Abstract
Background
Acute pancreatitis is a painful inflammatory disorder known to occur in children. Recent reports, primarily on the basis of adult data, have suggested an increasing incidence. However, pediatric studies are limited.
Objective
The study was performed to examine the frequency of acute pancreatitis in a pediatric population from 1994 to 2007 and to characterize etiologies by age subsets.
Patients and Methods
In this retrospective study, cases of pancreatitis were identified by ICD-9 codes and subjected to inclusion criteria.
Results
Two hundred and seventy-one cases of pancreatitis met inclusion criteria. Mean age of the subjects was 13.1 ± 5.6 years. The recurrence rate was 15.3%. Biliary disease was the most common etiology (32.6%). Acute pancreatitis cases evaluated at a single tertiary care center increased 53% between 1995 to 2000 and 2001 to 2006 (P <0.02). However, when cases were normalized by all annual pediatric emergency department visits for all medical reasons, the increase was reduced to 22% and lost statistical significance (P = 0.16). The rise was not associated with a change in etiologies or body mass index (BMI).
Conclusions
This is the first report demonstrating that an increase in pediatric pancreatitis may in part be due to growing referrals to tertiary care centers. The data on etiologies, particularly with regard to differing ages, may be helpful in managing children who present with acute pancreatitis.
Keywords: Biliary, Etiology, Pediatric, Tertiary referral
Acute pancreatitis is a painful inflammatory disorder with a major health impact (1,2). It accounts for more than 230,000 adult hospitalizations annually in the United States at an estimated cost of $4.6 to 4.8 billion/year (3). Even mild disease may require analgesia, nutritional support, and hospital stay ranging from 3 to 10 days (4). Data in children with acute pancreatitis are limited, but they suggest important differences vis-à-vis adults (1,5). Although alcohol and biliary tract disease cause most cases in adults, etiologies in children are more variable. Adult pancreatitis has been increasing in the last 4 decades (6). This has been attributed to the increasing incidence of gallstone pancreatitis. In children, recent reports indicate an increase of acute pancreatitis within the last decade (7–9). However, these data are confined to limited series from specific geographic locations. It is unclear whether the increase is attributable to local factors or a general shift in the epidemiology of this disease.
Our primary objective was to analyze the frequency of pediatric pancreatitis in the last 12 years at Yale-New Haven Children’s Hospital. Our secondary objective was to perform detailed analysis on etiologies of pancreatitis in children. If changes in frequency were present, then we sought to attribute causes for observed trends by analyzing shifts in etiologies, body mass index (BMI), or referral patterns during the study period.
PATIENTS AND METHODS
Study Group and Inclusion Criteria
The study was a retrospective chart review conducted at Yale-New Haven Children’s Hospital, New Haven, CT. This is a tertiary care teaching hospital whose catchment area is broad. It is 1 of only 2 children’s hospitals in the state of Connecticut and the only level 1 pediatric trauma center. The study was approved by the institutional review board. Our center sees pediatric patients from birth to age 21 years. Patients within this age range seen from August 1994 to July 2007 were screened using ICD-9 codes for acute pancreatitis. Records were reviewed for inclusion criteria relevant to acute pancreatitis. To be included in the study group, patients needed any 1 of the following 3 features:
Serum amylase or lipase greater than 3 times the upper limit of normal
Radiographic evidence of acute pancreatitis on computed tomography (CT) and ultrasound (U/S) demonstrating a minimum of pancreatic parenchymal changes or peripancreatic fluid
Serum lipase greater than 1.5 times the upper limit of normal that could not be explained by nonpancreatic causes of hyperlipasemia, and the presence of 2 out of 3 clinical features—abdominal pain characteristic of acute pancreatitis, nausea and vomiting, or epigastric tenderness
These criteria were modified from diagnostic guidelines published by the Acute Pancreatitis Classification Working Group (10). Less emphasis was placed on the presence of abdominal pain, which may be difficult to assess in children presenting with pancreatitis. Patients were excluded if they had chronic pancreatitis (documented by calcifications on CT or evidence of chronic pancreatic duct abnormality by endoscopic retrograde cholangiopancreatography). Incomplete records were also excluded. For patients with multiple presentations of acute pancreatitis, at least 4 weeks had to elapse after the prior discharge for the subsequent visit to be counted as recurrent pancreatitis.
