Abstract
Objective
Previous studies have uncovered a PTEN-dependent interaction between the sphingolipid agonist, dhS1P, and the TGF-β/Smad3 signaling pathway. Here we sought to examine responses of SSc fibroblasts to S1P and dhS1P and to gain further insight into the regulation of the S1P/dhS1P/PTEN pathway in SSc fibrosis.
Methods
Fibroblast cultures were established from skin biopsies of patients with SSc and matched healthy controls. Western blotting and qPCR were used to measure protein and mRNA levels, respectively. PTEN protein was examined in skin biopsies by immunohistochemistry.
Results
PTEN protein levels are low in SSc fibroblasts and correlated with elevated levels of collagen and phospho-Smad3 and reduced levels of MMP1. DhS1P treatment restored PTEN levels and normalized collagen and MMP1 expression, as well as Smad3 phosphorylation status in SSc fibroblasts. S1P was strongly pro-fibrotic in SSc and control fibroblasts. Distribution of S1P receptor isoforms was altered in SSc fibroblasts with reduced levels of S1P1 and S1P2 receptors and elevated level of S1P3 receptors. Depletion of a single, S1P1 receptor abrogated effects of dhS1P and S1P in control dermal fibroblasts. In contrast, depletion of either S1P1 or S1P2 receptor prevented the effects of S1P and dhS1P in SSc fibroblasts.
Conclusion
Our findings demonstrate that PTEN deficiency is a critical determinant of the pro-fibrotic phenotype of SSc fibroblasts. The anti-fibrotic effect of dhS1P is mediated through normalization of PTEN expression levels, suggesting that dhS1P or its derivatives may be effective as therapeutic anti-fibrotic agents. The distribution and function of S1P receptors differ in SSc and healthy fibroblasts, suggesting that alteration in sphingolipid signaling pathway may contribute to SSc fibrosis.
INTRODUCTION
SSc is a connective tissue and autoimmune disease of unknown etiology characterized by severe and often progressive cutaneous and visceral fibrosis, pronounced alterations in the microvasculature, and numerous cellular and humoral immune abnormalities (1). Excessive scarring due to overproduction of extracellular matrix (ECM) proteins is a hallmark of SSc (1). The molecular basis of fibrosis is still incompletely understood, however, there is a general consensus that TGF-β plays a central role in the development of SSc and other fibrotic diseases (2). TGF-β is a potent inducer of extracellular matrix (ECM) and is required under physiologic conditions, such as wound repair, to induce fibroblasts to produce and contract ECM. The canonical TGF-β signaling is a simple cascade that is initiated by ligand binding to the TGF-β receptor type II, which phosphorylates receptor type I, resulting in binding and phosphorylation of signal transducers R-Smads, which then interact with common Smad4, translocate to the nucleus and regulate target gene expression (3). Furthermore, more recent studies uncovered additional modes of TGF-β signaling that involve non-canonical, non-Smad pathways, including MAP kinase, Rho-like GTPase signaling, and the PI3 Kinase/Akt pathway (4). Activation of canonical and non-canonical pathways have been reported in SSc fibroblasts, including elevated levels of phosphorylated Smad3, as well as Smad1 and constitutive activation of downstream effectors of PI3K signaling such as Akt and c-Abl (2).
Sphingosine kinase (SK) is a lipid kinase that catalyzes formation of two bioactive lipid mediators, sphingosine-1 phosphate (S1P) and dihydrosphingosine-1 phosphate (dhS1P). SK and its metabolite, S1P, have emerged as important regulators of a wide range of biological processes, including cell growth and proliferation, cell survival and apoptosis, calcium homeostasis, angiogenesis and vascular remodeling (5). Recent evidence suggests that S1P may also play an important role in fibrosis through cross-talk with the TGF-β pathway. It was initially reported that in keratinocytes and mesangial cells, S1P mimics the effects of TGF-β through cross-activation of Smad signaling (6, 7). In mesangial cells these effects of S1P were mediated through the S1P3 receptor and were dependent on the presence of TGFβRII (7). Mimetic of S1P, FTY720 (Fingolimod), was shown to have similar pro-fibrotic effects, including Smad phosphorylation, and CCN2 and collagen upregulation (8). Furthermore, similar to TGF-β, both S1P and FTY720 induced fibroblast to myofibroblast differentiation via activation of the S1P3 receptor (9). Other studies have demonstrated that SK was upregulated during bleomycin-induced lung fibrosis and contributed to the TGF-β-induced myofibroblast differentiation of lung fibroblasts (10).
