Abstract
Background
Infantile hemangioma (IH) is a most common tumor of infancy. Using infantile hemangioma-derived stem cells (HemSCs), we recently demonstrated that corticosteroids suppress the expression of VEGF-A, monocyte chemoattractant protein-1 (MCP-1), urokinase plasminogen activator receptor (uPAR), and interleukin-6 (IL-6); each of these are known targets of the transcription factor nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). In the present study, we examined the expression of these NF-κB target genes in IH tissue specimens and the effect of NF-κB regulation on the expression of pro-angiogenic cytokines, and in particular VEGF-A, in HemSCs.
Materials and methods
RNA extracted from IH tissue and hemangioma-derived stem cells (HemSCs) was used to analyze NF-κB target gene expression by reverse transcription–quantitative PCR (RT-qPCR). The effects of NF-κB blockade were examined in HemSCs. Immunostaining, immunoblotting and ELISA were used to assess protein expression.
Results
MCP-1, uPAR, and IL-6 were found to be differentially expressed in proliferating versus involuting IH. Corticosteroids suppressed NF-κB activity of HemSCs. Velcade (Bortezomib), a proteosome inhibitor that can indirectly inhibit NF-κB, impaired HemSCs viability and expression of pro-angiogenic factors. Furthermore, specific inhibition of NF-κB resulted in suppression of VEGF-A.
Conclusions
We demonstrate expression of NF-κB target genes in proliferating IH. In addition, we show that the expression of several pro-angiogenic factors in HemSCs, and in particularVEGF–A, is regulated by NF-κB activity.
Keywords: Hemangioma-derived stem cells, infantile hemangioma, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), urokinase plasminogen activator receptor (uPAR), VEGF
Introduction
Infantile hemangioma (IH) is a common tumor composed of disorganized blood vessels and immature cells(1, 2). It has an incidence 5–7% in newborns of European descent; the prevalence is higher in females and in premature, low birth weight infants (3). Although a benign and usually harmless, some IHs cause deformation and destruction of facial features, and obstruction of vision and breathing. Many tumors result in permanent cutaneous residua, which can be disfiguring. The tumor has three distinctive clinical and histological phases. The proliferating phase is characterized by undeveloped blood channels interspersed with immature, proliferating cells. Transition to the involuting phase represents vascular differentiation; nascent channels develop into well-formed endothelial-lined and pericyte-invested blood vessels. In the final involuted phase, the tumor is replaced with fibro-fatty tissue with scattered capillaries and large draining vessels. The spontaneous regression of blood vessels is the defining feature of IH. In sum, hemangioma-genesis represents human vascular differentiation, followed by vascular regression and adipogenesis.
Hemangioma-derived stem cells (HemSCs) are multipotential cells isolated from specimens of human proliferating IH (4). HemSCs exhibit a mesenchymal morphology and robust proliferation when cultured in vitro. In contrast to endothelial cells (ECs) and endothelial progenitor cells isolated from IH, HemSCs form human blood vessels with the immunophenotype and dynamics of IH when injected subcutaneously into nude mice (4). Thus, HemSCs are the putative progenitors of IH.
Recently we showed that corticosteroids, the traditional first-line therapy for IH, inhibit the ability of HemSCs to form blood vessels in vivo by suppressing VEGF-A production by HemSCs. There was no effect on the secretion of VEGF-A from hemangioma-derived ECs or normal human cord blood-derived endothelial progenitor cells (cbEPCs) (5). A human angiogenesis antibody array enabled us to detect the expression of 43 different pro-angiogenic cytokines, and we identified four additional cytokines that are down-regulated by corticosteroids: uPAR,, MCP-1, IL-6 and matrix metalloproteinase-1 (MMP-1). A common feature of these cytokines is regulation by NF-κB(6–10).
The NF-κB family of transcription factors is known to regulate the expression of multiple genes involved in immune and stress responses, inflammation, cell proliferation and cell survival. Constitutive activation of NF-κB is associated with diseases of chronic inflammation and immune dysfunction as well as cancer (11, 12). NF-κB plays a complex role in angiogenesis. Although its activity was shown to be critical for maintaining endothelial cell survival (13–15), activation of NF-κB in endothelial cells induces expression of both angiogenic and angiostatic factors (16, 17). Endothelial repression of NF-κB has been shown to increase the size and vascularity of tumor metastases (18).
