also suggest that ErbB3 may be involved in the development and/or function of a subset of enteric neurons. ErbB3 expression has also been observed in a subset of CNS neurons (Thompson et al., 2007) and ErbB3 is important in the development of dorsal root and sympathetic ganglia (Britsch et al., 1998; Honjo et al., 2008).
The fetal bowel, moreover, contained transcripts encoding the ErbB3 ligand, GGF2. GGF2 expression was detected in fetal rat gut as early as E12 but increased significantly at E14 and E16. GGF2 expression in the developing bowel thus lags behind that of GDNF and corresponds well in its timing to the abilities of BMPs and GGF2 to promote gliogenesis from isolated ENCDC, both of which were found to increase significantly between E12 and E14. GGF2 increased proliferation of glial precursors as well as their commitment to a glial lineage; therefore, addition of GGF2 to cultures of ENCDC immunoselected from fetal rat gut skewed the glia/neuron ratio toward glia. Conversely, GDNF treatment of ENCDC skewed the developing glia/neuron ratio toward neurons. At E12, cultures tended to be more responsive to GDNF, in that GDNF-induced neuronal development was not inhibited by co-addition of GGF2 and the co-addition of GDNF prevented GGF2-simulated glial development. In contrast, at E16, when GGF2 expression in vivo is maximal, the co-addition of GGF2 counteracted the enhancement of neurogenesis by GDNF and GDNF no longer inhibited GGF2-mediated gliogenesis. These data are consistent with prior observations that GDNF inhibits gliogenesis in ENCDC cultures (Chalazonitis et al., 1998a), an observation that was interpreted to mean that GDNF is unable to maintain glial progenitors. Alternatively, this might reflect the ability of GDNF to skew development of E14–16 ENCDC toward neurons.
Survival of committed cells often requires continued ligand stimulation of receptors that cells acquire as a result of their differentiation. By promoting commitment of ENCDC precursors, therefore, BMPs might induce cells to become dependent on particular growth factors for survival. BMPs have been demonstrated, for example, to promote the expression of TrkC by a subset of ENCDC; this subset of cells become responsive to NT-3 and dependent on it for survival (Chalazonitis et al., 2004). We therefore questioned whether BMPs, by promoting ErbB3 expression in developing ENCDC, render the cells not only GGF2-responsive, but also GGF2-dependent. In fact, glia developing in vitro from immunoisolated ENCDC underwent apoptosis upon withdrawal of GGF2 indicating that newly-generated glia are GGF2-dependent. When GGF2 was withdrawn following the co-exposure of immunoisolated ENCDC to BMPs, the proportion of total cells in the cultures represented by apoptotic glia increased significantly. BMPs thus increased the overall GGF2-dependency of glial cells developing from ENCDC. This increase, however, was not apparent when the numbers of apoptotic glia were expressed as a percent of total glia, probably because BMP signaling also decreased glial proliferation.
In summary, the current investigation supports the concept that BMPs promote the differentiation of ENCDC. Early in development, this effect enhances the ability of GDNF to drive neurogenesis and to suppress GGF2-promoted development of glia. Later in ontogeny, BMP-promoted differentiation leads to an increased ErbB3 expression and enhanced GGF2-driven enteric gliogenesis with downregulation of the neurogenic response to GDNF. It thus appears that BMPs are called upon to play multiple roles during development, exerting different effects on cells depending on the time and site of their action. In the bowel, BMPs are evidently important for the differentiation of both neurons and glia. Because transgenic overexpression of the BMP antagonist, noggin leads to an overabundance of neurons and a reduction of glia with a distortion of the neuron/glia ratio in favor of neurons, it is likely that the direct involvement of BMPs in promoting ErbB3 expression and consequent gliogenesis is required and cannot be compensated for by other gliogenic factors such as the neuropoietic cytokines (Chalazonitis et al., 1998b).