Studies from our laboratory showed that in contrast to the pro-fibrotic function of S1P, dhS1P elicits potent anti-fibrotic effects through several mechanisms. dhS1P inhibits TGF-β/Smad signaling and collagen synthesis in dermal fibroblasts through a mechanism that involves the tumor suppressor, PTEN (11). We have uncovered a novel function of PTEN as a co-factor of the Smad3 phosphatase, PPM1A. Upon translocating into the nucleus, PTEN forms complexes with PPM1A and protects it from degradation in response to TGF-β signaling, thus resulting in Smad2/3 dephosphorylation (11). We have also shown that S1P and dhS1P have opposing roles in the regulation of the MMP1/TIMP1 pathway in dermal fibroblasts (12, 13). TGF-β enhanced SK1 activity and S1P production, and induced prolonged up-regulation of SK1 expression levels. In addition, we demonstrated that SK1 was required for the TGF-β-induced upregulation of the tissue inhibitor of metalloprotease-1, TIMP1 (13). Conversely, dhS1P upregulated MMP1 (matrix metalloproteinase-1) via activation of ERK/Ets1 signaling and was required for the TNF-α induced production of MMP1 (12).
Existing evidence suggests that the sphingosine kinase metabolites, S1P and dhS1P, have distinct and often opposite effects on the TGF-β signaling pathway. Given the importance of TGF-β signaling in SSc fibrosis, the goal of this study was to evaluate the influence of dhS1P and S1P on the fibrotic features of SSc fibroblasts. Our study shows that significantly reduced protein levels of PTEN are found in SSc fibroblasts. Treatment with dhS1P normalized the pro-fibrotic characteristics of SSc fibroblasts through the upregulation of PTEN protein, while having only small effects in healthy dermal fibroblasts. S1P induced pro-fibrotic changes in healthy, as well as SSc fibroblasts. We have also observed alterations in the distribution and utilization of the S1P receptor isoforms in healthy and SSc fibroblasts suggesting that deregulation of sphingolipid signaling may contribute to SSc fibrosis.
MATERIALS AND METHODS
Cell Culture
Human dermal fibroblast cultures were established from skin biopsy specimens obtained from the dorsal forearm of patients with diffuse cutaneous systemic sclerosis (dcSSc) and from age, race and gender matched healthy controls, upon informed consent and in compliance with the Institutional Review Board. All patients fulfilled the American College of Rheumatology criteria for the diagnosis of dcSSc (14). Dermal fibroblasts were cultured from the biopsy specimens as described previously (15). Normal and SSc skin fibroblasts were cultured in DMEM supplemented with 10% FBS and 1% antibiotic antimycotic solution. For experiments, cells were incubated with serum-free media for 24h before specific treatments.
Immunohistochemistry
The study group consisted of 7 patients with dcSSc and 7 healthy volunteers (Table 1). Skin biopsies were embedded in paraffin and used for immunohistochemistry. Immunohistochemical staining of paraffin-embedded sections was performed using a Vecstain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Four-micrometer thick sections were mounted on silane-coated slides, then deparaffinized with histoclear and rehydrated through a graded series of solutions of ethyl alcohol and PBS. Skin sections were treated with hydrogen peroxide for 30 min. to block endogenous peroxidase activity and then subjected to a 45 min. antigen-retrieval treatment with antigen unmasking solution (Vector Laboratories). Incubation with PTEN antibody (Cell Signaling Laboratories, Beverly, MA), was performed overnight in a humidified chamber at 4°C as previously described (16). After three rinses in PBS, binding sites of the primary antibodies were detected with biotinylated IgG, and the sites of peroxidase activity were visualized by using diaminobenzidine. The sections were then counterstained with hematoxylin. Immunostaining was detected by light microscopy. Normal rabbit IgG was used as a negative control (not shown).
Table 1.
Comparison of PTEN levels in dermal fibroblasts between normal and scleroderma skin
| Age | Sex | Race | Disease duration |
TSS | The levels of PTEN in dermal fibroblasts |
|
|---|---|---|---|---|---|---|
| NS 352 | 37 | F | AA | +++ | ||
| SSc 351 | 38 | F | AA | 2y | 3 | + |
|
| ||||||
| NS 356 | 60 | F | C | ++ | ||
| S5c 355 | 63 | F | C | 4M | 37 | +/− |
|
| ||||||
| NS 360 | 40 | F | AA | + | ||
| SSc 359 | 42 | F | AA | 3y | 15 | +/− |
|
| ||||||
| NS 362 | 44 | F | AA | +/− | ||
| SSc 361 | 49 | F | AA | 4y | 24 | +/− |
|
| ||||||
| NS 364 | 54 | M | C | ++ | ||
| SSc 363 | 50 | M | C | 1.5y | 3 | + |
|
| ||||||
| NS 366 | 48 | M | C | +++ | ||
| SSc 365 | 42 | M | C | 2y | ND | − |
|
| ||||||
| NS 368 | 45 | F | C | + | ||
| SSc 367 | 50 | F | C | 13y | 34 | − |
(−) - none or little staining in < 10% of cells
(−/+) - faint, partial staining in > 20% of cells
(+) - moderate, complete staining in > 20% of cells
(++) – moderate to strong staining in > 50% of cells
(+++) – strong staining in > 50%
Reagents
The following antibodies were used: anti-phospho Smad3, Smad2/3, and PTEN (Cell Signaling, Beverly, MA), S1P1 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), MMP1 (Chemicon, Temecula, CA), collagen (Southern Biotech Birmingham, AL), PPM1A (Abcam Inc, Cambridge, MA 02139), and β-actin (clone AC-150) (Sigma, St. Louis, MO). Recombinant human TGF-β1 was obtained from Ramp;D Systems (Minneapolis, MN), S1P and dhS1P were from Avanti (Alabaster, AL), PTEN siRNA was from Cell Signaling Technology (Beverly, MA), S1P1, S1P2 and S1P3 receptor siRNAs were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Tissue culture reagents, DMEM and 100x antibiotic antimycotic solution (penicillin streptomycin and amphotericin B) were obtained from Gibco BRL (Grand Island, NY) and fetal bovine serum was purchased from HyClone (Logan, UT). Enhanced chemiluminescence reagent and BSA protein assay reagent were obtained from Pierce Chemical Co. (Rockford, IL). TriReagent was purchased from the Molecular Research Center Inc. (Cincinnati, OH). Primers were purchased from Operon (Huntsville, AL).
