Abstract
The chemical composition of herbal medicines is very complex, and their therapeutic effects are determined by multi-components with sophisticated synergistic and/or suppressive actions. Therefore, quality control of herbal medicines has been a formidable challenge. In this work, we describe a fast analytical method that can be used for quality assessment of herbal medicines. The method is based on ligand fishing using human-serum-albumin-functionalized magnetic nanoparticles (HSA-MNPs) and mass spectrometry. To demonstrate the applicability of the proposed method, eight samples of Dioscorea panthaica were analyzed. The sampled plants were of both wild and cultivated origins. They grew at different geographical locations and were harvested at different times. The ligands bound to HSA-MNPs were isolated from the plant extracts and detected by using direct infusion electrospray ionization mass spectrometry (DI–ESI–MS). Chemical identity has been confirmed for five of the ligands isolated. From more than 15 peaks in the ESI–MS spectrum, 11 common peaks were selected for calculating the correlation coefficient and cosine ratio. The values of correlation coefficient and cosine ratio were >0.9824 and >0.9988, respectively, for all the samples tested. The results indicated a high level of similarity among the eight D. panthaica samples. Compared with chromatographic fingerprint analysis, the proposed HSA-MNP-based DI–ESI–MS/MS approach was not only fast and easy to carry out but also biological-activity-oriented, promising a more effective data interpretation and thus reliable assessment conclusions.
Keywords: Magnetic nanoparticles, Ligand fishing, ESI—MS/MS, Quality assessment of herbal medicines, Dioscorea panthaica
Introduction
Herbal medicines have been used to prevent and cure diseases for several thousand years. Over the past decades, they are gaining more and more attention all over the world due to their reliable therapeutic efficacies and low side effects, especially for chronic diseases [1, 2]. However, unlike modern therapeutic drugs that are single active pure compounds, herbal medicines are often used as mixtures of several herbal plants that contain many active ingredients, and the chemical compositions always vary depending on the breed, origin, and processing technique. This characteristic makes the quality control of herbal medicines a big challenge. Intensive studies have been done to fingerprint (i.e., profile) the active ingredients of herbal medicines by utilizing hyphenated techniques such as liquid chromatography–mass spectrometry (LC–MS), liquid chromatography–nuclear magnetic resonance (LC–NMR), and two-dimensional gas chromatography–mass spectrometry (2D GC–MS) [3–6]. Chromatographic fingerprinting has become the most commonly used approach for assessing the quality of herbal medicines [7–10]. It is very useful as it involves multi-compounds in the view of holistic approaches and integrated evaluation models [11]. However, it has no reflection of the bioactivity of the compounds included. As a result, the fingerprinting normally becomes very complicated in order to obtain a reliable evaluation.
To solve this problem, biofingerprint chromatogram analysis was proposed for the screening and analysis of multiple bioactive compounds in herbal extracts [12]. In the analysis, fingerprinting chromatograms obtained before and after the interaction between an herbal medicine and a biological system (e.g., protein, enzyme, receptor, cell, etc.) were compared. Sample preparation techniques including dialysis and cell extraction were so far employed in fingerprinting of herbal medicines. Dialysis is a time-consuming method for sample preparation as it uses the semi-permeable membrane to differentiate bound and unbound constituents [13–16]. Cell extraction technique is based on centrifugal differentiation and is not able to reflect the direct interaction between small molecules and macro-biomolecules such as protein, enzyme, and receptor [17–19]. Thus, the development of simple and fast methods for fingerprint analysis is of high significance.
Ligand fishing is an extraction technique based on the receptor theory. It is widely used to screen biological complex matrices that are potential sources for ligands of known or orphan receptors [20, 21]. Magnetic nanoparticles (MNPs) have been widely used in biological and chemical sciences due to its excellent suspension stability, easy surface modification, and, especially, convenient solid–liquid separation. MNP-based ligand fishing has been predominantly applied for protein purification purposes [22, 23]. Several concept-proof studies on MNP-based ligand fishing of small molecules were also reported [24–27]. We recently demonstrated that ligand fishing with the use of human serum albumin (HSA)-functionalized MNPs (HSA-MNPs) was a convenient approach to isolate/identify small bioactive molecules from botanical extracts [28]. HSA, the most abundant protein in blood plasma, plays a major role in the transportation and disposition of endogenous and exogenous ligands [29] and thus has been used as a model protein for diverse biophysical and physiochemical studies. It binds a wide variety of drug compounds in two primary binding sites (I and II) and can have a significant impact on their pharmacokinetics. It is believed that the affinity of ligands towards HSA determines their overall distribution, thus influencing their pharmaceutical efficacy and metabolism. Based on these facts, we expect that HSA-functionalized MNPs may serve well as a biological system for fingerprint analysis of herbal medicines.