Data Collection
Relevant clinical data were collected from patients’ charts in those who met inclusion criteria. These included patient demographics, BMI percentile, weight-for-age percentile, clinical presentation, and clinical course of pancreatitis.
Etiologic Classification
Patients were assigned etiologies on the basis of the reported cause of pancreatitis from the chart record. Etiologies were not mutually exclusive, because some patients could have several suggested etiologies in their record. Etiologies in patients with recurrent pancreatitis were recorded on their first visit unless they were idiopathic, in which case the final diagnosis on the last episode of recurrence was recorded. Idiopathic pancreatitis was defined as pancreatitis that could not be assigned an etiology after work-up during multiple presentations.
Biliary pancreatitis was defined as a spectrum of gallstone pancreatitis, microlithiasis/sludge, pancreatic divisum, sphincter of Oddi dysfunction, and “other/structural.” Microlithiasis, or biliary sludge, was defined as common bile duct (CBD) dilatation with sludge in the CBD or gallbladder in the absence of stones, as seen on U/S. “Other/structural” biliary pancreatitis was defined as an anatomic abnormality interfering with normal pancreaticobiliary function.
Medication-related pancreatitis etiology was assigned only if the patient was taking a medication listed in the AGA Technical Bulletin on Acute Pancreatitis as having “definite” association or “probable” association with acute pancreatitis (11). In addition, the medication had to be actively taken before the onset of acute pancreatitis.
Patients with a systemic disease associated with pancreatitis are also often taking medications for that systemic condition. The medications may be independently associated with pancreatitis. In such cases, we limited the etiologic classification to the systemic causes alone. However, patients taking a medication unrelated to the systemic illness were counted in both medication-related and systemic-associated categories. Inflammatory bowel disease (IBD) was considered a systemic disease-associated pancreatitis etiology; however, it was assigned only if the disease was active during the presentation of pancreatitis.
Viral etiology was associated with a prodromal presentation in the absence of another identifiable etiology. Alcoholic pancreatitis cases required etiologic assignment with documented evidence of heavy alcohol abuse in the chart record.
Outcomes
The primary outcome of the study was total number of cases of acute pancreatitis in 2 time blocks, 1995 to 2000 and 2001 to 2006. The cohort was divided into 2 time groups at the midpoint of the retrospective analysis (2000–2001). Secondary outcomes were etiologies and patient characteristics including age, sex, ethnicity, BMI, and weight-for-age percentiles.
Statistical Analysis
Continuous variables were analyzed using Student t test, whereas discrete variables were analyzed using χ2 test. A P value of <0.05 denotes statistical significance. Age-adjusted percentiles for BMI and weight-for-age were calculated with SAS (SAS Institute, Cary, NC) using a program provided by the Centers for Disease Control and Prevention.