Immunoblotting
Whole cell extracts were prepared from fibroblasts using lysis buffer with the following composition: 1% Triton X-100, 50mM Tris-HCl pH7.4, 150mM NaCl, 3mM MgCl2, 1mM CaCl2, proteinase inhibitor cocktail (Roche), 1mM phenylmethyl sulfonyl fluoride. Protein extracts were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated overnight with primary antibody, washed, and incubated for 1 h with secondary antibody. After washing, visualization was performed by enhanced chemiluminescence (Pierce).
siRNA silencing
For the inhibition of gene expression using specific siRNA reagents, dermal fibroblasts were grown to 80–90% confluence, serum starved for 4 h and transiently transfected using HiPerFect (Qiagen, Valencia, CA) with 20μm of the gene specific siRNA, or the corresponding concentration of scrambled non-silencing siRNA (Scrm). Twenty-four hours later, medium was changed to 10% fetal bovine serum and cells were harvested 72 h after transfection.
Real-Time PCR
Total RNA was isolated from dermal fibroblasts using TriReagent (MRC Inc.) according to the manufacturer’s instructions. 2 μg of RNA was reverse transcribed in a 20-μl reaction using random primers and Transcriptor First Strand synthesis kit (Roche Applied Sciences). qPCR was carried out using IQ SYBR Green mixture (Bio-Rad) on an iCycler PCR machine (Bio-Rad) using 1 μl of cDNA in triplicate with β-actin as the internal control. The primers used are as follows: S1P1 forward: aacttcgccctgcttgag, reverse: tccaggctttttgtgtagctt; S1P2 forward: ggccttcatcgtcatcctc, reverse: cgcaatgagcaccagaag; S1P3 forward: tgatgagatgaaacctatttgtaagg, reverse: caagaaggcaacagaaatgct.
RESULTS
Treatment of SSc fibroblasts with dhS1P normalizes the fibrotic phenotype
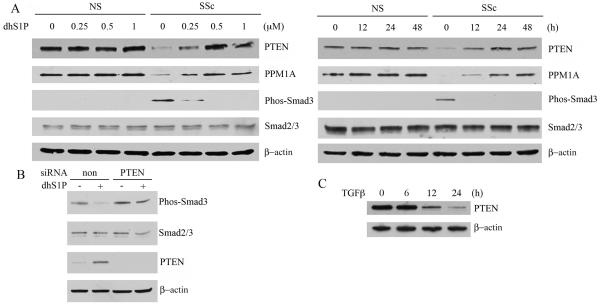
We have recently reported that dhS1P inhibits TGF-β-induced Smad3 signaling and collagen upregulation in human foreskin fibroblasts through a PTEN/PPM1A-dependent pathway (11). Since SSc fibroblasts are characterized by constitutively activated TGF-β signaling, we asked whether dhS1P would be effective in inhibiting this pathway in SSc fibroblasts. SSc and closely matched healthy control fibroblasts were treated with increasing doses (0-1 μM) of dhS1P for 12-24 hours. As previously reported, SSc fibroblasts expressed elevated levels of phospho-Smad3 (17, 18). Treatment with dhS1P abrogated Smad3 phosphorylation in a dose- and time-dependent manner (Fig. 1A). PTEN or PPM1A expression levels have not been previously reported in SSc fibroblasts. As shown in Fig. 1A SSc fibroblasts expressed low protein levels of PTEN and PPM1A as compared to control cells. Treatment with dhS1P normalized PTEN and PPM1A protein levels in SSc fibroblasts in a dose- and time-dependent manner, while dhS1P did not affect PTEN or PPM1A levels in control fibroblasts. Importantly, constitutive phosphorylation of Smad3 in SSc fibroblasts inversely correlated with the increase in PTEN levels. To examine whether PTEN is responsible for the dhS1P mediated inhibition of Smad3 phosphorylation, PTEN was depleted from SSc fibroblasts using siRNA as previously described (11). In the absence of PTEN, dephosphorylation of Smad3 by dhS1P was abrogated, indicating that PTEN is required for this process (Fig. 1B). These experiments establish for the first time that the phosphorylation status of Smad3 in SSc fibroblasts depends on endogenous PTEN levels.