The role of NF-κB in the pathogenesis of IH and in the tumor’s response to corticosteroids is unknown. In the current work, we tested the expression levels several NF-κB targets in proliferating versus involuting IH. We also investigated the effect of corticosteroids on NF-κB activity in HemSCs. Finally, we tested the effect of NF-κB inhibition on the secretion of pro- angiogenic factors by HemSCs, and in particular VEGF-A.
Materials and Methods
Cell isolation and culture
Specimens of proliferating IH were obtained from the Department of Plastic Surgery, Children’s Hospital Boston, under a human subject protocol approved by the Committee on Clinical Investigation. The clinical diagnosis was confirmed in the Department of Pathology, Children’s Hospital Boston. Informed consent was obtained for use of the IH specimens, according to the Declaration of Helsinki. HemSCs were isolated as described previously (4). The derivation, sources, and culture conditions for the HemSCs and the other cells used in this study have been reported previously (4, 19, 20).
mRNA expression of NF-κB target genes in IH
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. A quantitative real-time reverse transcriptase–polymerase chain reaction (RT-qPCR) was done with the following primers
| Gene | Forward | Reverse |
|---|---|---|
| uPAR | TTGTGGGAAGAAGGAGAAGAG | AAATGCATTCGAGGTAAC |
| MCP-1 | CACCTGCTGTTATAACTTCAC | AATGGTCTTGAAGATCACAG |
| IL-6 | GAGAAGATTCCAAAGATGTAGCC | GCAAGTCTCCTCATTGAATCC |
| GAPDH | TGCACCACCAACTGCTTAG | GATGCAGGGATGATGTTC |
| CD31 | CAGTCAGAGTCATTCTTGCC | GTTGTTGGAGTTCAGAAGTGG |
Reactions were carried out for 35 cycles at an annealing temperature of 58°C.
Immunohistochemical and immunofluorescence analyses
Human-specific CD31 (DakoCytomation,) and uPAR immunostaining were performed as described (19, 21). uPAR/CD31 double immunofluorescence was done on cryosections and on HemSCs in vitro. Slides were fixed with acetone, blocked with 1% BSA and incubated with mouse anti-uPAR (American Diagnostica Inc., Stamford, CT, 1:100) over-night, followed by Alexa-Fluor 488-conjugated anti-mouse, and goat anti-human CD31 (Santa Cruz Biotechnology Inc.,Santa Cruz, CA) followed by biotinylated anti-goat and Streptavidin-Texas Red (1:200; Vector Laboratories, Burlingame CA).
Western blot for phosphorylated p65 and phosphorylated IκB
To test for dexamethasone effect on p65 phosphorylation, HemSCs were plated on 100mm dishes and incubated with dexamethasone (200nM) for 96 hours. To test for the effect of Velcade on IkB phosphorylation cells were plated on 60mm dishes and incubated with Velcade or dexamethasone for 24 or 72 hours. Treated cells and control, untreated cells were lysed and 50μg protein was separated by SDS-gel electrophoresis and transferred to a membrane. The membrane was blotted with anti-phosphorylated p65(Ser468) (rabbit, Cell Signaling, Beverly MA 1:1000), anti-phosphorylated-IκBα (Ser32/36) (mouse (clone 5A5) Cell Signaling or with anti-β actin (mouse, Sigma-Aldrich, St Louis, MO).
NF-κB DNA-Binding activity assay
Nuclear protein extracts were prepared using the NucBuster Protein Extraction Kit (Novagen, Madison, WI) according to the manufacturer’s instructions. DNA-binding activity of NF-κB was assayed colorimetrically, utilizing the NoShift Transcription Factor Assay Kit and NoShift NF-κB (p65) reagents (both Novagen) according to the manufacturer’s instructions.
Viability and proteosome activity assays
Velcade (Bortezomib) was a kind gift from Dr. Alfred Goldberg, Department of Cell Biology, Harvard Medical School. Drug was dissolved in DMSO and aliquots were kept at −20ºC. For proliferation assays, HemSCs and human bone marrow-derived mesenchymal stem cells (BM-MSCs) were plated at 4000cells/well in a 96-well plate. Twenty-four hours later, medium was replaced with EBM-2 containing Velcade at the indicated concentration. Cell viability was determined seventy-two hours later by CellTiter-Glo® (Promega, Madison, WI) according to the manufacturer’s instructions.