RESULTS
Participant Flow and Inclusion Criteria
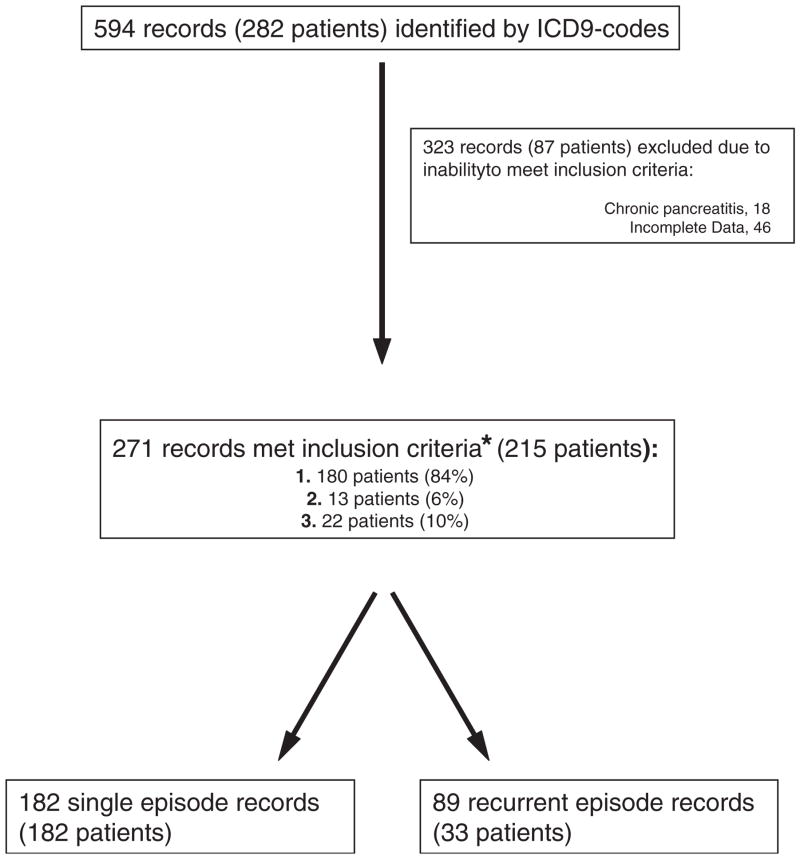
Patient flow and inclusion are shown in Figure 1. The majority of patients met inclusion by the first criteria (ie, biochemical evidence; 180 patients or 84%). Eighty-eight percent of these (n = 155) had abdominal pain characteristic of acute pancreatitis, whereas 84% (n = 152) had 2 out of the 3 typical clinical features of acute pancreatitis (abdominal pain, epigastric tenderness, and nausea or vomiting).
FIG. 1.
Study flow. The chart begins with total number of patient records initially screened. Patients that met inclusion criteria were further divided on the basis of having had single episodes versus recurrent pancreatitis. *Methods for details of each inclusion criteria.
Patient Characteristics
Patient characteristics are summarized in Table 1. Data were available for calculation of BMI in 142 (67%) patients and verified by calculating weight-for-age percentile, for which data were available in 193 (90%) patients. Although 4 patients (1.9%) died during hospitalization, only 1 of the deaths was directly related to acute pancreatitis.
TABLE 1.
Characteristics of children presenting with acute pancreatitis
| No. patients | 215 |
| No. episodes | 271 |
| Mean episodes per patient | 1.26 |
| Recurrence rate, % (n) | 15.3 (33) |
| Mean age (SD) | 13.1 (5.64) |
| Male, % | 86 (40) |
| Median BMI percentile* (lower–upper quartile) | 60.2 (17.8–89.5) |
| >95 percentile, n (%) | 24 (16.7) |
| 85–95 percentile, n (%) | 19 (13.3) |
| 0–85 percentile, n (%) | 100 (70) |
| Median weight-for-age percentile* (lower–upper quartile) | 63.35 (26.8–90.6) |
| >95 percentile, n (%) | 28 (14.5) |
| 85–95 percentile, n (%) | 28 (14.5) |
| 0–85 percentile, n (%) | 137 (71) |
| Ethnicity, n (%) | |
| White | 112 (53) |
| Black | 49 (23) |
| Hispanic | 40 (19) |
| Other | 11 (5) |
Age adjusted.
Frequency of Acute Pancreatitis
There was a statistically significant 53.1% increase in pancreatitis cases between the two 6-year time groups, (16 ± 3.35 cases/year during 1995–2000 versus 24.5 ± 5.5 cases/year during 2001–2006, P <0.02). There were no significant differences in BMI, weight-for-age percentiles, patient demographics, etiologies, or radiographic testing over time (Table 2). Amylase and lipase levels are not a component of routine metabolic panels at our hospital and because of the nature of our institutional databases, testing rates for these biochemical markers of pancreatitis could not be assessed during the study period.