An endemic Chinese medicinal plant, Dioscorea panthaica, is distributed widely at altitudes from 1,000 to 3,500 m in central southern and southwestern China. Its rhizomes have long been used for treating anthrax, gastropathy, rheumatic heart disease, and rheumarthritis in traditional Chinese medicine [30]. A few phytochemical and characterization studies on this medicinal plant were reported [31–35]. However, the information about its bioactive constituents is inadequate so far, particularly for the quality control purpose. In this work, analysis of this medicinal plant based on HSA-MNP ligand fishing and direct infusion electrospray ionization mass spectrometry (DI–ESI–MS) was demonstrated. Firstly, ligand fishing was performed to isolate the bioactive compounds in D. panthaica that bound to HSA-MNPs. Secondly, the small-molecule ligands fished out were detected/identified by DI–ESI–MS. Eleven ligands detected in the plant samples were selected for the similarity assessment. The results suggested that the affinity interaction between different ligands and HSA-MNPs varied significantly depending on the structural characteristics. Using the proposed method, eight D. panthaica samples of both wild and cultivated origins collected at different geographical locations and times were comparatively studied. Correlation coefficients and cosine ratios of the samples were determined from the fingerprinting obtained. The similarity of these samples was assessed.
Experimental section
Reagent and materials
HPLC-grade acetonitrile (ACN, Fisher, USA) and deionized water obtained from a Milli-Q water system (Millipore Corp., Bedford, MA, USA) were used during sample preparation procedures and analysis. Eight D. panthaica samples were collected from different areas in Sichuan and Yunnan provinces, China. HSA and 25% glutaraldehyde (GD) solution were purchased from Sigma-Aldrich (MO, USA). Ginsenoside Rb1 (internal standard) was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Tetraethyl ortho-silicate (TEOS) and 3-aminopropyltrimethoxysilane (APTMS) were purchased from TCI (Tokyo, Japan). The other chemicals and solvents were of analytical reagent grade and were obtained from Chengdu Chemical Factory (Chengdu, China).
ESI–MS analysis
ThermoQuest Finnigan LCQDECA system equipped with an electrospray ionization source (ThermoQuest LC/MS Division, San Jose, CA, USA) was used for mass spectrometric analysis. The DI–ESI–MS operating conditions were optimized in negative mode as follows: sheath gas flow rate, 35 units; auxiliary gas flow rate, 0 units; capillary temperature, 250 °C; capillary voltage, 4.5 kV. The samples were introduced into the ESI source by continuous infusion at a flow rate of 10 μL/min by a syringe pump.
Preparation of HSA-MNPs
HSA-MNPs were prepared following the procedures reported previously [28]. Briefly, Fe3O4 nanoparticles (MNPs) were prepared by co-precipitation with a molar ratio of Fe2+/Fe3+= 1:2. MNPs were firstly coated with SiO2 using TEOS. Secondly, the particles were dispersed in APTMS solution to add –NH2 to the SiO2; the resultant particles were then dispensed in the GD solution to provide –CHO to the silica coating. Finally, the silica-coated MNPs with –CHO functionality was incubated with HSA to obtain HSA-MNPs. The HSA-MNPs prepared (∼20 nm in diameter) were characterized by transmission electron microscopy. HSA-MNPs were suspended in NH4Ac solution and kept at 4 °C before use.
Ligand fishing
Eight samples of D. panthaica were collected. The sampled plants were of both wild and cultivated origins. They grew at different geographical locations throughout southwestern China and were harvested at different times. The sample collection information is shown in Table 1. To 0.5 g dried powder of D. panthaica, 20 mL water was added. The mixture was sonicated for 15 min. After being centrifuged, the supernatant was kept aside for ligand fishing. The solution was referred as S0.
Table 1. D. panthaica sample collecting information.
| Sample | Origin | Growing location | Harvesting time |
|---|---|---|---|
| DP#1 | Wild | Jiulong, Sichuan | Dec. 2006 |
| DP#2 | Wild | Maoxian, Sichuan | April 2008 |
| DP#3 | Cultivated | Xichang, Sichuan | March 2010 |
| DP#4 | Cultivated | Xichang, Sichuan | March 2009 |
| DP#5 | Wild | Xichang, Sichuan | March 2008 |
| DP#6 | Wild | Ninglang, Yunnan | May 2007 |
| DP#7 | Cultivated | Ninglang, Yunnan | May 2007 |
| DP#8 | Cultivated | Xiaojin, Sichuan | April 2008 |
To carry out ligand fishing, a portion of S0 (1 mL) was mixed with 100 μL HSA-MNPs suspension prepared above in a 4-mL Eppendorf tube. The mixture was vigorously shaken for 5 min using a vortex oscillator and then put aside for magnetic separation. The supernatant (S1) was carefully removed. The HSA-MNPs were washed three times with 1 mL of ammonium acetate buffer solution (10 mM, pH 7.4), each by vigorously shaking for 30 s, and the supernatants (referred as S2, S3, and S4) were carefully removed after magnetic separation. The fourth wash of the HSA-MNPs was done with 1-mL buffer containing 50% ACN for 1 min. The supernatant was carefully removed and saved (referred as S5). Ginsenoside Rb1 solution (7 μg/mL) was added to S0 and S5 (1:1). They were then analyzed by DI–ESI–MS.