For measuring the chymotrypsin-like peptidase activity of the 20S proteasome, HemSCs were plated at 104 cells/well at 12-well dishes. Twelve hours later medium was replaced with the indicated concentrations of Velcade in fresh media. After seventy-two hours, the cells were lysed with RIPA buffer and 20μg of protein was added to each well of 96-well dark plate with LLVY buffer (Boston Biochem, Cambridge, MA #S-280). Fluorescence rate was measured by plate reader with λex 380nm: 460nm 37º 30 cycles.
HemSCs VEGF quantitative RT-PCR and ELISA
A RT-qPCR assay was used to measure VEGF-A mRNA (22). An enzyme-linked immunoassay (ELISA) was performed using Quantikine Human VEGF (R&D Systems, Inc., Minneapolis, MN).
Statistical Analysis
Data was analyzed by Mann-Whitney. Differences were considered significant at P < 0.05
Results
MCP-1, IL-6 and uPAR are differentially expressed in proliferating versus involuting IH
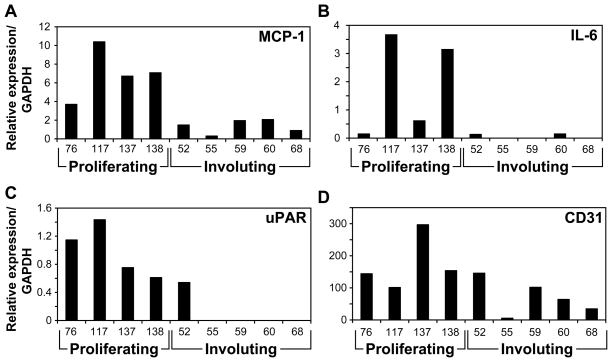
As an indirect measure of NF-κB activity in proliferating IH, we analyzed nine IH tissues, four proliferative and five involuting specimens, for the mRNA expression of three NF-κB target genes: MCP-1, uPAR, and IL-6. Clinical data on the IH patients with IH is provided in Table 1. RT-qPCR analyses revealed higher expression of all three genes in proliferating versus involuting IH (Figure 1A–C). The difference between the groups was statistically significant with p= 0.016, p=0.03 and p=0.016 for MCP-1, uPAR, and IL-6, respectively. CD31 expression analysis was done as a measure of the tissue endothelial content. As shown in Figure 1D, proliferating IH had higher CD31 expression than involuting IH (average of 173.9 vs. 70.3, respectively). However, the differences did not reach significance (p=0.09), indicating that the involuting tissues are still vascular. The differential expression of three NF-κB target genes in proliferating versus involuting IH suggested that NF-κB activity may play a role in IH vasculogenesis.
Table 1.
Clinical data for proliferating and involuting IH tissue samples used to analyze MCP-1 , IL-6, uPAR and CD31 expression.
| Patient number | Gender | Age | Location | Steroid treatment (systemic or local) | |
|---|---|---|---|---|---|
| Proliferating | 76 | F | 6.5 months | Upper eyelid | No |
| 117 | M | 9 weeks | Left shoulder | No | |
| 137 | F | 6 months | Behind L ear, proliferating | No | |
| 138 | F | 3 months | Upper right chest | No | |
| Involuting | 52 | F | 14 months | Forehead | No |
| 55 | M | 6 years | Upper lip | No | |
| 59 | F | 3.5 years | Lower lip | Yes | |
| 60 | F | 3.5 years | Upper lip | No | |
| 68 | F | 3 years | Forehead | No |
Figure 1.
mRNA expression of NF-κB target genes in proliferating and involuting IH tissues. RNA was extracted from homogenized IH tissues and tested by RT-qPCR for mRNA expression of MCP-1 (A), IL-6 (B), uPAR (C). CD31 expression (D) was used to meaure endothelial content of the specimen.