TABLE 2.
Comparison of patients presenting with acute pancreatitis between 1995 to 2000 and 2001 to 2006
| Characteristics | 1995–2000 | 2001–2006 |
|---|---|---|
| No. patients (%) | 74 (34.4) | 119 (55.3) |
| No. episodes (%) | 96 (35.4) | 147 (54.2) |
| No. episodes per patient | 1.3 | 1.24 |
| Mean age (SD) | 12.6 (6) | 13.6 (5) |
| Male (%) | 27 (36.5) | 50 (42) |
| Ethnicity | ||
| White (%) | 39 (54.2) | 63 (53.4) |
| Black (%) | 17 (23.6) | 27 (22.9) |
| Hispanic (%) | 14 (19.4) | 21 (17.8) |
| Other (%) | 2 (2.8) | 7 (5.9) |
| Median BMI percentile* (lower–upper quartile) | 50 (20.1–91.4) | 58.9 (17.7–89.8) |
| >95 percentile (%) | 9 (22) | 15 (16.7) |
| 85–95 percentile (%) | 4 (9.8) | 12 (13.3) |
| 0–85 percentile (%) | 28 (68.3) | 63 (70) |
| Median weight-for-age percentile† (lower–upper quartile) | 68.5 (25.4–91.2) | 58.6 (25.4–83.2) |
| >95 percentile (%) | 11 (17.2) | 13 (11.8) |
| 85–95 percentile (%) | 11 (17.2) | 13 (11.8) |
| 0–85 percentile (%) | 42 (65.6) | 84 (76.4) |
| Mean CT scan rate (%) | 35.0 | 38.7 |
| Mean US scan rate (%) | 70.9 | 68.8 |
| Most common etiologies, (n) % | Idiopathic (17) 23 | Medication (35) 29 |
| Medication (13) 17.6 | Idiopathic (26) 21.8 | |
| Biliary (12) 16.2 | Biliary (24) 20.2 | |
| Viral (10) 13.5 | Systemic (13) 11 | |
| Trauma (7) 9.5 | Trama (9) 7.6 | |
Data available for age-adjusted BMI percentile in 55.4% and 75.6% of patients, in 1995–2000 and 2001–2006 groups, respectively.
Data available for weight-for-age percentile in 86.5% and 92.4% of patients, in 1995–2000 and 2001–2006 groups, respectively.
At Yale-New Haven Hospital, there was a concurrent 27.5% increase in average pediatric emergency department (ED) visits during the 2 time blocks (21,853 ± 2120.6 visits/year to 27,856 ± 1356.0 visits/year; P <0.0004). When the yearly frequency of acute pancreatitis cases was normalized using annual pediatric ED visits, the percent increase during the study period decreased from 53.1% to 21.9% and lost statistical significance (7.27 ± 0.97 cases per 10,000 ED visits/year in 1995–2000 versus 8.86 ± 2.3 cases per 10,000 ED visits/year in 2001–2006, P = 0.16).
Etiologies of Acute Pancreatitis
Etiologies of acute pancreatitis are listed in Table 3. Forty-five (21%) patients had more than 1 etiology identified. Biliary causes were most common, found in 70 (32.6%) cases. The 11 cases classified as other/structural etiology included 4 with pancreatic mass or cyst compressing the pancreatic duct, 2 with choledochal cyst, 1 with pancreatic duct stenosis of unknown cause, 1 with annular pancreas, 1 with self-limited intussusception, and 2 with postspinal fusion pancreatitis. There were significantly more females (23%) than males (11.6%) with gallstone pancreatitis (P <0.04).
TABLE 3.