Method validation
Assay reproducibility was evaluated by repeatedly analyzing an S5 solution for six times. The method repeatability was assessed by analyzing six S5 solutions separately prepared. Relative standard deviation (RSD) of the abundance was calculated for each compound (m/z). Sample stability was investigated with a mixture solution of authentic compounds. The solution (referred as M0) contained progenin α at 3.8 μmol/L, dioscin at 4.6 μmol/L, and gracillin at 5.4 μmol/L. Portions (1 mL) of the M0 solution and 100-μL HSA-MNP suspension were mixed in a 4-mL Eppendorf tube. Similarly, M1, M2, M3, M4, and M5 solutions were obtained and analyzed by DI–ESI–MS.
Quality assessment of medicinal plants
Each of the S5 solutions of the eight D. panthaica samples was analyzed using DI–ESI–MS. To assess the similarity of the eight samples, correlative coefficient and cosine ratios were calculated using Microsoft Excel 2003 software [36–38]. Correlation coefficient (rir) measures the strength and the direction of a linear relationship between two groups of variables. rir is calculated by using Eq. 1.
| (1) |
where Xik is the value of variable k in sample i; X̄i is the average of all variables in sample i; Xrk is the value of variable k in common mode; X̄r is the average of all variables in common mode. Cosine ratio (Cir) is a vector that calculates the angle between two groups of variables in Euclidian geometry. Cir is defined by Eq. 2, and the parameters are the same as in Eq. 1.
| (2) |
Results and discussions
Ligand fishing
In previous phytochemical studies on the chemical constituents of D. panthaica, steroidal saponins were isolated by using a tedious chromatographic procedure [31–33]. Because the chemical structures of these saponins are very similar, separating them from one another was difficult that it restricted the study on the medicinal benefits of individual saponins. MNP-based ligand fishing may serve as an effective and convenient separation procedure. The high selectivity is based on the receptor theory, and the liquid–solid phase separation involved can be carried out easily.
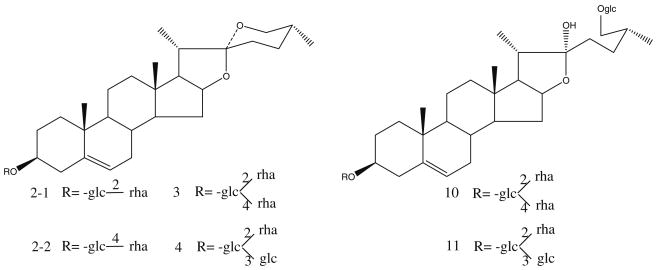
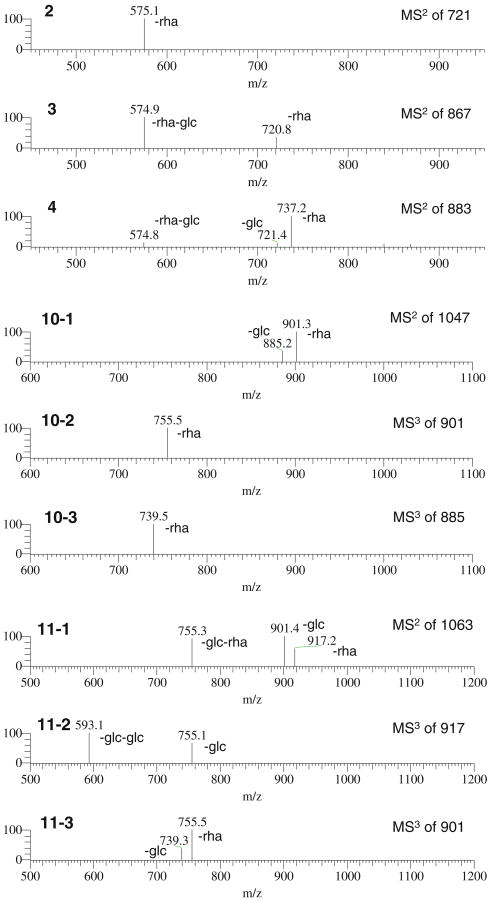
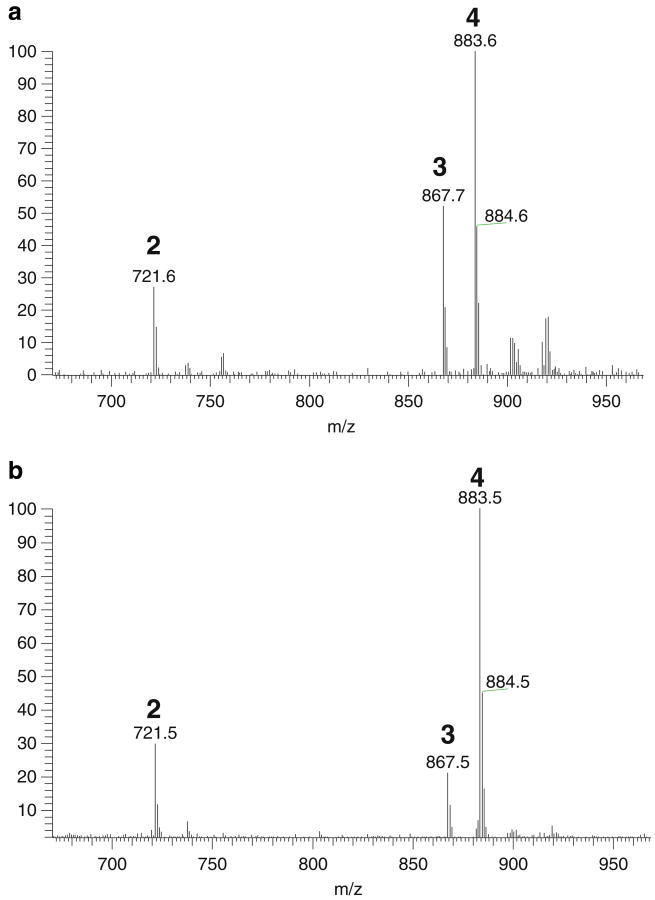