Research Highlights
BMPs regulate enteric gliogenesis in vivo and act directly in cultured ENCDC
Nrg1/GGF2 and its binding receptor ErbB3 are expressed in the fetal and adult ENS
BMPs increase expression of ErbB3 in enteric glia and their dependency on GGF2
GGF2 promotes glial precursors proliferation and counteracts GDNF neurogenic effect
BMPs and GGF2 are major determinants in the final enteric glia/neuron ratio
Supplementary Material
The culture media in the illustrated experiment contained GDNF, GGF2, and a high concentration of BMP2. Cells were cultured for 5 days. Preparations were subjected to double label immunocytochemistry to demonstrate cells developing in glial (BFAPB) and neuronal (HuC/D) lineages. A. Field illuminated to reveal BFABP-immunoreactive cells (green) in the absence of neurons. The glia have not yet extended processes and are small round cells. DNA was stained with bisbenzimide (blue). B. The same field that is shown in A, but with images merged to illustrate neurons, marked with antibodies to HuC/D (red) and the glia (green). Note that the primordial glial are distinct and have not yet become intimately associated with neurons. Bar = 10 µm
The ability of a prior exposure to BMP4 to enhance the subsequent gliogenic effects of GGF2 was assessed in cultures of ENCDC immunoisolated from fetal rat gut at E16. Mature glia was defined as cells that co-express the immunoreactivities of Sox10 (red fluorescence) and GFAP (green fluorescence). Sox10 is a transcription factor; therefore its immunoreactivity is nuclear, while GFAP is present in intermediate filaments and thus is cytoplasmic. A. ENCDC pre-exposed to vehicle for 3 days followed by exposure to GGF2 for a final 2 days of culture. A limited subset of cells with Sox10-immunoreactive nuclei contain GFAP-immunoreactive cytoplasmic filaments. B. ENCDC pre-exposed to BMP4 for 3 days followed by exposure to GGF2 for a final 2 days of culture. Almost all cells with Sox10-immunoreactive nuclei contain GFAP-immunoreactive cytoplasmic filaments. The pre-exposure to BMP4 has thus enhanced the gliogenic effects of the subsequent exposure to GGF2. Scale bar = 20 µm.
Acknowledgments
This study was supported by NIH grant numbers DK58056 (to A.C.), NS15547 (to M.D.G.) and NS 20778 and NS20013 (to J.A.K.). We thank Dr. Daniel Roman, Ms Valerie Boone and Wanda Setlik for valuable technical assistance, Dr. Udayan Guha for making available the noggin overexpressing animals, and Dr. William Gomes for the BMP-4 overexpressing mice. Thanks are due to Dr. Marc Marchionni, at Cambridge Neuroscience, MA, and Dr. Lena Hofer at Acorda Therapeutics Inc. Hawthorne, NY for supplying GGF-2; Dr. David Anderson, HHMI, California Institute of Technology for the gift of Sox10 antibodies and Dr. Thomas Müller, Max Delbruck Center for Molecular Medecine, Berlin, for the gift of the BFABP antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., III BMPR-1A signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–618. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–6400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development. 2006;133:2075–2086. doi: 10.1242/dev.02375. [DOI] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. The sacral crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, D'Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J. Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, D'Autréaux F, Pham TD, Kessler JA, Gershon MD. Divergence of enteric neuronal and glial lineages from uncommitted progenitors: roles of bone morphogenetic proteins, neuregulins, neuropoietic cytokines and glial cell derived neurotrophic factor. Neurogastroenterol Motil. 2009;21:223-iii. [Google Scholar]
- Chalazonitis A, d'Autréaux F, Roman D, Pham T, Guha U, Kessler JA, Gershon MD. Neuroscience. San Diego, CA: Society for Neuroscience; 2007. Neuregulin1/GGF2 and BMP2 and 4 regulate gliogenesis in the enteric nervous system (ENS) abstracts, Vol. Neuroscience abstracts online. www.sfn.org.Program #546.1. [Google Scholar]
- Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Rothman TP, DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L, Gershon MD. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21:5620–5636. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998a;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Vinson EN, MacLennan AJ, Gershon MD. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: interactions with neurotrophin-3. Dev Biol. 1998b;198:343–365. [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J. Biol. Chem. 1984;259:6882–6889. [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- D'Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development. 2007;134:2237–2249. doi: 10.1242/dev.003814. [DOI] [PubMed] [Google Scholar]
- Deal KK, Cantrell VA, Chandler RL, Saunders TL, Mortlock DP, Southard-Smith EM. Distant regulatory elements in a Sox10-beta GEO BAC transgene are required for expression of Sox10 in the enteric nervous system and other neural crest-derived tissues. Dev Dyn. 2006;235:1413–1432. doi: 10.1002/dvdy.20769. [DOI] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Furness J. The Enteric Nervous System. Malden, MA: Blackwell Publishing; 2006. [Google Scholar]
- Gershon MD, Ratcliffe EM. Development of the enteric nervous system. In: Johnson LR, Barrett KE, Ghishan FK, Mechant JL, Said HM, Wood JD, editors. Physiology of the gastrointestinal tract. Vol. 1. Burlington, MA: Elsevier Academic Press; 2006. pp. 499–521. [Google Scholar]
- Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The Serotonin Signaling System: From Basic Understanding To Drug Development for Functional GI Disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O'Guin WM, D'Vizio D, Pestell RG, Paus R, Kessler JA. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009;13:1193–1210. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev. Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Kniss J, Eisen JS. Neuregulin-mediated ErbB3 signaling is required for formation of zebrafish dorsal root ganglion neurons. Development. 2008;135:2615–2625. doi: 10.1242/dev.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin gene occur by different mechanisms. Dev. Biol. 1995;167:237–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Nägler K, Pfrieger FW, Göttman K. Purification of embryonic stem cell-derived neurons by immunoisolation. FASEB J. 2003;7(14):2100–2102. doi: 10.1096/fj.03-0118fje. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev Biol. 2000;227:146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- Karaosmanoglu T, Aygun B, Wade PR, Gershon MD. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat. Rec. 1996;244:470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signaling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnütgen F, Brüning G, Spener F, Müller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev Biol. 2002;246:245–258. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, Dormand E, Greenwood A, Anderson DJ. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuan JJ, Totty NF, Otsu M, McBurney RN, Waterfield MD, Stroobant P, Gwynne D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–168. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system. J. Comp. Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pomeranz HD, Gershon MD. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev. Biol. 1990;137:378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- Pomeranz HD, Rothman TP, Gershon MD. Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development. 1991;111:647–655. doi: 10.1242/dev.111.3.647. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol. 1998a;200:234–246. doi: 10.1006/dbio.1998.8963. [DOI] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA. Specification and migration of melanoblasts at the vagal level and in hyperpigmented Silkie chickens. Dev Dyn. 1998b;213:476–485. doi: 10.1002/(SICI)1097-0177(199812)213:4<476::AID-AJA12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaal T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Ritch PA, Carroll SL, Sontheimer H. Neuregulin-1 enhances motility and migration of Human Astrocytic Glioma Cells. J. Biol.Chem. 2003;278:20971–20978. doi: 10.1074/jbc.M213074200. [DOI] [PubMed] [Google Scholar]
- Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–120. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. J. Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Evolution of the neural crest viewed from a gene regulatory perspective. Genesis. 2008;46:673–682. doi: 10.1002/dvg.20436. [DOI] [PubMed] [Google Scholar]
- Shah NM, Anderson DJ. Integration of multiple instructive cues by neural crest stem cells reveals cell-intrinsic biases in relative growth factor responsiveness. Proc Natl Acad Sci U S A. 1997;94:11369–11374. doi: 10.1073/pnas.94.21.11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor rectricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Stanchina L, Baral V, Robert F, Pingault V, Lemort N, Pachnis V, Goossens M, Bondurand N. Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol. 2006;295:232–249. doi: 10.1016/j.ydbio.2006.03.031. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Thompson M, Lauderdale S, Webster MJ, Chong VZ, McClintock B, Saunders R, Weickert CS. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S, Nagaya M. Mutations in SIP1, encoding Smad interacting -protein 1, cause a form of Hirschprung disease. Nature Genet. 2001;27:369–370. doi: 10.1038/86860. [DOI] [PubMed] [Google Scholar]
- Wilkinson LD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. J. Comp. Neurol. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999;216:137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res. 2005;320:1–9. doi: 10.1007/s00441-004-1057-5. [DOI] [PubMed] [Google Scholar]
- Ziller C, Dupin E, Brazeau P, Paulin D, Le Douarin NM. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983;32:627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The culture media in the illustrated experiment contained GDNF, GGF2, and a high concentration of BMP2. Cells were cultured for 5 days. Preparations were subjected to double label immunocytochemistry to demonstrate cells developing in glial (BFAPB) and neuronal (HuC/D) lineages. A. Field illuminated to reveal BFABP-immunoreactive cells (green) in the absence of neurons. The glia have not yet extended processes and are small round cells. DNA was stained with bisbenzimide (blue). B. The same field that is shown in A, but with images merged to illustrate neurons, marked with antibodies to HuC/D (red) and the glia (green). Note that the primordial glial are distinct and have not yet become intimately associated with neurons. Bar = 10 µm
The ability of a prior exposure to BMP4 to enhance the subsequent gliogenic effects of GGF2 was assessed in cultures of ENCDC immunoisolated from fetal rat gut at E16. Mature glia was defined as cells that co-express the immunoreactivities of Sox10 (red fluorescence) and GFAP (green fluorescence). Sox10 is a transcription factor; therefore its immunoreactivity is nuclear, while GFAP is present in intermediate filaments and thus is cytoplasmic. A. ENCDC pre-exposed to vehicle for 3 days followed by exposure to GGF2 for a final 2 days of culture. A limited subset of cells with Sox10-immunoreactive nuclei contain GFAP-immunoreactive cytoplasmic filaments. B. ENCDC pre-exposed to BMP4 for 3 days followed by exposure to GGF2 for a final 2 days of culture. Almost all cells with Sox10-immunoreactive nuclei contain GFAP-immunoreactive cytoplasmic filaments. The pre-exposure to BMP4 has thus enhanced the gliogenic effects of the subsequent exposure to GGF2. Scale bar = 20 µm.