Abstract
Background
Liver allografts are accepted across major histocompatibility complex (MHC) barriers in mice and induce donor-specific tolerance without requirement for immunosuppressive therapy. There is evidence that passenger leukocytes may play a key role in tolerance induction. Flt-3 ligand (FL) is a recently cloned hematopoietic cytokine that strikingly augments functional dendritic cells (DCs) within lymphoid and nonlymphoid tissue.
Methods
The expression of costimulatory molecules and MHC class II antigen on DCs isolated from livers of FL-treated B10 (H2b) mice (10 μg/day; 10 days) was examined by flow cytometric analysis, and their allostimulatory activity assessed in primary mixed leukocyte cultures. B10 livers from FL-treated donors were transplanted orthotopically into naive C3H (H2k) recipients. Donor cells (MHC class II+) in recipient spleens were identified by immunohistochemistry. Antidonor cytotoxic T lymphocyte activity, and both natural killer and lymphokine-activated killer cell activities of graft nonparenchymal cells and host splenocytes were determined using isotope release assays. Apoptotic activity within liver grafts was determined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling.
Results
DCs isolated from livers of FL-treated donor mice exhibited increased cell surface expression of CD40, CD80, CD86, and IAb, and augmented T cell allostimulatory activity compared with controls. Within 24 hr of organ transplantation, the numbers of donor IAb+ cells within recipient spleens was augmented substantially compared with normal liver recipients. Livers from FL-treated donors were rejected acutely (median survival time, 5 days), whereas control B10 liver allografts survived >100 days. Nonparenchymal cells from rejecting grafts 4 days after transplantation exhibited increased antidonor cytotoxic T lymphocyte, natural killer, and lymphokine-activated killer cell activities compared with cells from spontaneously accepted grafts. This augmented cytotoxic reactivity was associated with histologic evidence of injury to bile duct epithelium and vascular endothelium that was not readily evident in controls.
Conclusion
Thus, although normal livers provide allostimulatory signals sufficient to elicit an antidonor immune response, regulatory mechanisms that may include apoptosis of graft-infiltrating T cells, and that are overcome by augmenting the number of functional donor DCs, may account for inherent liver tolerogenicity.
In most mouse and certain rat strain combinations, liver allografts are accepted across major histocompatibility complex (MHC*) barriers, and induce donor-specific tolerance, without the need for immunosuppressive therapy (1). Why this occurs frequently with the liver (and only exceptionally for mouse heart or kidney) is poorly understood. Mechanisms suggested to account for liver tolerogenicity (reviewed in 2 and 3) include low constitutive MHC antigen expression by hepatocytes, immunoregulatory effects of soluble hepatocyte products or donor MHC class I antigens, “insufficiency” of T helper-1 cell cytokines (4), “tolerogenic” donor-derived antigen-presenting cells (APCs) (5), and contrastingly, “superinduction” of T cell responses (resulting in proliferative clonal exhaustion and deletion; 6). There is evidence that “passenger” leukocytes may play a key role in determining the outcome of allogeneic liver transplantation. Compared with the heart or kidney, the liver is comparatively rich in interstitial leukocytes, including APCs, and in particular, dendritic cells (DCs) (7, 8). Persistence of multilineage donor-derived leukocytes, including DC progenitors, in recipient tissues is associated with liver transplant tolerance (1, 5, 9-12). Moreover, in spontaneous liver acceptance models, depletion of passenger leukocytes from the donor organ results in allograft rejection (13). We have suggested recently (14) that apoptosis of alloactivated T cells infiltrating liver portal areas (that are rich in DCs; 8) may be a mechanism underlying liver transplant tolerance.
DCs represent only a small proportion of the interstitial leukocytes within normal tissue (15). Unlike DCs resident within lymphoid tissue such as spleen, freshly isolated MHC class II+ DCs from nonlymphoid organs are poor allostimulators (16, 17). This is consistent with the apparent lack of invariant chain (18), and low or absent costimulatory molecule expression (19) that suggests these nonlymphoid tissue DCs may have tolerogenic properties. Indeed, DC progenitors propagated from normal liver and administered systemically can prolong allograft survival in mice (20). Other evidence points to potential tolerogenicity of DCs (21-24). These and other findings (2, 5) indicate that liver tolerogenicity may reflect quantitative and/or qualitative deficiencies in the capacity of the donor APC population, in particular DCs, to elicit an effective immune response.
Flt-3 ligand (FL) is a recently cloned hematopoietic growth factor (25), the cognate ligand of Flt-3, a receptor-type III tyrosine kinase (26) that is expressed on CD34+ stem cells. FL administration is highly effective in mobilizing stem and progenitor cells in vivo (27) and results in their accumulation in tissues. Unlike granulocyte-macrophage colony-stimulating factor, the essential myeloid DC growth factor in vitro, FL dramatically and selectively increases the number of functional DCs in mouse lymphoid and nonlymphoid tissues (28). Recently, we reported that increasing the number of liver passenger leukocytes (in particular, the proportion of potential allostimulatory DCs) by donor pretreatment with FL prevented the induction of liver transplant tolerance (29). We now show that augmentation of functional donor DCs in this passenger leukocyte population is associated with large increases in the number of donor-derived APCs within recipient lymphoid tissue, and with augmentation of antidonor cytotoxic T lymphocyte (CTL), natural killer (NK), and lymphokine-activated killer (LAK) cell activities of graft-infiltrating cells. Transplantation of livers from FL-treated donors was also associated with an altered pattern of in situ apoptotic activity. Thus, rather than inducing high dose unresponsiveness, a large increase in delivery/presentation of donor alloantigen can overcome tolerance-inducing mechanisms, and promote the expression of immune effector mechanisms consistent with bile duct and vascular cell injury leading to acute liver rejection.