In this work, HSA-MNPs were used to isolate ligands from D. panthaica extract. DI–ESI–MS analysis of the extract (solution S0) resulted in a complicated ESI–MS spectrum, indicating numerous components present in the extract (Fig. 1a). In the spectrum, five peaks could be assigned as follows: m/z 721 [M–H]− progenin α (2-1) and/or progenin β (2-2), m/z 867 [M–H]− to dioscin (3), m/z 883 [M–H]− to gracillin (4), m/z 1,047 [M–H]− to protodioscin (10), and m/z 1,063 [M–H]− to protogracillin (11) (see Fig. 2 for the structures). Since no chromatographic separation was performed here, interferences from other substances with the same m/z might be possible. However, neither the components detected in D. panthaica by using an LC–MS/MS method [34] nor those identified in our previous systematic phytochemical study on this plant showed any isomers of the saponins we “fished out” in this work. Further, the structure verification was carried out by MSn (shown in Fig. 3). After ligand fishing, the three predominant peaks were assigned to compounds 2, 3, and 4 (Fig. 1b). Comparing the mass spectrum of the extract solution (solution S0, Fig. 1a) with that of the resultant ligand fishing eluate (solution S5, Fig. 1b), it can be seen that the peak abundance increased dramatically for these three compounds after ligand fishing. These results indicated that compounds 2, 3, and 4 were the stronger HSA-MNP-bound compounds in the extract. On the contrary, those major components detected in solution S0 (Fig. 1a) such as 10, 11, etc. showed a greatly reduced abundance after ligand fishing (Fig. 1b). Therefore, these compounds were the weaker HSA-MNP-bound compounds. The above results combined suggested that the representative compounds 2-4, 10, and 11 were ligands for HSA and might be biologically active even though they showed significant differences in the affinity to HSA-MNPs. In fact, previous studies showed that these compounds possessed various biological activities such as antitumor [32, 39–41], anti-leukemia [42], antifungal [43, 44], antivirus [45], inhibition of P-glycoprotein-mediated drug efflux [46], affection on the Ca2+ release activity of cardiomyocytes [47], etc. Further, there was another group of compounds in solution S0 (Fig. 1a) such as m/z 901 [M–H]−, etc. showing no affinity towards HSA-MNPs as they were not detected in solution S5 (Fig. 1b). In view of the receptor theory, they were not ligands for HSA and thus probably make no contributions for the biological activities of this medicinal plant.
Fig. 1.
ESI–MS analysis of D. panthaica extract (solution S0) (a) and the 50% ACN eluent from HSA-MNPs after ligand fishing (solution S5) (b). Peak identifications: (2) progenin α or/and β, (3) dioscin, (4) gracillin, (10) protodioscin, (11) protogracillin, and (IS) internal standard ginsenoside Rb1.