Figure 1. DhS1P reverses constitutive phosphorylation of Smad3 in SSc fibroblasts through up-regulation of PTEN/PPM1A protein levels.
A. Healthy (NS) and SSc fibroblasts were treated with increasing doses of dhS1P for 24 hours (left panel) or were treated with 0.5 μM of dhS1P for the indicated time points (right panel). PTEN, PPM1A, Phospho-Smad3, and total Smad3 were analyzed by western blotting. β-actin was used as loading control. B. SSc fibroblasts were treated with PTEN or non-silencing siRNA for 24 hours and stimulated with dhS1P for additional 24 hours. Phospho-Smad3, total Smad3, PTEN and β-actin were analyzed by western blotting. C. Healthy control fibroblasts were treated with TGF-β (2.5 ng/ml) for the indicated time points. PTEN and β-actin levels were analyzed by western blotting.
We have previously shown that PPM1A protein is rapidly degraded in response to TGF-β (11). To determine whether TGF-β regulates PTEN expression, dermal fibroblasts were stimulated with TGF-β for 6-24 hours. As shown in Fig. 1D, TGF-β reduced PTEN expression after 12 hours of treatment, suggesting that reduced levels of this protein in SSc fibroblasts may be due to the constitutive activation of TGF-β signaling.
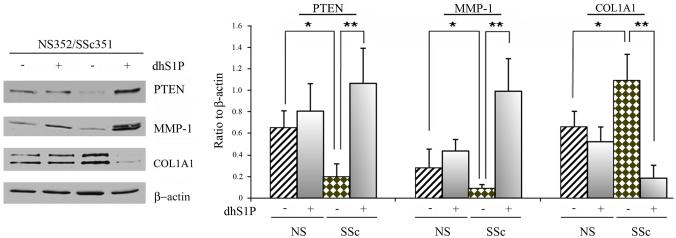
We next examined 6 pairs of SSc and closely matched healthy control fibroblasts to determine the effects of dhS1P on PTEN, collagen and MMP1 production (Fig. 2 and Fig. 1S, supplemental data). Consistent with previous reports, all SSc cell strains produced more collagen and had reduced levels of MMP1. In addition, all SSc cell strains demonstrated significantly reduced PTEN protein levels as compared to healthy fibroblasts. Treatment with dhS1P significantly increased the levels of PTEN in SSc fibroblasts, whereas only slight stimulatory effects were seen in control fibroblasts. Furthermore, dhS1P significantly increased MMP1 levels and decreased collagen levels in SSc fibroblasts. In contrast, the effects of dhS1P on MMP1 and collagen levels in control fibroblasts were insignificant. Together, these data suggest that dhS1P has a dual anti-fibrotic effect in SSc fibroblasts by decreasing collagen and increasing MMP1 production.
Figure 2. SSc fibroblasts show increased sensitivity to the anti-fibrotic effects of dhS1P.
Six pairs of SSc and closely matched control fibroblasts were stimulated with 0.5 μM of dhS1P for 48 hours. Left panel depicts the protein levels of PTEN, MMP-1, and collagen determined by western blotting in a representative pair. β-actin was used to normalize protein levels. Lines 1 and 2 represent NS fibroblasts, lanes 3 and 4, SSc fibroblasts. Right panel represents graphical summary of the experiment. Bar graph depicts mean±SD from all pairs tested expressed as a ratio of each protein level to β-actin level. *p<0.05, **p<0.01, ***p<0.001
Fibroblasts from patients with SSc exhibit heightened sensitivity to the pro-fibrotic effects of S1P
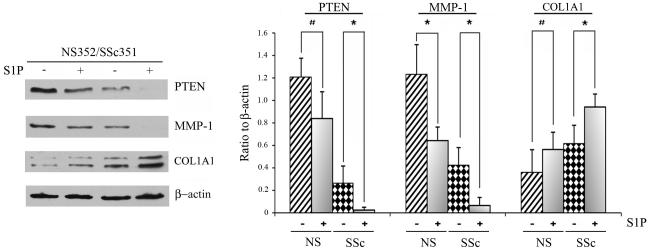
S1P has been shown to mimic the pro-fibrotic effects of TGF-β in several cell types, including foreskin fibroblasts (7, 10, 11). We next compared the effects of S1P on PTEN, MMP-1 and collagen production in four pairs of SSc and control fibroblasts. Although PTEN was already expressed at a relatively low level in SSc fibroblasts, treatment with S1P further significantly reduced PTEN protein expression (Fig. 3 and Fig. 2S, supplemental data). Likewise, S1P treatment further significantly reduced MMP1 levels, whereas collagen levels were up-regulated in SSc fibroblasts. Similar trends were observed in control fibroblasts, but the response was more pronounced in SSc fibroblasts. Together these data indicate that dhS1P and S1P have opposite effects on expression of several pro-fibrotic genes in SSc fibroblasts.