MATERIALS AND METHODS
Animals
Male C57BL/10J (B10; H2b, IAb, IE−) and C3H/HeJ (C3H; H2k, lEk, IAk) mice, 8–12 weeks of age, were purchased from The Jackson Laboratory, Bar Harbor, ME.
FL treatment
Recombinant human FL derived from Chinese hamster ovary cells (28) (Immunex Research and Development Corp., Seattle, WA; 10 μg in 200 μ1 of sterile Hanks’ balanced salt solution) was given by daily intraperitoneal injection for 10 days. Control animals received Hanks’ balanced salt solution (200 μl), using an identical regimen. Organs were harvested for analysis 18–24 hr after the last treatment.
Liver nonparenchymal cell (NPC) isolation and DC culture
Livers from FL-treated or control mice, or liver allografts were pooled (three to five animals per experiment). NPCs were isolated by collagenase digestion, followed by Percoll centrifugation (30). The NPCs comprised predominantly CD45+ leukocytes (at least 95% in both control and FL-treated animals). Metrizamide-purified DC preparations were obtained by overnight (18 hr) culture of liver NPCs, followed by centrifugation of the nonadherent cells over metrizamide (14.5% w/v, Sigma Chemical, St. Louis, MO), as described (8).
Immunophenotypic analysis of cell suspensions
Immunofluorescence staining of cell-surface antigens for flow cytometric analysis, including double-labeling to identify the origin of the cells, was performed as described previously (30), using monoclonal antibodies purchased from PharMingen (San Diego, CA). CD11c and DEC-205 antigens were detected using hybridoma N418 (31) and NLDC-145 (32) monoclonal antibody supernatants, respectively, that were provided by Dr. R.M. Steinman (Rockefeller University, New York, NY). Appropriate rat, hamster, or mouse Ig isotype controls were included in each experiment. Flow cytometric analysis was performed using a EPICS Elite flow cytometer (Coulter Corp., Hialeah, FL). Five thousand events were acquired for each sample. Cytocentrifuge preparations were stained with Giemsa or by immunocytochemistry (30, 33).
Allostimulatory activity
One-way mixed leukocyte reaction cultures were established in 96-well round-bottom microculture plates. Graded doses of γ-irradiated (20 Gy; x-ray source) syngeneic or allogeneic (B10) stimulator cells were added to 2×105 nylon wool-eluted C3H spleen cells (8) used as responders. Cultures were maintained for 72 hr; [3H]thymidine (1 μCi/well) was added for the final 18 hr, and incorporation of [3H]thymidine into DNA was assessed by liquid scintillation counting. Results were expressed as mean counts per minute (cpm) ± lSD.
Organ transplantation
Orthotopic liver transplantation was performed from either FL-treated or control B10 donors to C3H recipients, as described (34). No immunosuppressive therapy was given. Rejection was confirmed histologically.
Immunohistology
Staining for donor MHC class II+ (IAb+) cells in cryostat sections of recipient spleens was performed as described previously in detail (30).
CTL assays
Freshly isolated liver NPCs or spleen cells from either allograft recipients or control C3H mice were used as effectors at various effector-to-target cell ratios. The EL4 (H2b) lymphoma cell line (TIB39; American Type Culture Collection [ATCC], Rockville, MD) was used as a source of donor-specific target cells (ATCC) in 4-hr 51Cr release assays, as described (14). The percent specific cytotoxicity was calculated using the following formula: % cytotoxicity = 100 × [experimental (cpm) – spontaneous (cpm)/maximum (cpm) – spontaneous (cpm)]. Results are expressed as means ±1SD of percent specific 51Cr release in triplicate cultures.
Quantitation of NK and LAK cell activities
Lysis of YAC-1 tumor cells detected by 4-hr 51Cr release assay was used as an indicator of NK cell activity, whereas LAK cell activity and cytotoxicity towards third-party targets was determined by lysis of NK-resistant 51Cr-labeled P-815 (H2d) tumor cells (ATCC) (35). The tumor cell lines were grown in RPMI-1640 complete medium (Life Technologies, Gaithersburg, MD) and were subcultured two to three times per week.
In situ detection of apoptotic cells in liver sections. Diced samples of freshly isolated liver were placed in embedding medium (Tissue-Tek O.C.T. Compound; Miles Inc., Elkhart, IN). The tissue was snap-frozen in liquid nitrogen, and stored at −70°C until sectioned. Cryosections (4 μm) were mounted on precleaned slides, air-dried overnight at room temperature (RT), then fixed for 10 min at RT in 10% neutral buffered formaldehyde (pH 7.4), followed by two washes (5 min each) in phosphate-buffered saline (PBS). DNA strand breaks were identified by in situ terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL), as described (36), with some modifications. Sections were immersed in 2% H2O2 in PBS for 5 min at RT to quench endogenous peroxidase activity, then incubated with 20 μg/ml proteinase K (Sigma) for 15 min at RT, washed in PBS, and immersed in reaction buffer supplemented with TdT and biotinylated dUTP. Sections were incubated in a humid atmosphere at 37°C for 60 min. Each experiment was performed with a negative (without biotinylated dUTP) and a positive control (10 min pretreatment of sections with 1 mg/ml DNase dissolved in reaction buffer). Irradiated (5 Gy) thymocytes incubated for 18 hr and then cytocentrifuged onto glass slides (>90% apoptotic cells) also served as positive controls. After washing in stop/wash buffer (30 min at 37°C), antidigoxigenin-peroxidase (Oncor, Gaithersburg, MD) was added (30 min at RT). The sections were washed in PBS, and the reaction developed with 3-amino-5-ethycarbizol. Slides were counterstained with Harris’ hematoxylin and mounted with Crystal mount (Biomeda Corp., Foster City, CA).