Note: b also served as the fingerprinting of D. panthaica in this work
Fig. 2.
Chemical structures of compounds 2-4, 10, and 11: (2-1) progenin α, (2-2) progenin β, (3) dioscin, (4) gracillin, (10) protodioscin, and (11) protogracillin
Fig. 3.
ESI–MSn spectra of compounds 2-4, 10, and 11. (2: MS/MS spectrum of m/z 721→575 for progenin α or/and β; 3: MS/MS spectrum of m/z 867→721, 575 for dioscin; 4: MS/MS spectrum of m/z 883→737, 721, 575 for gracillin; 10-1, 10-2, and 10-3: MS/MS spectrum of m/z 1,047→901, 885 and MS/MS/MS spectrum of m/z 901→755, and m/z 885→739 for protodioscin; 11-1, 11-2, and 11-3: MS/MS spectrum of m/z 1,063→917, 901, 755 and MS/MS/MS spectrum of m/z 917→755, 593, and m/z 901→755, 739 for protogracillin, respectively
Fingerprint analysis based on ligand fishing and DI–ESI–MS
DI–ESI–MS has been used for fingerprint analysis [48–50]. DI–ESI–MS fingerprinting has advantages including short analysis times (1–2 min), thereby permitting a rapid screening of samples and high sample throughput over other techniques such as LC–MS, LC–NMR, GC–MS, FT-IR, etc. However, fingerprints obtained by DI–ESI–MS are normally complex and, more importantly, contain many peaks from compounds that have no bioactivity, which makes the data interpretation complicated and prone to bias. In this work, fingerprint analysis was carried out by DI–ESI–MS after ligand fishing. The analysis only involved the ligands “fished out” by HSA from the sample matrix, which was simple and quick to perform. In order to evaluate the applicability of the proposed method for assessing the quality of herbal medicines, eight samples of D. panthaica were analyzed. The sampled plants were of both wild and cultivated origins. They grew at different geographical locations and were harvested at different times. A typical fingerprinting of D. panthaica is shown in Fig. 1b. More than 15 peaks appeared in the ESI–MS spectrum. Eleven were found to be common peaks for all the eight D. panthaica samples, which were m/z 653 (1), 721 (2), 867 (3), 883 (4), 889 (5), 905 (6), 919 (7), 987 (8), 1,003 (9), 1,047 (10), and 1,063 (11) [M–H]−. Some of them (i.e., m/z 721, 867, 883, 1,047, and 1,063 [M–H]−) were identified and reported in previous phytochemical studies. The others were detected for the first time in this work and have not yet been identified. Quantification of the selected 11 common peaks for each sample was carried out by means of the relative abundance of ginsenoside Rb1 (m/z 1,107 [M–H]−) as internal standard. The results are shown in Table 2.
Table 2. Relative abundance of common peaks from the eight samples of D. panthaica.
| No. | DP #1 | DP #2 | DP #3 | DP #4 | DP #5 | DP #6 | DP #7 | DP #8 | Average |
|---|---|---|---|---|---|---|---|---|---|
| 1 (m/z 653) | 0.2700 | 0.2049 | 0.1296 | 0.1377 | 0.1463 | 0.1596 | 0.3019 | 0.2182 | 0.1960 |
| 2 (m/z 721) | 0.8800 | 0.5902 | 0.5741 | 0.4638 | 0.7683 | 0.6383 | 0.7358 | 0.6182 | 0.6586 |
| 3 (m/z 867) | 1.8800 | 1.6393 | 1.8519 | 1.4493 | 2.4390 | 2.1277 | 1.8868 | 1.8182 | 1.8865 |
| 4 (m/z 883) | 2.0000 | 1.1967 | 1.3611 | 1.1377 | 2.0366 | 1.4468 | 1.5472 | 1.4000 | 1.5158 |
| 5 (m/z 889) | 0.2400 | 0.2049 | 0.1944 | 0.1667 | 0.2683 | 0.2766 | 0.2453 | 0.2273 | 0.2279 |
| 6 (m/z 905) | 0.2300 | 0.1557 | 0.1667 | 0.1667 | 0.3171 | 0.2660 | 0.2736 | 0.2182 | 0.2242 |
| 7 (m/z 919) | 0.3000 | 0.1803 | 0.1111 | 0.1449 | 0.3415 | 0.2128 | 0.3019 | 0.2909 | 0.2354 |
| 8 (m/z 987) | 0.2200 | 0.1803 | 0.1296 | 0.1232 | 0.1829 | 0.1915 | 0.2453 | 0.2364 | 0.1887 |
| 9 (m/z 1,003) | 0.2800 | 0.1475 | 0.1296 | 0.1159 | 0.1829 | 0.1915 | 0.3019 | 0.2364 | 0.1982 |
| 10 (m/z 1,047) | 0.3200 | 0.1803 | 0.1944 | 0.2971 | 0.3537 | 0.2021 | 0.3868 | 0.2364 | 0.2714 |
| 11 (m/z 1,063) | 0.3000 | 0.1475 | 0.1759 | 0.2681 | 0.2561 | 0.1702 | 0.3113 | 0.2000 | 0.2287 |
| IS | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Angle cosine | 0.9903 | 0.9980 | 0.9966 | 0.9972 | 0.9983 | 0.9957 | 0.9964 | 0.9988 | |
| Correlation coefficient | 0.9821 | 0.9966 | 0.9982 | 0.9948 | 0.9979 | 0.9935 | 0.9987 | 0.9983 |