Figure 3. SSc fibroblasts show increased sensitivity to the pro-fibrotic effects of S1P.
Four pairs of SSc and closely matched control fibroblasts were stimulated with 0.5 μM of S1P for 48 hours. Left panel depicts the protein levels of PTEN, MMP-1, and collagen determined by western blotting in a representative pair. β-actin was used to normalize protein levels (left panel). Bar graph represents Mean±SD from all pairs tested expressed as a ratio of each protein level to β-actin level. *p<0.05, #-non significant.
PTEN expression is reduced in SSc skin biopsies
To further investigate the involvement of the PTEN pathway in dermal fibrosis in SSc, we examined the distribution of PTEN in skin specimens from 7 SSc patients and 7 healthy controls. Representative staining from the skin of SSc and healthy controls is shown in Fig. 3S A-D (supplemental data). PTEN positive fibroblasts were counted in each specimen and the summary of the results is included in Table 1. The analysis revealed heterogeneity of PTEN expression among SSc and control skin sections. While majority of the SSc skin fibroblasts showed either absent or low levels of PTEN expression, similar pattern was also observed in some of the healthy skin biopsies. This finding is in agreement with other studies that found low to moderate expression of PTEN in dermal fibroblasts in vivo (19). However, comparison of closely matched SSc and control skin specimen showed that, with the exception of one pair, a significantly higher proportion of PTEN positive fibroblasts was present in healthy skin, suggesting that downregulation of the PTEN pathway may contribute to the development of fibrosis in SSc.
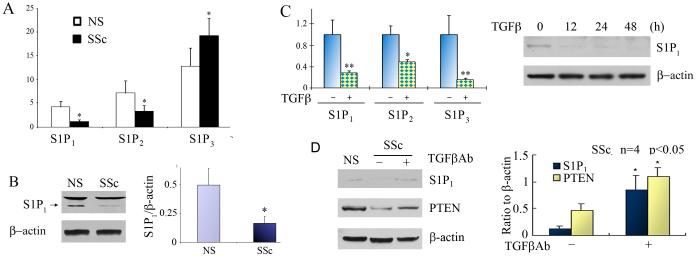
S1P1 and S1P2 receptor expression is decreased in SSc dermal fibroblasts
We have previously shown that effects of S1P and dhS1P on the TGF-β-induced Smad3 phosphorylation are mediated via a single S1P1 receptor in foreskin fibroblasts (11). We reasoned that increased sensitivity of SSc fibroblasts to dhS1P and S1P could be due to increased levels of S1P1 receptor. The distribution of S1P receptor subtypes in SSc and control fibroblasts was examined using qPCR. Unexpectedly, SSc fibroblasts showed reduced mRNA expression of S1P1 and S1P2 receptor, however mRNA expression of S1P3 receptor was increased (Fig. 4A). The expression of S1P1 receptor protein was further investigated in SSc and control fibroblasts. Consistent with mRNA levels, S1P1 receptor protein is expressed at the lower level in SSc fibroblasts (Fig. 4B). We were unable to measure the protein levels of other S1P receptor isoforms, because of the lack of suitable antibodies. We next investigated whether TGF-β signaling regulates S1P receptor expression. As shown in Fig 4C, expression of all three S1P receptor isoforms was significantly down-regulated by TGF-β. Down-regulation of S1P1 receptor was further confirmed at the protein level. These data suggest that the distribution of S1P receptor isoforms differs in SSc and healthy control fibroblasts. To test the possibility that downregulation of S1P1 and S1P2 receptor isoforms in SSc fibroblasts may be in part mediated by TGF-β signaling, we blocked autocrine TGF-β signaling using anti-TGF-β neutralizing antibody. Addition of the TGF-β neutralizing antibody completely abrogated TGF-β induced phosphorylation of Smad3 (Fig. 4S A, supplemental data). Treatment of SSc fibroblasts with the neutralizing antibody resulted in upregulation of S1P1 receptor, as well as PTEN, suggesting that reduced level of these genes in SSc fibroblasts may be in part mediated by the autocrine TGF-β signaling (Fig. 4D and Fig. 4S B, supplemental data).
Figure 4. Distribution of S1P receptor isoforms differs in SSc and control fibroblasts.