Statistical analyses
Statistical analyses were performed using Student’s t test or the Mann-Whitney U test, as appropriate.
RESULTS
Markedly increased numbers of allostimulatory DCs can be isolated from liver NPCs of FL-treated mice
As reported previously (29), there was a large increase in the absolute number of liver leukocytes (NPCs) in FL-treated animals, including a substantial 30–50-fold increase, compared to controls, in cells bearing the myeloid markers, Gr-1 and MAC-1 (CD11b). There was also a large increase both in the relative and absolute numbers of cells that were positive for the DC-restricted antigens, CD11c or DEC 205 (data not shown). To investigate the liver DCs, highly purified preparations were obtained after overnight (18 hr) culture of NPCs, and centrifugation of nonadherent cells over metrizamide. Compared to control animals, from which only approximately 3×104 DCs could be harvested per liver, about 6×106 DCs could be recovered from livers of 10-day FL-treated mice. The cells displayed morphologic and surface phenotypic features characteristic of DCs (Fig. 1), and were DEC 205+, F4/80−, CD40hi, CD80+, CD86hi, and MHC class II (IAb)hi. The extent of lymphocyte contamination was very low. Significantly, an additional 3×106 DCs/liver could be harvested after a second day of culture of cells from FL-treated mice in the absence of granulocyte-macrophage colony-stimulating factor. Additional flow cytometric and immunocytochemical analyses indicated that overnight-cultured DCs from NPCs of both control and FL-treated mouse livers expressed similar levels of MHC class II (IAb) (Fig. 2), but that higher levels of CD40, CD80 and CD86 were expressed on DCs from FL-treated mice (Fig. 3). This suggested that the DC population present in FL-treated livers was in a later stage of phenotypic maturation than that in controls. Consistent with their higher levels of expression of costimulatory molecules, DCs from FL-treated livers exhibited significantly increased allostimulatory activity for naive T cells compared to DCs from controls (Fig. 4).
Figure 1.
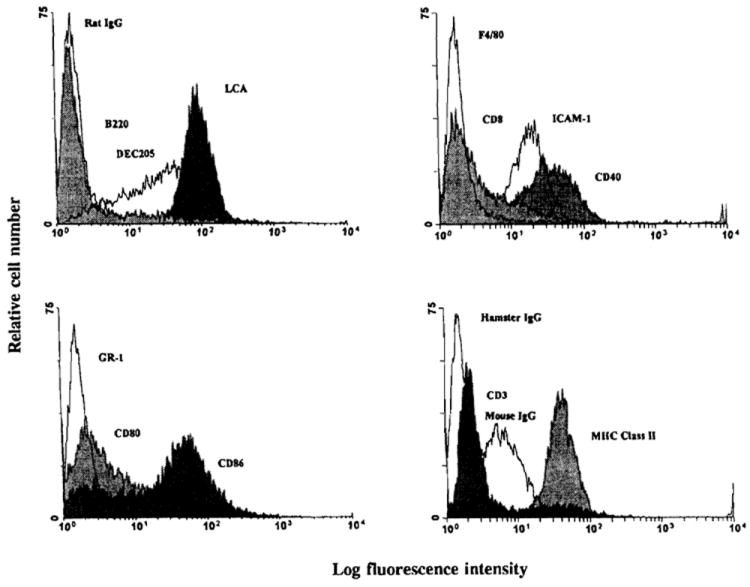
Flow cytometric analysis of overnight (18 hr)-cultured, metrizamide-purified nonadherent cells isolated from the NPC population of FL-treated and control B10 mouse livers. The results are representative of two separate experiments.
Figure 2.