Etiologies of acute pancreatitis, * n (%)
| Overall cohort | Subgroups | |
|---|---|---|
| Biliary causes | 70 (32.6) | |
| Gallstone pancreatitis | 40 (57) | |
| Microlithiasis/biliary sludge | 16 (22.9) | |
| Other/structural | 11 (15.7) | |
| Pancreatic divisum | 2 (2.9) | |
| Sphincter of Oddi dysfunction | 1 (1.4) | |
| Medication | 55 (25.6) | |
| Valproic acid | 13 (24) | |
| Prednisone | 12 (22) | |
| Mesalamine | 9 (16) | |
| Trimethoprim/sulfamethoxazole | 7 (13) | |
| 6-Mercaptopurine/azathioprine | 7 (13) | |
| Asparaginase | 4 (7.3) | |
| Furosemide | 2 (3.6) | |
| Tacrolimus | 2 (3.6) | |
| Antiretrovirals | 2 (3.6) | |
| Idiopathic | 44 (20) | |
| Systemic | 22 (10.2) | |
| Sepsis/shock | 14 (63.6) | |
| Other systemic causes | 8 (36.4) | |
| Trauma | 20 (9.3) | |
| Viral infection | 17 (7.9) | |
| Metabolic condition | 11 (5.1) | |
| Diabetic ketoacidosis | 5 (45) | |
| Hypertriglyceridemia | 3 (27.3) | |
| Inborn error of metabolism | 2 (18.2) | |
| Hypercalcemia | 1 (9.1) | |
| ERCP | 8 (3.7) | |
| Cystic fibrosis | 4 (1.9) | |
| Alcohol | 2 (0.9) |
ECRP = endoscopic retrograde cholangiopancreatography.
Etiologies are not mutually exclusive.
Medications were the second most common etiology for acute pancreatitis, found in 55 (25.6%) patients (Table 3). Trauma patients included those with motor-vehicle accidents, sports injury, accidental fall, and child abuse. Systemic etiology was typically seen in critically ill intensive care unit patients who developed sepsis and/or shock (n = 14, 63.6% of systemic causes). Other systemic causes included systemic lupus erythematous (n = 1), HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome (n = 1), acute liver failure (n = 1), sarcoidosis (n = 1), and active IBD (n = 2, 1 each with ulcerative colitis and Crohn disease).
Eleven (5.1%) patients had acute pancreatitis associated with a metabolic condition; these included diabetic ketoacidosis (n = 5) and hypertriglyceridemia (n = 3). Mean triglyceride levels in the latter were 986 mg/dL. Both cases of inborn error of metabolism occurred in females younger than 2 years of age with propionic acidemia. One of these patients has been previously reported (12).
Forty-four (20%) patients were idiopathic. Seven (25%) of these had recurrent pancreatitis. There were no statistically significant differences in age, sex, BMI, or weight-for-age between idiopathic and nonidiopathic cases. Four recurrent cases initially presented as idiopathic but their final etiologies were hypercalcemia due to hyperparathyroidism, pancreas divisum, sphincter of Oddi dysfunction, and microlithiasis.
Etiologies by Age and Recurrent Pancreatitis
An age-stratified etiologic analysis is summarized in Table 4. Under age 6 years, causes were diverse and an idiopathic diagnosis was rare. After age 11 years, biliary was the most common etiology and 62% (n = 34) of these cases were due to gallstone pancreatitis. The next most common biliary cause in that age group was microlithiasis (24%, n = 13 cases). Inborn errors of metabolism were diagnosed only in the 0 to 2 years age group. All biliary causes in the 0 to 2 years group were due to sludge (n = 2) or a biliary structural problem (n = 2, intussuception and pancreatic mass).
TABLE 4.