A. mRNA expression of S1P1-3 receptor isoforms were measured in 5 pairs of SSc and NS fibroblasts by qPCR (* p<0.05). B. S1P1 receptor protein level was analyzed in 3 pairs of SSc and control fibroblasts. Left panel shows representative western blot (note that only lower band is specific). Bar graph represents Mean±SD from all pairs tested (* p<0.05). C. Control fibroblasts were stimulated with 2.5 ng/ml of TGF-β. mRNA expression of S1P1-3 receptor isoforms were measured in 3 fibroblast strains by qPCR (* p <0.05, ** p<0.01). Representative western blot for S1P1 receptor is shown on the right. D. Autocrine TGF-β signaling contributes to downregulation of S1P1 receptor and PTEN in SSc fibroblasts. SSc fibroblasts were treated for 48 h with 10 μg/ml of anti -TGF-β Antibody. Left panel shows representative western blot of S1P1 receptor and PTEN levels in SSc fibroblasts treated with the antibody as compared to levels in closely matched healthy control fibroblasts (NS) used as a reference. Bar graph represents Mean±SD from 4 SSc cell strains (* p<0.05).
The effects of dhS1P and S1P are mediated through S1P1 and S1P2 receptor in SSc fibroblasts
To investigate S1P/dhS1P signaling in SSc and healthy fibroblasts, we next focused on the function of the individual S1P receptor isoforms. To determine which receptor mediates the effects of S1P and dhS1P in control and SSc dermal fibroblasts, S1P1, S1P2, and S1P3 receptor were individually depleted >80% using specific siRNAs (Fig. 5A). Cells were then stimulated with 0.5 μM S1P or with a combination of TGF-β (2.5 ng/ml) and 0.5 μM dhS1P. In healthy adult dermal fibroblasts S1P stimulated phosphorylation of Smad3, while dhS1P prevented TGF-β-induced Smad3 phosphorylation in the presence of non-silencing siRNA (Fig. 5A, left panel). Depletion of S1P1 receptor inhibited the effects of S1P and dhS1P on Smad3 phosphorylation, while depletion of S1P2 or S1P3 receptor did not have any appreciable effect on these responses. These results are consistent with our previous observations in foreskin fibroblasts.
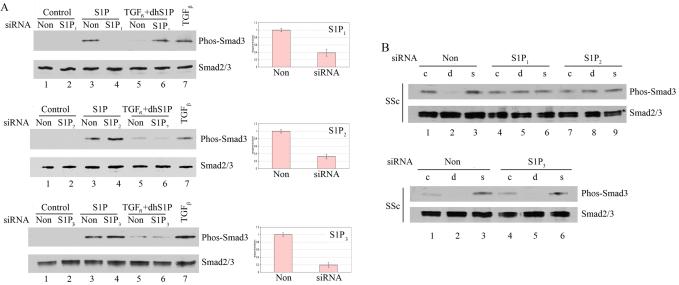
Figure 5. Depletion of distinct endogenous S1P receptor isoforms abrogates effects of dhS1P and S1P on Smad phosphorylation levels in normal and SSc fibroblasts.
Cells were transfected with 30 nM of S1P1, S1P2, or S1P3 receptor siRNA or non-silencing siRNA for 24 h, then serum starved overnight. Depletion of S1P receptor isoforms was assessed by qPCR. A. Control cells were treated with 1 μM S1P or 2.5 ng/ml of TGF-β plus 0.5 μM dhS1P for 30 min. to assess Smad3 phosphorylation level. B. SSc fibroblasts were treated with S1P(s) or dhS1P (d) for 30 min. to assess Smad3 phosphorylation level. Phospho-Smad3, total Smad3, and β-actin were detected by western blotting.
We next examined the involvement of S1P receptors in response to S1P or dhS1P in SSc fibroblasts. S1P1, S1P2, and S1P3 were individually depleted using siRNA followed by stimulation with the agonists (Fig. 5B). Interestingly, depletion of either S1P1 or S1P2 receptor abrogated responses to dhS1P (d) and S1P (s), while depletion of S1P3 receptor had no effect. Together these data suggest that normal fibroblasts mediate their responses to S1P and dhS1P through a single S1P1 receptor, whereas SSc fibroblasts require two receptors, S1P1 and S1P2, for their responses.