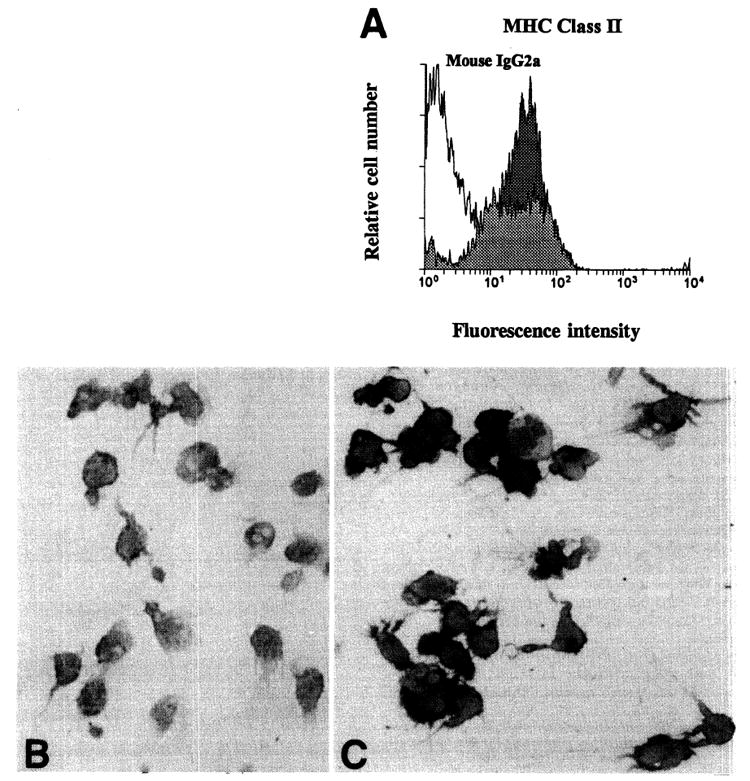
(A), Expression of MHC class II (IAb) on DCs isolated from livers of control (pale shading) and FL-treated mouse donors (dark shading); (B), DEC 205 (DC marker) and (C), MHC class II (IAb) expression on cytospin preparations of metrizamide-purified DCs isolated from the NPC population of FL-treated mouse livers. (Avidin-biotin-peroxidase stain; original magnification, ×400.)
Figure 3.

Expression of costimulatory molecules on overnight (18 hr) cultured, metrizamide-purified nonadherent cells isolated from the NPC population of normal (pale shading) or FL-treated mouse livers (dark shading). The results are representative of two separate experiments.
Figure 4.
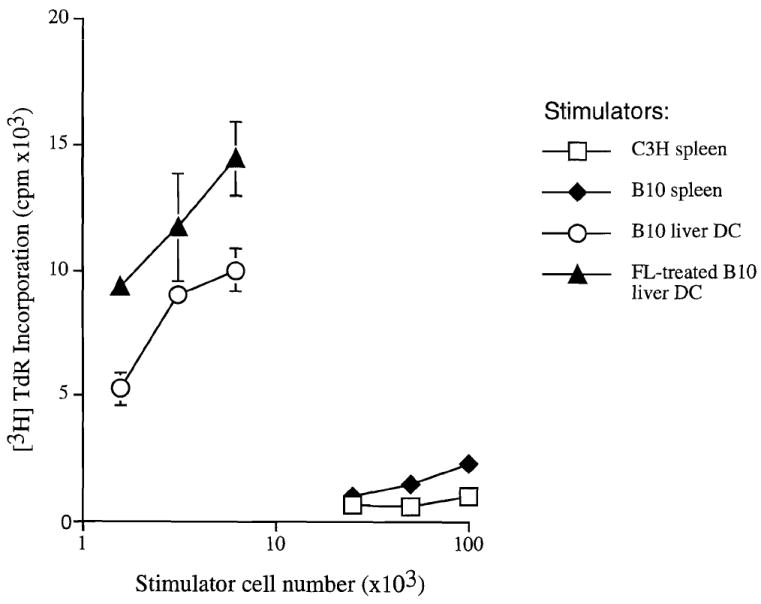
Allostimulatory activity for naive C3H splenic T cells of DCs isolated from the livers of control and FL-treated B10 mice. The mixed leukocyte reaction cultures were maintained for 72 hr. The stimulatory activity of freshly isolated bulk allogeneic (B10) and syngeneic spleen cells is also shown. The results are means±SD of triplicate cultures.
FL treatment of organ donors results in acute liver allograft rejection
In the B10 to C3H strain combination, orthotopic liver allografts are accepted spontaneously in nonimmunosuppressed recipients. Treatment of liver donors with FL for 10 days, however, resulted in the acute rejection of livers in all allograft recipients (median survival time, 5 days) (Table 1). Liver allograft rejection was confirmed histologically.
Table 1.
Donor pretreatment with FL prevents “spontaneous” liver transplant toleranceaa
| Donor treatment | n | Survival (days) | Median survival time |
|---|---|---|---|
| Control | 5 | >100 (×5) | >100 |
| FLc (10 μg/day) × 10 days | 5 | 4, 4, 5, 5, 6 | 5 |
Strain combination B10 (H2b) → C3H (H2k).
Increased donor MHC class II+ cells are observed in spleens of recipients of livers from FL-treated donors
Immunohistochemical staining of spleens from recipients of hepatic allografts 24 hr after transplant revealed substantial increases in donor MHC class II+ (IAb+) cells in those mice given livers from FL-treated donors compared with controls (data not shown).
FL pretreatment of liver donors augments CTL, NK, and LAK cell activities of graft-infiltrating cells
Similar numbers of graft-infiltrating cells with comparable immunophenotypic characteristics were isolated from the transplanted livers of control and FL-treated donors. By day 4 after transplant, >80% of these cells expressed recipient MHC class I phenotype (H2Kk); of these, approximately 25% were CD4+ and 30% CD8+. It has been shown previously that freshly isolated graft-infiltrating cells of normal liver allografts exhibit ex vivo antidonor CTL activity for a short period after transplantation (14). This activity gradually subsides to normal levels as the grafts are accepted permanently. To test the antidonor responses of graft-infiltrating cells within livers from FL-treated donors, CTL, NK, and LAK cell activities were evaluated 4 days after transplantation. As shown in Figure 5, there were increases in all three activities when compared with those exhibited by cells isolated from control liver allografts. Corresponding activities of host spleen cells were also evaluated. There was increased antidonor CTL activity of spleen cells from mice given livers from FL-treated donors compared with the modest activity of control liver recipients’ splenocytes. However, no alteration in the low NK and LAK cell activities of corresponding host spleen cells was observed.