Etiologies by age of presentation
| Age group, y | 0–2 | 3–5 | 6–10 | 11–15 | 16–20 |
|---|---|---|---|---|---|
| No. patients (%) | 12 (5.6) | 16 (7.4) | 39 (18) | 52 (24.2) | 96 (44.7) |
| Male (%) | 4 (33) | 7 (43.7) | 12 (30.8) | 24 (42.6) | 39 (40.6) |
| Most common etiologies, n (%) | Bilary, 4 (33.3) | Medication, 7 (44) | Idiopathic, 13 (33) | Biliary, 23 (44) | Biliary, 32 (33) |
| Systemic, 3 (25) | Systemic, 4 (25) | Biliary, 10 (26) | Medication, 17 (33) | Idiopathic, 27 (28) | |
| Trauma, (3) 25 | Trauma, (3) 18.8 | Medication, (8) 21 | Idiopathic, (7) 13 | Medication, (21) 22 | |
| Inborn error of metabolism, (2) 16.7 | Biliary, 2 (12.5) | Viral, 7 (18) | Metabolic, 6 (12) | Systemic, 10 (10) | |
| Medication, (2) 16.7 | Metabolic, 2 (12.5) | Trauma, 5 (13) | Trauma, 3 (6) | Trauma, 7 (7) | |
| Viral, 2 (12.5) | Systemic, 3 (6) | Viral, 6 (6) | |||
| Idiopathic, 2 (12.5) |
Thirty-three (15.3%) patients had recurrence at an average of 2.7 episodes per patient. These were compared to 182 patients who had single episodes. A significantly higher percentage of patients with recurrent episodes were white (76%, n = 25 vs 48.9%, n = 87 among single-episode patients; P <0.05). There were no significant differences in age, sex, BMI, weight-for-percentile, or etiologies. The top 3 causes of pancreatitis among patients with recurrence and those with single presentations were gallstones, idiopathic, and medications.
DISCUSSION
In this study, we report a rise in pediatric acute pancreatitis evaluated at a single tertiary care center on the basis of a 12-year retrospective analysis. There was a 53% increase in cases from the latter half of the 1990s to the first half of the current decade. This was largely accounted for by an increase in pediatric ED visits in general for patients during the same time period. To further evaluate the increase we examined several factors.
The increasing BMIs of children have been well documented (12) and an association has been noted between obesity and severity of pancreatitis in adults (13). However, the contribution of BMI to the frequency of pancreatitis in children has not been previously investigated. Thirty percent of patients in the study had BMIs at the 85th percentile or higher, indicating at-risk status or clinical obesity. This observation was consistent with trends in the pediatric population of Connecticut (12). However, there was no statistically significant increase in BMI percentiles between patients in 1995 to 2000 and 2001 to 2006. This was confirmed with weight-for-age data.
The cohort was ethnically similar to the patient population seen at our hospital and this did not change over time. Careful etiologic analysis could not account for the increase in frequency and showed the most common etiologies in both time groups to be biliary tract disease, medications, and idiopathic.
Increased clinical awareness or referral bias can account for rising disease rates in retrospective studies. A national trend toward regionalization of pediatric emergency care among tertiary centers has created increased referral load at children’s hospitals throughout the United States. (14). The effect of this phenomenon on the incidence of pediatric pancreatitis has not been previously investigated. We found that pediatric ED visits increased by more than 25%, whereas pancreatitis cases rose by 53% between 1995 to 2000 and 2001 to 2006. To assess the effect of referral patterns, we calculated incidence by dividing annual case frequency by ED visits. As a result, the observed increase between 1995 to 2000 and 2001 to 2006 was reduced by more than half, to 22%. Concurrent reanalysis of data collected over a similar time period from the Children’s Hospital of Wisconsin corroborated the effect of referral bias. At Wisconsin, a previously reported rise in pediatric pancreatitis was reduced by nearly half after normalizing for a concurrent increase in ED visits (8, S. Werlin, personal communication, 2008).