DISCUSSION
Persistent TGF-β signaling is a major factor in the activation of lesional SSc fibroblasts (2). Cultured SSc fibroblasts maintain an “activated phenotype”, which is characterized by overexpression of collagen and other ECM proteins and reduced expression of the principal collagen-degrading enzyme, MMP-1. This study demonstrates that treatment of SSc fibroblasts with dhS1P effectively reverses this phenotype, including inhibition of phospho-Smad3, down-regulation of collagen, and up-regulation of MMP-1. Importantly, we show that the anti-fibrotic effects of dhS1P in SSc fibroblasts are mediated through the modulation of PTEN expression levels and that activation of Smad3 pathway in SSc fibroblasts is directly linked to the reduced levels of PTEN. Furthermore, our data suggest that the autocrine TGF-β signaling contributes to the downregulation of PTEN in SSc fibroblasts. DhS1P had little effect on matrix related genes in healthy dermal fibroblasts, consistent with its previously described role as an inhibitor of TGF-β/Smad3 signaling (11). S1P mimicked the effects of TGF-β by down-regulating PTEN and MMP1 and upregulating collagen protein levels. Interestingly, despite evidence of constitutive activation of the TGF-β signaling pathway, S1P effects were even more pronounced in SSc fibroblasts, suggesting that TGF-β and S1P may have an additive pro-fibrotic effect.
There is increasing evidence that PTEN deficiency is associated with fibrosis in different organs. In patients with idiopathic pulmonary fibrosis, expression of PTEN was diminished in lung myofibroblasts within fibroblastic foci (20). The authors also demonstrated that inhibition of PTEN function is necessary and sufficient for myofibroblasts differentiation of lung fibroblasts. A similar role of PTEN was reported in activation of cultured hepatic stellate cells (21). Our study demonstrates a significantly lower level of PTEN in cultured SSc fibroblasts and a decreased presence of PTEN-positive fibroblasts in SSc skin in vivo. Restoration of PTEN levels in SSc fibroblasts correlated with normalization of collagen and MMP-1 expression. The anti-fibrotic role of PTEN is not well understood. PTEN encodes a lipid phosphatase that dephosphorylates PtdIns(3,4,5)P3 (PIP3) leading to the inhibition of PI3 Kinase/Akt signaling. There is also evidence that PTEN, through a protein-protein interaction involving its C terminal domain, has cellular functions that do not depend on its lipid phosphatase activity (22). Previous studies uncovered a novel function of nuclear PTEN as a chaperone of Smad3 phosphatase PPM1A. PPM1A is rapidly degraded in response to TGF-β signaling, and PTEN stabilizes PPM1A protein through formation of PTEN/PPM1A complexes (11). Accordingly, this study shows that normalization of PTEN, as well as PPM1A levels in SSc fibroblasts leads to dephosphorylation of Smad3, suggesting that this may be one of the mechanisms whereby PTEN deficiency exerts fibrogenic effects. It is also likely that PTEN deficiency contributes to fibrosis through activation of other fibrogenic pathways such as Akt. In dermal fibroblasts, Akt induces collagen gene expression and inhibits MMP-1 production through a TGF-β independent mechanism (23). Constitutive activation of the Akt pathway, which plays a central role in regulating cell growth and survival, has been reported in SSc fibroblasts in vitro and in vivo, however the pathway responsible for Akt activation in SSc was not examined (24). A recent study performed in glomerular mesangial cells has delineated the mechanism governing TGF-β activation of Akt (25). It was shown that TGF-β induces two microRNAs (miRNAs) miR-216a and miR-217, which target PTEN. The decrease in PTEN, increases PIP3, and leads to Akt activation. Further studies are needed to determine whether miRNA-dependent mechanisms are responsible for the down-regulation of PTEN and activation of Akt in SSc fibroblasts.
S1P/dhS1P signal through S1P1-5 receptors, which belong to the G protein coupled receptor (GPCR) family (26). Different receptor subtypes couple to different G proteins, with S1P1 receptor coupling exclusively to Gi and S1P2, and S1P3 receptor coupling to Gi, Gq, and G12/13 (26). This study examined for the first time the distribution and function of S1P receptors in SSc fibroblasts. Our data show that reduced levels of S1P1 and S1P2 receptors and elevated levels of the S1P3 receptors characterize SSc fibroblasts. Conversely, treatment of healthy fibroblasts with TGF-β resulted in the downregulation of all three receptor isoforms; thus altered distribution of S1P receptor isoforms in SSc fibroblasts could only be partially dependent on the activation of autocrine TGF-β signaling. Interestingly, SSc fibroblasts differ from control cells in the utilization of S1P receptor isoforms. In SSc fibroblasts, S1P and dhS1P signal through S1P1 and S1P2 receptors, while in healthy fibroblasts these agonists mediate their effects via a single, S1P1 receptor. The basis for this difference is not known however, in other cell types, including cardiac fibroblasts, mesangial cells, and lung fibroblasts, pro-fibrotic effects of S1P are mediated through S1P2 and S1P3 receptors (7, 10, 27). S1P2 and S1P3, but not S1P1 receptor couple to G12/13, the only G protein that activates the Rho pathway. Rho kinase was shown to contribute to the TGF-β induced myofibroblast differentiation in several experimental models, including SSc fibroblasts (28). Thus, it is possible that differential S1P/dhS1P signaling in SSc and healthy fibroblasts is related to the myofibroblast characteristics of SSc cells. While further studies are needed to fully understand the significance of these novel observations, this study points out a previously unappreciated role of sphingolipid signaling in SSc fibrosis.