Figure 5.
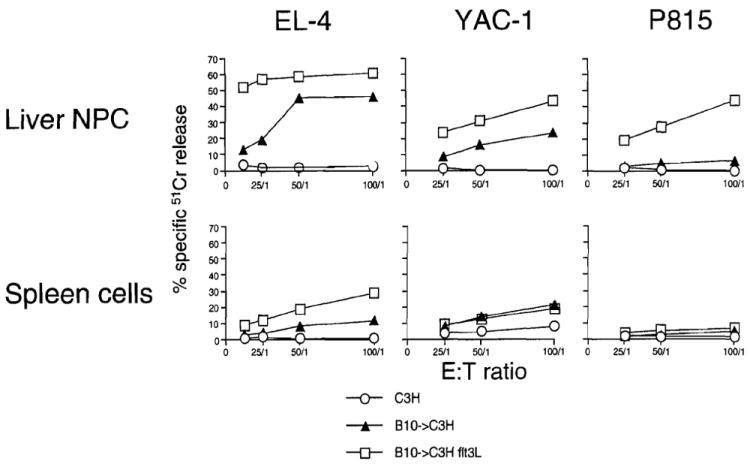
CTL, NK, and LAK cell activities (specific 51Cr release) of FL-treated donor liver graft-infiltrating cells and host spleen cells against EL-4, YAC-1, and P815 target cells, respectively, 4 days after transplant. The results are representative of two separate experiments.
Increased bile duct and hepatocellular injury in liver grafts from FL-treated donors
Liver grafts harvested from experimental and control animals were examined histologically 4 days after transplantation. As reported previously (1), there was little evidence of damage to bile duct epithelium, hepatocytes or vascular endothelial cells in control liver grafts, despite a prominent (mainly periportal/perivascular) inflammatory cell infiltrate. In contrast, liver grafts from FL-treated donors, examined at the same time after transplant, exhibited an extensive, generalized mononuclear cell infiltrate, with clear evidence of bile duct damage, and vascular endothelial cell injury (Fig. 6, A–D). These findings were consistent with acute liver rejection (median survival time, 5 days) in recipients of grafts from FL-treated donors.
Figure 6.
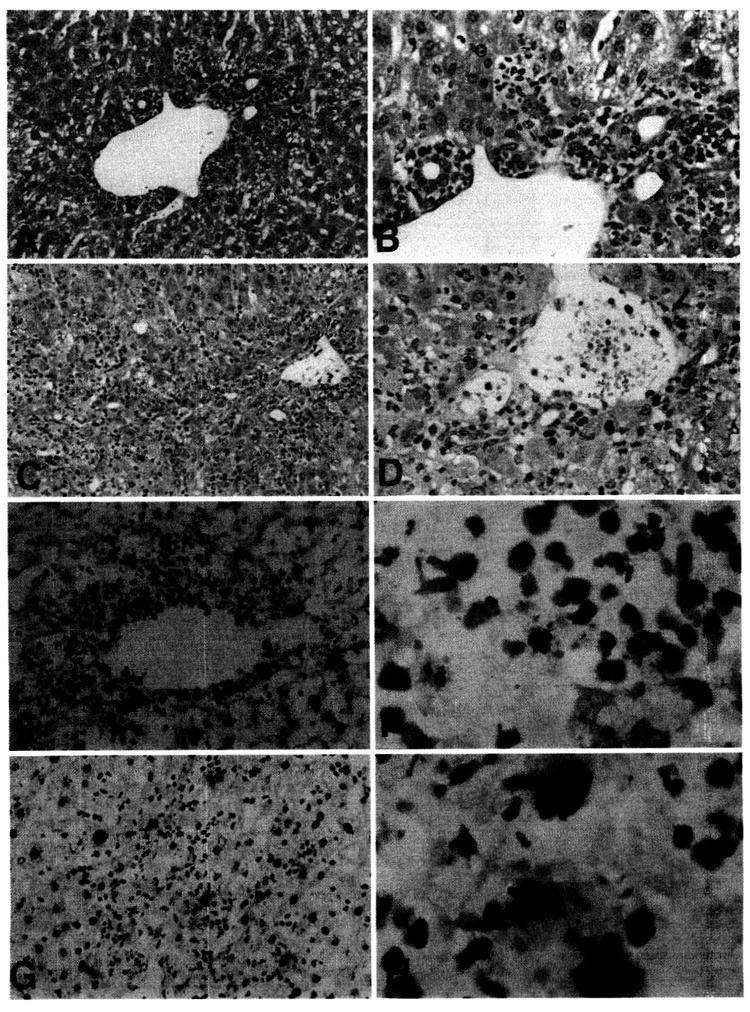
(A–D) Histological appearance of FL-treated B10 donor liver allografts 4 days after transplantation into normal C3H recipients. (A and B) normal liver allograft, showing predominantly periportal inflammation (A), with absence of damage to bile duct epithelium (B). (C and D) FL-treated donor liver allograft, showing diffuse periportal and lobular inflammatory cell infiltration, with focal bile duct injury (arrowheads) and evidence of endothelial cell separation from a portal vein (arrow). (Hematoxylin and eosin stain; A and C, original magnification, ×100; B and D, original magnification, ×200.) (E–H) TUNEL staining for apoptotic cells in liver allografts, 4 days after transplantation. (E and F) normal liver allograft, showing apoptotic cells within the periportal inflammatory cell infiltrate (compare with A). (G and H) FL-treated donor liver allograft, showing larger, TUNEL-positive cells (presumptive apoptotic hepatocytes) throughout the lobular area. (Counterstained with hematoxylin; E and G, original magnification, ×100; F and H, original magnification, ×400.)