To date there are no population-based studies assessing pediatric acute pancreatitis. Reports are limited to a handful of case series and retrospective studies (5,7–9,15–18). Because of the effect on our data of increasing visits to our ED during the time periods studied, we are unable to compare our results with those of other retrospective studies not reporting simultaneous changes in ED visits. These studies were done during similar time periods in Dallas (9), Wisconsin (8), Melbourne (7), and Mexico (18). The Melbourne study (7) found a significant increase in incidence between 1993 to 1997 and 1998 to 2002. This was attributed to an absolute rise in systemic-associated and idiopathic etiologies. There were no differences in etiology subsets between the 2 time groups in our study. The Dallas study (9) reported a significant increase in cases per year from 1993 to 1998. They examined the hypothesis of increased clinical awareness by assessing amylase and lipase testing rates. However, they noted a paradoxical decrease in testing per 100 ED visits from 1993 to 1998. This suggests that the rise in disease could not be attributed to an increase in testing. Because of the nature of laboratory databases at our institution, we were unable to study biochemical testing rates over time. A potential testing bias not previously assessed is radiography. However, there were no significant changes in CT and U/S scanning rates between 1995 to 2000 and 2001 to 2006.
Another unique aspect of the present report is the analysis of etiologies by age subsets. Under the age of 6 years, etiologies were highly diverse and idiopathic pancreatitis was uncommon, with most children obtaining a diagnosis. After age 11 years, biliary was the most common etiology and more than half of these cases were due to gallstones. The higher diversity of etiologies we observed in comparison with adults is consistent with other pediatric studies. However, our findings overall are in contrast to most pediatric reports, which have typically found acute pancreatitis in children most often associated with systemic or “multisystem” disease (5,8,9,17). Kandula et al (17) analyzed etiologies in children 0 to 3 years in a retrospective analysis and found that systemic disease was the most common cause of pancreatitis, followed by infections, idiopathic, medications, trauma, and biliary disease. We analyzed 0 to 2 years and 3 to 5 years patients separately, hence a direct comparison is difficult. In our 0 to 2 years cohort, there were no idiopathic cases and biliary disease was the primary etiology. However, the number of patients in our 0 to 2 years cohort was small compared to the number of patients in the Kandula study (12 vs 87).
There are several important limitations of this study. First, it is a retrospective analysis and suffers from the limitations inherent in such a study design. Also, we were unable to calculate true incidence because the pediatric population in our region has historically been divided among several referral centers whose catchment areas overlap. Only 1 other study in this field has been able to calculate true incidence (7), and it was outside the United States.
Our finding that referral patterns may account for a rise in pancreatitis is limited because the data are confined to Yale-New Haven Children’s Hospital. However, there were no shifts in patient flow among the pediatric referral centers in our area during the study. No centers opened or closed, nor were there expansions or eliminations of service at existing institutions. In addition, the referral pattern cannot be attributed to overall growth in the pediatric population because the number of children in Connecticut decreased during the study period (19,20).
Etiologic classifications and inclusion criteria vary widely among previous reports in this field because unlike adults, there is no evidence-based standard in diagnosis and treatment of children with pancreatitis. Our inclusion parameters were modified from definitions by the Acute Pancreatitis Classification Working Group (10) to deemphasize clinical features because abdominal pain “characteristic” of pancreatitis is problematic to assess in children and there are data to suggest that patients can be diagnosed by biochemical changes alone (21). Our etiologic classifications were not mutually exclusive, on the basis of the emerging concept that acute pancreatitis is the result of multiple factors that sensitize the pancreas to injury (17).
Despite its limitations, the study confirms that acute pancreatitis in children evaluated at a single tertiary care center has been increasing during the last 2 decades. It is the first to assess the role of BMI and referral patterns in this regard, finding that the observed rise may be partly explained by higher referral rates at tertiary care centers. Regionalization of pediatric care has been encouraged by the National Institutes of Medicine (14) and, therefore, our report may have wider applicability to other areas of the country. Subsequent studies from tertiary centers may need to control for referral patterns or include nontertiary institutions. Additional studies, population based if possible, are necessary to further confirm the rising frequency of pancreatitis. Our work also highlights the need for an evidence-based standard in diagnosis and etiologic classification of pediatric pancreatitis, particularly at a time when it appears that there is a rising burden of disease.
Acknowledgments
We thank Drs Pram Mistry and Phillip Tarr for their helpful suggestions at the inception of the study.
Footnotes
The authors report no conflicts of interest.