To further evaluate the applicability of the proposed method for quality assessment of medicinal plants, assay reproducibility, method repeatability, and sample stability were determined. The reproducibility was determined by six replicate analyses of an S5 solution. The RSD of the respective peak abundance for the 11 common components was found in the range of 1.3% to 2.9%. Method repeatability was assessed by analyzing six S5 solutions from six different portions of a D. panthaica sample. The RSD of the respective peak abundance was in the range of 1.9–4.6%. Sample stability study was done with a standard solution containing progenin α (2), dioscin (3), and gracillin (4). This solution was referred as M0. Ligand fishing was carried out with this solution. The eluent was referred as M5. The DI–ESI–MS spectra from M0 and M5 solutions are shown in Fig. 4. As can be seen, all these three compounds were effectively extracted from the M0 solution by the ligand fishing procedure. Also, importantly, no unwanted modifications of the bound compounds were observed during ligand fishing. The above results indicated that the proposed ligand fishing–mass spectrometric method was useful for fingerprint analysis of D. panthaica.
Fig. 4.
DI–ESI–MS analysis of a standard mixture solution (solution M0) (a) and the 50% ACN eluent from HSA-MNPs after ligand fishing (solution M5) (b). Peak identifications: (2) progenin α, (3) dioscin, and (4) gracillin
Similarity of herbal fingerprinting can be evaluated by the correlative coefficient (rir) and/or angle cosine (Cir) values, both ranging from 0 to 1. The larger the values are, the higher the similarity of the target samples is. When they equal to 1, the targets are identical. In this work, the two mathematic parameters were calculated using Excel 2003 [36–38]. The data, as shown in Table 2, indicated that the values of rir and Cir in each mass spectrum to their simulative mean were approximate to 1.0000. The values of the correlation coefficient and the cosine ratio were above 0.9824 and 0.9988, respectively. These results showed a high level of similarity among the eight D. panthaica samples tested. The similar profiles of fingerprinting indicated that the quality of D. panthaica was stable, in view of the holistic approaches and integrated evaluation models. Based on these results, it was expected that these D. panthaica samples might have similar medicinal properties.
Conclusions
Ligand fishing with HSA-functionalized MNPs coupled with DI–ESI–MS has been proven an effective and fast approach for fingerprint analysis of D. panthaica (an endemic Chinese medicinal plant). HSA-functionalized MNPs were very useful for isolating ligands from the herbal extract. By using the proposed method, eight samples of D. panthaica were analyzed to determine their similarity. The fingerprint analysis found that the correlation coefficient and cosine ratio values were >0.9824 and >0.9988, respectively, indicating a high level of similarity among the herbal samples tested. These results suggested that the method might serve as a powerful tool for quality control of herbal medicines. As MNPs can be functionalized by various biological macromolecules of interests (such as protein, enzyme, receptor, cell, etc.), it is expected that the proposed approach is applicable to fingerprint analysis of many other herbal medicines for quality assessment purposes.
Acknowledgments
Financial support from the National Natural Science Foundation of China (20872137/B020402 to XL) and the US National Institutes of Health (SC1 GM089557 to YML) is gratefully acknowledged.
Contributor Information
Lin-Sen Qing, National Engineering Research Center for Natural Medicines, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China.
Ying Xue, National Engineering Research Center for Natural Medicines, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China.
Wen-Long Deng, Sichuan Key Laboratory of Quality and Innovation Research of Chinese Materia Medica, Sichuan Academy of Chinese Medicine Sciences, Chengdu 610041, China.
Xun Liao, Email: liaoxun@cib.ac.cn, National Engineering Research Center for Natural Medicines, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China.
Xue-Min Xu, Sichuan Key Laboratory of Quality and Innovation Research of Chinese Materia Medica, Sichuan Academy of Chinese Medicine Sciences, Chengdu 610041, China.