Apoptotic activity of liver parenchymal cells is enhanced and that of inflammatory cells decreased in liver grafts from FL-treated donors
It has been shown recently that spontaneous liver allograft acceptance in mice is associated with a relatively high incidence of apoptotic cells within the periportal inflammatory cell infiltrate, that may be linked to deletion of donor-reactive CTL (14). In view of these observations, we compared apoptotic activity in allografts from FL-treated donors with those from control liver grafts, using the TUNEL assay for in situ DNA fragmentation. As shown in Figure 6 (E–H), there was a striking increase in the number of large apoptotic cells (presumed hepatocytes) throughout the parenchyma of livers from FL-treated donor compared with control liver grafts. The differential increase in apoptotic death of parenchymal cells was associated with a reduced incidence of TUNEL-positive inflammatory cells. This was consistent with the elevated levels of antidonor CTL activity of freshly isolated graft-infiltrating cells, and with increased numbers of inflammatory cells throughout the liver lobules compared with controls.
DISCUSSION
The present study describes a model of mouse liver allograft rejection that is dependent on donor pretreatment with the potent hematopoietic growth factor FL. Rejection is associated with: (i) dramatic increases in the numbers of potential allostimulatory DCs that can be recovered from the donor organ using conventional DC isolation procedures; (ii) substantial increases in donor MHC class II+ cells within recipient lymphoid tissue 24 hr after transplant; (iii) potentiation of antidonor CTL, NK, and LAK cell activities within the graft; and (iv) reduced apoptotic activity of graft-infiltrating cells. In virtually all H2-incompatible normal mouse strain combinations, liver transplant tolerance is achieved without the need for host immunosuppression (1). In contrast, skin allografts in the same combinations are rejected acutely. The mechanisms underlying “spontaneous” liver acceptance are poorly understood (2, 3). Liver acceptance occurs despite early intragraft mononuclear leukocyte infiltration (1), that appears identical histologically to that observed in organ rejection, and that may reflect the “active” nature of liver-induced tolerance. Indeed, it has been suggested that, because liver tolerance in rats can be inhibited by steroid administration for the first 2 days after transplant, immune activation is key to the induction of donor-specific unresponsiveness (6). With the acceptance of liver allografts, the recipients become permanently tolerant to donor-type challenge grafts, such as those of heart and even skin, indicating the robust nature of the tolerance (1, 38, 39). The process of tolerance induction can be prevented, however, by presensitization of the liver recipient to donor alloantigens, usually by skin grafting 2 weeks or more before organ transplantation (40). With recipient presensitization, the liver is rejected acutely (within 4–5 days). Mouse liver allograft rejection also results from recipient treatment with recombinant mouse interleukin (1L)-2 (14) or IL-12 (41).
In recipients of normal liver allografts, graft NPCs isolated during the first week after transplant exhibit strong, donor-specific CTL responses. This “paradoxical” CTL activity, detected using donor MHC-positive cells as targets, correlates well with the extent of the histologic infiltrate that peaks between days 4 and 7, then diminishes rapidly and progressively to normal levels. Despite these findings, there is little evidence of either bile duct or hepatocyte injury in the normal mouse liver allograft recipient. Taken together, these observations suggest that, although donor-specific immunity is induced initially, it wanes rapidly as a result of the elimination of donor-specific CTL within the graft. Apoptosis of graft-infiltrating cells (14) by mechanisms yet to be elucidated may play a key role in the process. It is conceivable that donor liver-derived NPCs or DCs (that may express Fas ligand [CD95L]) (42) could play a key role in the apoptosis of recipient activated T cells. The capacity of exogenous IL-2 to induce normal liver allograft rejection (41) may reflect protection of graft-infiltrating T cells from apoptosis (14). By contrast, the results of the present study show that dramatic increases in donor leukocytes, in particular functional DCs, leads to augmented early antidonor CTL activity of liver graft-infiltrating cells, hepatocellular destruction, and allograft rejection. The observed increase in apoptosis of parenchymal cells is most likely due, predominantly, if not exclusively, to the cytotoxic activity of host immune effector cells. The stimulus provided to unmodified hosts by substantially increased numbers of immunostimulatory donor DCs and probably other APCs is sufficient to overcome other mechanisms that generally contribute to the success of liver allografts.