uPAR is expressed by HemSCs and in proliferating IH
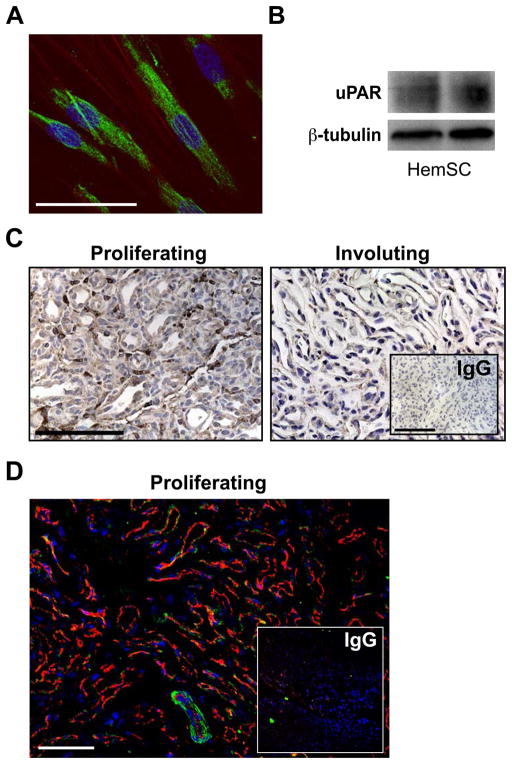
MCP-1 has been reported to be differentially expressed in proliferating versus involuting IH (23) and IL-6 was down-regulated following corticosteroid treatment in one patient (24). In contrast, uPAR expression has not been reported in IH. Thus, we focused on testing uPAR expression in IH tissues and HemSCs. uPAR is a multidomain cell surface glycoprotein that has been implicated in cellular growth, metastasis, and angiogenesis in several solid and hematologic malignancies (25–27). HemSCs expressed uPAR protein as demonstrated by immunofluorescence (Figure 2A) and by Western blot (Figure 2B). We then tested whether uPAR is expressed at the protein level in IH tissue specimens. We found a strong cellular staining in almost every vessel of the proliferating IH (Figure 2C). In contrast, involuting IH expressed very little uPAR (Figure 2C). To better localize uPAR expressing cells within the proliferating IH, we performed double-label immunofluorescence. uPAR expression was noted at the periphery of the vascular channels. There was only occasional co-localization with the endothelial marker CD31 (Figure 2D).
Figure 2.
uPAR expression in HemSCs and in proliferating versus involuting IH. Panel A, immunofluorescence of uPAR in HemSCs, scale bar: 50 μm. Nuclei are stained blue with DAPI, uPAR positive cells are stained green. Panel B, immunoblotting of HemSCs with anti-uPAR antibody. Panel C, anti-uPAR immunostaining shows uPAR in proliferating IH but not involuting IH. Scale bar: 100 μm. Panel D, immunofluorescence of uPAR (green) and CD31 (red) in a proliferating IH tissue section.. Nuclei are stained blue with DAPI. Scale bar: 100 μm.
Dexamethasone suppresses NF-κB activity of HemSCs
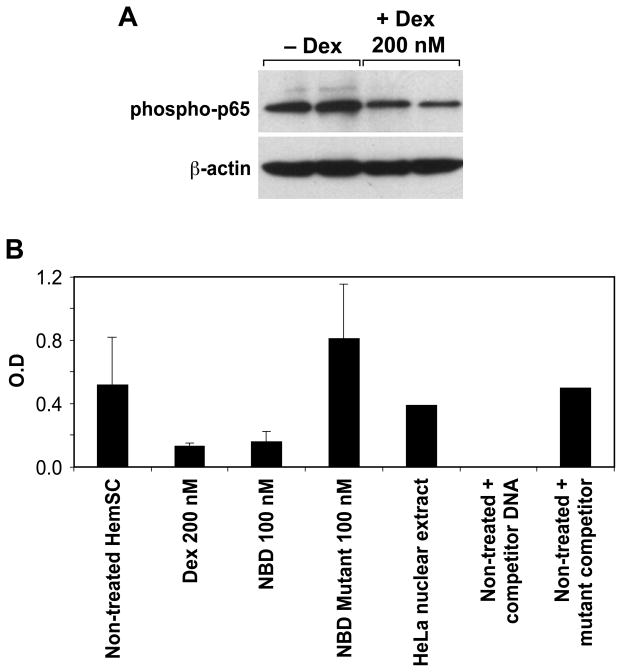
Our findings suggest increased NF-κB activity in proliferating versus involuting IH. We hypothesized that corticosteroids, the traditional pharmacological treatment for IH, work by suppressing this function, as this could account for reduced expression of VEGF-A, MCP-1, IL-6, uPAR and MMP-1 in HemSC treated with dexamethasone(5). Thus, we tested the effect of dexamethasone on NF-κB activity of HemSCs. First, we analyzed the expression of the phosphorylated p65(S468) subunit of NF-κB. The p50 (NFKB1)/p65 (RELA) heterodimer is the most abundant form of NF-κB(28). Phosphorylation of p65 by IKK at S468 occurs after TNF-α or IL-1β stimulation, mainly in the nucleus(29, 30), and thus this form represents a good correlate of activated p65. As shown in Figure 3A, dexamethasone (200 nM) down-regulated phosphorylated p65. Next, we directly assessed NF-κB DNA-complexing activity in nuclear extracts of HemSCs treated with dexamethasone by assaying the binding of NF-κB to oligonucleotides containing the consensus binding site (31, 32). We found that dexamethasone suppressed NF-κB binding activity by 73% (Figure 3B). As a control, we used HemSCs treated with NEMO-binding domain (NBD) peptide or mutant NBD. NBD is a selective inhibitor of NF-κB activation (33, 34).. Treatment of HemSCs with NBD, but not with mutant NBD, decreased NF-κB binding activity (Figure 3B). To assess sequence-specific binding activity, nuclear extracts of untreated cells were incubated with NF-κB wild-type DNA, with or without either specific NF-κB consensus binding motif or non-specific mutant NF-κB consensus-binding motif. Specific competitor DNA, but not the nonspecific binding motif, significantly reduced the binding activity, confirming sequence-specificity of the assay for NF-κB binding. HeLa cell nuclear extract provided a positive control for NF-κB binding activity. In summary, these results suggest that treatment of HemSCs with dexamethasone suppressed NF-κB activity.