References
- 1.Lowe ME. Pancreatitis in childhood. Curr Gastroenterol Rep. 2004;6:240–6. doi: 10.1007/s11894-004-0014-5. [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865–8. doi: 10.1001/jama.291.23.2865. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson BC, Vander Vliet MB, Hughes MD, et al. A prospective, randomized trial of clear liquids versus low-fat solid diet as the initial meal in mild acute pancreatitis. Clin Gastroenterol Hepatol. 2007;5:946–51. doi: 10.1016/j.cgh.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weizman Z, Durie PR. Acute pancreatitis in childhood. J Pediatr. 1988;113(1 Pt 1):24–9. doi: 10.1016/s0022-3476(88)80523-7. [DOI] [PubMed] [Google Scholar]
- 6.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–30. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 7.Nydegger A, Heine RG, Ranuh R, et al. Changing incidence of acute pancreatitis: 10-year experience at the Royal Children’s Hospital, Melbourne. J Gastroenterol Hepatol. 2007;22:1313–6. doi: 10.1111/j.1440-1746.2007.04936.x. [DOI] [PubMed] [Google Scholar]
- 8.Werlin SL, Kugathasan S, Frautschy BC. Pancreatitis in children. J Pediatr Gastroenterol Nutr. 2003;37:591–5. doi: 10.1097/00005176-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr. 2002;140:622–4. doi: 10.1067/mpd.2002.123880. [DOI] [PubMed] [Google Scholar]
- 10.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Arch Surg; Summary of the International Symposium on Acute Pancreatitis; Atlanta, GA. September 11 through 13, 1992; 1993. pp. 586–90. [DOI] [PubMed] [Google Scholar]
- 11.Forsmark CE, Baillie J. AGA Institute Technical Review on acute pancreatitis. Gastroenterology. 2007;132:2022–44. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 12.Bultron G, Seashore MR, Pashankar DS, et al. Recurrent pancreatitis associated with propionic acidemia. J Pediatr Gastroenterol Nutr. 2008;47:370–1. doi: 10.1097/MPG.0b013e3181132252. [DOI] [PubMed] [Google Scholar]
- 13.Martinez J, Sanchez-Paya J, Palazon JM, et al. Obesity: a prognostic factor of severity in acute pancreatitis. Pancreas. 1999;19:15–20. [PubMed] [Google Scholar]
- 14.Committee on the Future of Emergency Care in the United States Health System BoHCS, Insititute of Medicine of the National Academies. . Emergency Care for Children: Growing Pains. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 15.Benifla M, Weizman Z. Acute pancreatitis in childhood: analysis of literature data. J Clin Gastroenterol. 2003;37:169–72. doi: 10.1097/00004836-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Haddock G, Coupar G, Youngson GG, et al. Acute pancreatitis in children: a 15-year review. J Pediatr Surg. 1994;29:719–22. doi: 10.1016/0022-3468(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 17.Kandula L, Lowe ME. Etiology and outcome of acute pancreatitis in infants and toddlers. J Pediatr. 2008;152:106, e1–10, e1. doi: 10.1016/j.jpeds.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Ramirez CA, Larrosa-Haro A, Flores-Martinez S, et al. Acute and recurrent pancreatitis in children: etiological factors. Acta Paediatr. 2007;96:534–7. doi: 10.1111/j.1651-2227.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 19.Poverty status by state and ten large metropolitan areas in 2001, people under 18. Child Welfare League of America; 2003. http://www.cwla.org/advocacy/statefactsheets/2003/connecticut.pdf. [Google Scholar]
- 20.Current Population Survey. Child Welfare League of America. 2007 http://www.cwla.org/advocacy/statefactsheets/2007/connecticut.pdf.
- 21.Lankisch PG, Burchard-Reckert S, Lehnick D, et al. Underestimation of acute pancreatitis: patients with only a small increase in amylase/lipase levels can also have or develop severe acute pancreatitis. Gut. 1999;44:542–4. doi: 10.1136/gut.44.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]