As reported previously (29), treatment with FL for 10 days resulted in a dramatic accumulation of myelomonocytic cells within the liver, both in portal areas (where DCs are normally found; 8), and throughout the parenchyma. Augmentation of the number of myeloid lineage cells far exceeded that of lymphocytes, although these too were increased significantly in absolute numbers. The largest increase (> 100-fold) was observed in the absolute number of cells bearing the DC-restricted cell surface antigenic markers DEC-205 or CD11c. The most striking evidence of the large increase in DCs within FL-treated mouse livers was, however, the huge increase in the absolute number of CD11c+ cells that could be recovered after overnight culture of liver NPCs. Evidence that these cultured cells had rapidly undergone functional maturation was provided by the high levels of surface MHC class II, CD40, CD80, and CD86, and their potent allostimulatory capacity. These findings are consistent with recent reports that FL may preferentially mobilize or enhance the development of myeloid (28, 29) or myelomonocytic lineage cells (43), and also enhance DC accumulation in mouse tissues (28). This in turn, may be associated with increased levels of secreted IL-12 and/or other cytokines/growth factors that promote graft immunogenicity, and possibly, DC proliferation. DC-derived IL-12 could augment CD4+ Th1 cell responses that have been linked previously to murine liver allograft rejection (4, 41). Although not examined in the present study, it is conceivable that a component of the multilineage NPC accumulation within the liver is derived from local lineage-committed progenitor cell proliferation and maturation (30). This could also be achieved through proliferation of stem cells in the liver (44, 45) induced or enhanced by FL administration.
As shown previously, both in mouse and rat models, systemic effects of normal liver transplantation include the capacity of liver leukocytes (including progenitor and stem cells) (1, 5, 46, 47), to migrate extensively to recipient lymphoid and nonlymphoid tissues, where they may persist indefinitely. These donor-derived cells may provide not only a continuing source of allostimulation, but may also have the capacity to modulate the survival and function of alloactivated T cells within the recipient lymphoid system (10, 48, 49). Key molecules that may be expressed by donor-derived DCs, including costimulatory molecules (22, 42), inducible nitric oxide synthase (37), and Fas ligand (42) could play important regulatory roles in these cell interactions. Recently, emphasis has been placed on multilineage donor hematolymphopoietic cell chimerism as the basis for organ allograft acceptance (9, 48, 49). Although tolerance and chimerism are spontaneous in the mouse liver transplant model (1,2), they require an umbrella of immunosuppression in most models (including human organ transplantation) to prevent one of the interacting donor and recipient immune populations from overwhelming the other (50). With an uncontrolled imbalance, the result is rejection or graft-versus-host disease (51). It has been emphasized as a therapeutic principle, that attempts to augment donor chimerism clinically with either adjunct donor leukocytes (i.e., bone marrow [BM] cells) or by administration of hematolymphopoietic growth factors, is predictably unsafe, unless it is done under immune suppression that affects both cell populations equally (49). The present study reinforces this warning by demonstrating that augmentation with FL treatment of potentially potent immunostimulatory cells in a liver allograft before its transplantation can lead to an enhanced rejection reaction and graft loss in an otherwise spontaneously tolerant host. In contrast, FL treatment of the mouse allogeneic BM cell recipient, rather than the donor under coverage of immunosuppression (tacrolimus) from the time of BM infusion, results in a striking enhancement of chimerism (52).
Studies such as those reported herein provide compelling in vitro immunologic evidence that the timing and circumstances of FL administration may determine the balance between transplant tolerance and immunity. Precisely how this balance is accomplished by the DC and other lineages, or influenced by growth factors or immunosuppression, remains speculative. Further studies of FL and other hematopoietic growth factors in the mouse liver transplantation model may help elucidate the role of donor-recipient hematopoietic cell interactions in determining graft acceptance. Our findings also support the prediction that under the appropriate circumstances, FL-mediated expansion of functional DCs in the liver may provide effective strategies to increase immunologic resistance to liver cancer and infectious diseases, such as viral hepatitis. It has been obvious since the studies of Knight et al. (53) that “blind” attempts to engineer such immunity could lead inadvertently to tolerance, and vice versa.
Acknowledgments
The authors thank the Immunex Research and Development Corp., Seattle, WA, for providing Flt-3 ligand, Alison Logar for skillful flow cytometric analyses, and Shelly L. Conklin for excellent secretarial assistance.
Footnotes
Presented at the 16th Annual Meeting of the American Society of Transplant Physicians, May 10–14, 1997, Chicago, IL.
This work was supported by NIH grants DK 49745, AI41011, and DK 29961.
Abbreviations: APC, antigen-presenting cell; BM, bone marrow; CTL, cytotoxic T lymphocyte; DC, dendritic cell; FL, Flt-3 ligand; IL, interleukin; LAK, lymphokine-activated killer; MHC, major histocompatibility complex; NPC, nonparenchymal cell; NK, natural killer; PBS, phosphate-buffered saline; RT, room temperature; TdT, terminal deoxynucleotidyltransferase; TUNEL, TdT-mediated dUTP-biotin nick end labeling.