Bo-Gang Li, National Engineering Research Center for Natural Medicines, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China.
Yi-Ming Liu, Email: yiming.liu@jsums.edu, Department of Chemistry, Jackson State University, 1400 Lynch St., Jackson, MS 39217, USA.
References
- 1.Harvey AL. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Xue T, Roy R. Science. 2003;300:740–741. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 3.Drasar P, Moravcova J. J Chromatogr B. 2004;812:3–21. doi: 10.1016/j.jchromb.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 4.de Rijke E, Out P, Niessen WMA, Ariese F, Gooijer C, Brinkman UAT. J Chromatogr A. 2006;1112:31–63. doi: 10.1016/j.chroma.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Oleszek W, Bialy Z. J Chromatogr A. 2006;1112:78–91. doi: 10.1016/j.chroma.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Willför SM, Smeds AI, Holmbom BR. J Chromatogr A. 2006;1112:64–77. doi: 10.1016/j.chroma.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 7.Cao YH, Gong WJ, Li N, Yin CN, Wang Y. Anal Bioanal Chem. 2008;392:1003–1010. doi: 10.1007/s00216-008-2337-9. [DOI] [PubMed] [Google Scholar]
- 8.Xie P, Chen S, Liang YZ, Wang X, Tian R, Upton R. J Chromatogr A. 2006;1112:171–180. doi: 10.1016/j.chroma.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 9.Qi LW, Yu QT, Li P, Li SL, Wang YX, Sheng LH, Yi L. J Chromatogr A. 2006;1134:162–171. doi: 10.1016/j.chroma.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 10.Gu M, Ouyang F, Su Z. J Chromatogr A. 2004;1022:139–144. doi: 10.1016/j.chroma.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Qi LW, Liu EH, Zhou JL, Wen XD. TrAC Trends Anal Chem. 2008;27:66–72. [Google Scholar]
- 12.Su X, Kong L, Li X, Chen X, Guo M, Zou H. J Chromatogr A. 2005;1076:118–126. doi: 10.1016/j.chroma.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Lei X, Kong L, Zou H, Ma H, Yang L. J Chromatogr A. 2009;1216:2179–2184. doi: 10.1016/j.chroma.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 14.Lei XY, Kong L, Su XY, Guo M, Zou HF. Chem Res Chin Univ. 2008;24:411–417. [Google Scholar]
- 15.Qi LW, Li P, Li SL, Sheng LH, Li RY, Song Y, Li HJ. J Sep Sci. 2006;29:2211–2220. doi: 10.1002/jssc.200600107. [DOI] [PubMed] [Google Scholar]
- 16.Guo M, Su X, Kong L, Li X, Zou H. Anal Chim Acta. 2006;556:183–188. doi: 10.1016/j.aca.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Dong ZB, Li SP, Hong M, Zhu Q. J Pharm Biomed Anal. 2005;38:664–672. doi: 10.1016/j.jpba.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Zhao J, Zhu Q, Li SP. J Pharm Biomed Anal. 2007;44:439–445. doi: 10.1016/j.jpba.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HY, Hu CX, Liu CP, Li HF, Wang JS, Yuan KL, Tang JW, Xu GW. J Pharm Biomed Anal. 2007;43:151–157. doi: 10.1016/j.jpba.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Fermas S, Gonnet F, Varenne A, Gareil P, Daniel R. Anal Chem. 2007;79:4987–4993. doi: 10.1021/ac070146h. [DOI] [PubMed] [Google Scholar]
- 21.Zou H, Zhang Q, Guo Z, Guo B, Chen X. Angew Chem Int Ed. 2002;41:646–649. [Google Scholar]
- 22.Widjojoatmodjo MN, Fluit AC, Torensma R, Verhoef J. J Immunol Meth. 1993;165:11–19. doi: 10.1016/0022-1759(93)90101-c. [DOI] [PubMed] [Google Scholar]
- 23.Ljungquist C, Lundeberg J, Rasmussen AM, Hornes E, Uhlen M. DNA Cell Biol. 1993;12:191–197. doi: 10.1089/dna.1993.12.191. [DOI] [PubMed] [Google Scholar]
- 24.Lin PC, Tseng MC, Su AK, Chen YJ, Lin CC. Anal Chem. 2007;79:3401–3408. doi: 10.1021/ac070195u. [DOI] [PubMed] [Google Scholar]