In conclusion, this study suggests that the sphingosine kinase metabolites, dhS1P and S1P, may have an important role in regulation of extracellular matrix in dermal fibroblasts through modulation of PTEN expression levels. The discovery that PTEN is directly involved in the regulation of Smad signaling, in addition to its well known role as lipid phosphatase, broadens the functional range of this tumor suppressor molecule and suggests that PTEN could also be called “fibrosis suppressor”. This study provides the evidence that PTEN deficiency is present in SSc and suggests that dhS1P or its derivatives may be effective as a therapeutic anti-fibrotic agent. Both S1P and dhS1P are present in the circulation, with levels of S1P being an order of magnitude higher than those of dhS1P (Trojanowska and Bielawska, unpublished observations). Interestingly, it was recently reported that serum levels of S1P are higher in SSc, while there was no difference in dhS1P levels (29). Given the enhanced responsiveness of SSc fibroblasts to the pro-fibrotic effects of S1P, these new data further underscore the potential contribution of S1P to SSc fibrosis and suggest that targeting the sphingolipid pathway may benefit patients with SSc.
Supplementary Material
Acknowledgements
This study was funded through National Institute of Health grant AR044883 to Maria Trojanowska.
REFERENCES
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin North Am. 2008;34(1):115–43. doi: 10.1016/j.rdc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–95. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB, et al. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004;279(37):38471–9. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 7.Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, et al. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem. 2004;279(34):35255–62. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 8.Xin C, Ren S, Eberhardt W, Pfeilschifter J, Huwiler A. The immunomodulator FTY720 and its phosphorylated derivative activate the Smad signalling cascade and upregulate connective tissue growth factor and collagen type IV expression in renal mesangial cells. Br J Pharmacol. 2006;147(2):164–74. doi: 10.1038/sj.bjp.0706452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller CD, Rivera Gil P, Tolle M, van der Giet M, Chun J, Radeke HH, et al. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am J Pathol. 2007;170(1):281–92. doi: 10.2353/ajpath.2007.060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37(4):395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 11.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem. 2008;283(28):19593–602. doi: 10.1074/jbc.M802417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu S, Yamanaka M, Pei H, Bielawska A, Bielawski J, Hannun YA, et al. Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via activation of ERK1/2-Ets1 pathway in human fibroblasts. FASEB J. 2006;20(1):184–6. doi: 10.1096/fj.05-4646fje. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka M, Shegogue D, Pei H, Bu S, Bielawska A, Bielawski J, et al. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 up-regulation. J Biol Chem. 2004;279(52):53994–4001. doi: 10.1074/jbc.M410144200. [DOI] [PubMed] [Google Scholar]
- 14.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 15.Pannu J, Asano Y, Nakerakanti S, Smith E, Jablonska S, Blaszczyk M, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58(8):2528–37. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Czuwara-Ladykowska J, Moussa O, Markiewicz M, Smith E, Silver RM, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163(2):571–81. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxford) 2006;45(2):157–65. doi: 10.1093/rheumatology/kei124. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48(7):1964–78. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 19.Tsao H, Mihm MC, Jr., Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol. 2003;49(5):865–72. doi: 10.1016/s0190-9622(03)02473-3. [DOI] [PubMed] [Google Scholar]
- 20.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173(1):112–21. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takashima M, Parsons CJ, Ikejima K, Watanabe S, White ES, Rippe RA. The tumor suppressor protein PTEN inhibits rat hepatic stellate cell activation. J Gastroenterol. 2009 doi: 10.1007/s00535-009-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120(Pt 23):4071–9. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 23.Bujor AM, Pannu J, Bu S, Smith EA, Muise-Helmericks RC, Trojanowska M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008;128(8):1906–14. doi: 10.1038/jid.2008.39. [DOI] [PubMed] [Google Scholar]
- 24.Jun JB, Kuechle M, Min J, Shim SC, Kim G, Montenegro V, et al. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J Invest Dermatol. 2005;124(2):298–303. doi: 10.1111/j.0022-202X.2004.23559.x. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11(7):881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watterson KR, Lanning DA, Diegelmann RF, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007;15(5):607–16. doi: 10.1111/j.1524-475X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 27.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc Res. 2009;82(2):303–12. doi: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhmetshina A, Dees C, Pileckyte M, Szucs G, Spriewald BM, Zwerina J, et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum. 2008;58(8):2553–64. doi: 10.1002/art.23677. [DOI] [PubMed] [Google Scholar]
- 29.Tokumura A, Carbone LD, Yoshioka Y, Morishige J, Kikuchi M, Postlethwaite A, et al. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int J Med Sci. 2009;6(4):168–76. doi: 10.7150/ijms.6.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.