References
- 1.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian S, Thai NL, Lu L, Fung JJ, Thomson AW. Liver transplant tolerance: mechanistic insights from animal models, with particular reference to the mouse. Transplant Rev. 1997;11:151. [Google Scholar]
- 3.Wood K, Farges O. Tolerance. In: Neuberger J, Adams D, editors. Immunology of liver transplantation. Boston: Little Brown & Co; 1993. p. 139. [Google Scholar]
- 4.Thai NL, Fu F, Qian S, et al. 1995. Cytokine mRNA profiles in murine orthotopic liver transplantation: graft rejection is associated with augmented Thl function. Transplantation. 1995;59:274. [PubMed] [Google Scholar]
- 5.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995;182:379. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop GA, Sun J, DeCruz DJ, et al. Tolerance to rat liver allografts, III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156:4925. [PubMed] [Google Scholar]
- 7.Hart DNJ, Fabre JW. Demonstration and characterisation of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;153:347. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo J, Lu L, Rao AS, et al. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Thomson AW, Murase N, Demetris AJ. Liver transplants contribute to their own success. Nat Med. 1996;2:163. doi: 10.1038/nm0296-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu Y, Arima T, Flye MW. Rejection of spontaneously accepted rat liver allografts with recipient interleukin-2 treatment or donor irradiation. Transplantation. 1997;63:177. doi: 10.1097/00007890-199701270-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, McCaughan GW, Gallagher N, Sheil AGR, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60:233. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Qian S, Lu L, Fu F, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654. [PMC free article] [PubMed] [Google Scholar]
- 15.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 16.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1995;161:526. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austyn JM, Hankins DF, Larsen CP, Morris PJ, Rao AS, Roake JA. Isolation and characterization of dendritic cells from mouse heart and kidney. J Immunol. 1994;152:2401. [PubMed] [Google Scholar]
- 18.Saleem M, Sawyer GJ, Schofield RA, Seymour ND, Gustafsson K, Fabre JW. Discordant expression of major histocompatibility complex class II antigens and invariant chain in interstitial dendritic cells. Transplantation. 1997;63:1134. doi: 10.1097/00007890-199704270-00013. [DOI] [PubMed] [Google Scholar]
- 19.Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7–1 and B7–2) expression on murine dendritic cells. J Immunol. 1994;152:5208. [PubMed] [Google Scholar]
- 20.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. GM-CSF stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366. [PMC free article] [PubMed] [Google Scholar]
- 21.Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in non-immunosuppressed recipients. Transplantation. 1996;62:659. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelman FD, Lees A, Bimbauni R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406. [PubMed] [Google Scholar]
- 24.Grohmann U, Bianchi R, Ayroldi E, et al. A tumor-associated and self-antigen peptide presented by dendritic cells may induce T cell anergy in vivo, but IL-12 can prevent or revert the anergic state. J Immunol. 1997;158:3593. [PubMed] [Google Scholar]
- 25.Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 26.Matthews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65:1143. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 27.Brasel K, McKenna HJ, Morrisey PJ, et al. Hematological effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004. [PubMed] [Google Scholar]
- 28.Maraskovsky E, Brasel K, Teepe M, et al. In vivo administration of flt3 ligand results in generation of large numbers of dendritic cells in the lymphoid tissue of mice. J Exp Med. 1996;184:1953. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steptoe RJ, Fu F, Li W, et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol. 1997;159:5483. [PubMed] [Google Scholar]
- 30.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metlay JP, Witmer PM, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraal G, Breel M, Janse M, Bruin G. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognised by a monoclonal antibody. J Exp Med. 1986;163:981. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L, Hsieh M, Oriss TB, et al. Generation of DC from mouse spleen cultures in response to GM-CSF: immunophenotypic and functional analyses. Immunology. 1995;84:127. [PMC free article] [PubMed] [Google Scholar]
- 34.Qian S, Fung JJ, Demetris AJ, Ildstad ST, Starzl TE. Orthotopic liver transplantation in mice. Transplantation. 1991;52:526. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vecchini F, Delfino D, Patrene D, et al. Generation of natural killer cells from long-term cultures of mouse bone marrow. Nat Immun. 1993;12:1. [PubMed] [Google Scholar]
- 36.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu L, Bonham CA, Chambers FG, et al. Induction of nitric oxide synthase in mouse dendritic cells by interferon γ, endotoxin and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577. [PubMed] [Google Scholar]
- 38.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 39.Kamada N, Davies H, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 40.Qian S, Fu F, Li Y, et al. Presensitization by skin grafting from MHC class I or MHC class II deficient mice identifies class I antigens as inducers of allosensitization. Immunology. 1995;85:82. [PMC free article] [PubMed] [Google Scholar]
- 41.Thai NL, Li Y, Fu F, et al. Interleukin-2 and interleukin-12 mediate distinct effector mechanisms of liver allograft rejection. Liver Transplant Surg. 1997;3:118. doi: 10.1002/lt.500030204. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, Qian S, Hershberger P, Rudert WA, Lynch DA, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676. [PubMed] [Google Scholar]
- 43.McKenna HJ, Williams DE, Morrissey PJ, Lyman SD. The hematopoietic effects of recombinant human (rh) flt3 ligand administered to non-human primates. Blood. 1996;86:424a. [Google Scholar]
- 44.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 46.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 47.Murase N, Starzl TE, Ye Q, et al. Multilineage hematopoietic reconstitution of supralethally irradiated rats by syngeneic whole organ transplantation: with particular reference to the liver. Transplantation. 1996;61:1. doi: 10.1097/00007890-199601150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. Response to Wood and Sachs. Immunol Today. 1996;17:588. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown Norway rats. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyengar AR, Bonham CA, Antonysamy MA, et al. Striking augmentation of hematopoietic cell chimerism in noncytoablated allogeneic bone marrow recipients by Flt3 ligand and tacrolimus. Transplantation. 1997;63:1193. doi: 10.1097/00007890-199705150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knight SC, Hung R, Dorse C, Medawar PB. Influence of dendritic cells on tumor growth. Proc Natl Acad Sci USA. 1985;82:4495. doi: 10.1073/pnas.82.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]