- 25.Moaddel R, Marszałł MP, Bighi F, Yang Q, Duan X, Wainer IW. Anal Chem. 2007;79:5414–5417. doi: 10.1021/ac070268+. [DOI] [PubMed] [Google Scholar]
- 26.Marszałł MP, Moaddel R, Kole S, Gandhari M, Bernier M, Wainer IW. Anal Chem. 2008;80:7571–7575. doi: 10.1021/ac801153h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonker N, Kretschmer A, Kool J, Fernandez A, Kloos D, Krabbe JG, Lingeman H, Irth H. Anal Chem. 2009;81:4263–4270. doi: 10.1021/ac9000755. [DOI] [PubMed] [Google Scholar]
- 28.Qing LS, Xue Y, Zheng Y, Xiong J, Liao X, Ding LS, Li BG, Liu YM. J Chromatogr A. 2010;1217:4663–4670. doi: 10.1016/j.chroma.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki K, Maruyama T, Kragh-Hansen U, Otagiri M. Biochim Biophys Acta. 1996;1295:147–157. doi: 10.1016/0167-4838(96)00013-1. [DOI] [PubMed] [Google Scholar]
- 30.JNM College . Chinese Materia Medica Dictionary. Shanghai Press of Science and Technology; Shanghai: 1977. [Google Scholar]
- 31.Dong M, Feng XZ, Wang BX, Ikejima T, Wu LJ. Pharmazie. 2004;59:294–301. [PubMed] [Google Scholar]
- 32.Dong M, Feng XZ, Wang BX, Wu LJ, Ikejima T. Tetrahedron. 2001;57:501–512. [Google Scholar]
- 33.Geng Y, Tan NH, Zhang J, Kang LY. Chin J Nat Med. 2004;2:3–7. [Google Scholar]
- 34.Li R, Zhou Y, Wu Z, Ding L. J Mass Spectrom. 2006;41:1–22. doi: 10.1002/jms.988. [DOI] [PubMed] [Google Scholar]
- 35.Jing WG, Zhang QW, Liu A. China J Chin Mater Med. 2009;29:20–28. [Google Scholar]
- 36.Gong W, Cao Y, Wang Y. Phytochem Anal. 2008;19:499–504. doi: 10.1002/pca.1074. [DOI] [PubMed] [Google Scholar]
- 37.Zhou XL, Sun PN, Bucheli P, Huang TH, Wang D. J Agric Food Chem. 2009;57:5121–5128. doi: 10.1021/jf803707a. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Wang L, Yu X, Ye J. J Pharm Biomed Anal. 2006;41:845–851. doi: 10.1016/j.jpba.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 39.Cai J, Liu M, Wang Z, Ju Y. Biol Pharm Bull. 2002;25:193–200. doi: 10.1248/bpb.25.193. [DOI] [PubMed] [Google Scholar]
- 40.Mi Q, Lantvit D, Reyes-Lim E, Chai H, Zhao W, Lee I, Peraza-Sanchez S, Ngassapa O, Kardono L, Riswan S. J Nat Prod. 2002;65:842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- 41.Liu M, Wang Z, Ju Y, Zhou J, Wang Y, Wong R. Biol Pharm Bull. 2004;27:1059–1063. doi: 10.1248/bpb.27.1059. [DOI] [PubMed] [Google Scholar]
- 42.Hu K, Yao X. Planta Med. 2002;68:297–301. doi: 10.1055/s-2002-26743. [DOI] [PubMed] [Google Scholar]
- 43.Sautour M, Mitaine-Offer A, Miyamoto T, Dongmo A, Lacaille-Dubois M. Planta Med. 2004;70:90–92. doi: 10.1055/s-2004-815467. [DOI] [PubMed] [Google Scholar]
- 44.Takechi M, Shimada S, Tanaka Y. Phytochemistry. 1991;30:2943–2951. doi: 10.1016/0031-9422(92)83496-l. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda T, Ando J, Miyazono A, Zhu X, Tsumagari H, Nohara T, Yokomizo K, Uyeda M. Biol Pharm Bull. 2000;23:363–364. doi: 10.1248/bpb.23.363. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen V, Darbour N, Bayet C, Doreau A, Raad I, Phung B, Dumontet C, Di Pietro A, Dijoux-Franca M, Guilet D. Fitoterapia. 2009;80:39–42. doi: 10.1016/j.fitote.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Wang HY, Yu BY, Yu B, Hui YZ. Chin J Nat Med. 2003;1:41–43. [Google Scholar]
- 48.Goodacre R, York EV, Heald JK, Scott IM. Phytochemistry. 2003;62:859–863. doi: 10.1016/s0031-9422(02)00718-5. [DOI] [PubMed] [Google Scholar]
- 49.Amorim ACL, Hovell AMC, Pinto AC. J Braz Chem Soc. 2009;20:313–317. [Google Scholar]
- 50.Li W, Song FR, Liu ZQ, Liu SY. Chem Res Chin Univ. 2008;24:162–167. [Google Scholar]