Figure 3.
Dexamethasone suppression of NF-kB activity in HemSCs. Panel A, immunoblotting of HemSCs, treated or untreated with dexamethasone, with anti phosphorylated p65 antibody. Panel B, NF-κB DNA-complexing activity assay. Nuclear extracts of HemSCs, treated or untreated with dexamethasone were assayed for the binding to a NF-κB consensus binding site. Positive and negative controls are detailed in the Results section.
Velcade (Bortezomib) inhibits HemSCs proteosome activity and impairs HemSCs viability
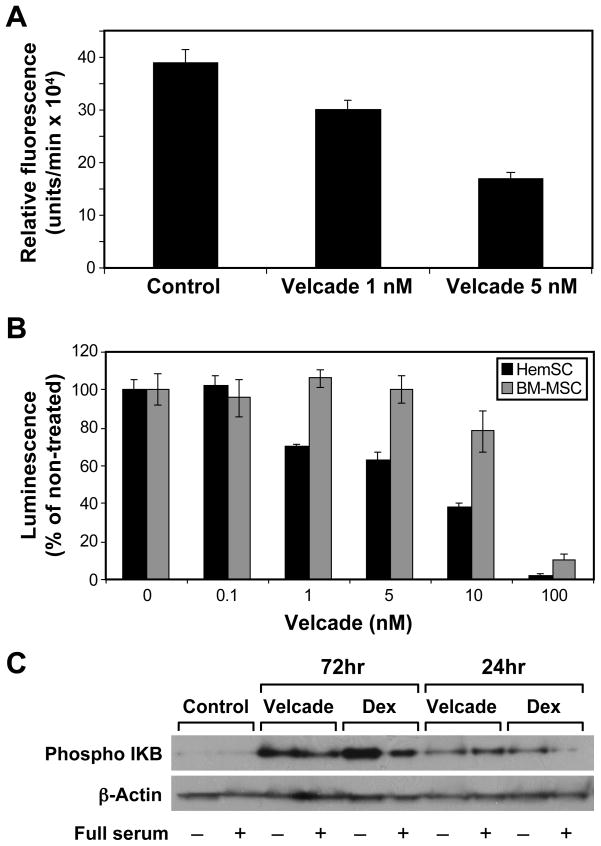
To study the role of NF-κB in HemSC survival and pro-angiogenic function, we used the selective proteosome inhibitor Velcade (Bortezomib) to block NF-κB activity. NF-κB is normally sequestered in the cytoplasm by the protein inhibitor of NF-κB (IκB). Velcade, by binding at high affinity to the proteasome, prevents IκB degradation. This allows IκB to accumulate in the cytoplasm and maintains NF-κB in its inactive state.(35, 36).(36). We verified the activity of Velcade on HemSCs by testing its chymotrypsin-like peptidase inhibitory effect of the 20S proteasome. Velcade, at 1nM and 5nM inhibited proteosome activity in HemSCs by 22.3% and 56.3%, respectively (Figure 4A). Subsequently, we tested the effect of Velcade on HemSCs viability. Velcade induces killing of tumor cells by diverse mechanisms, among them is the inhibition of the NF-κB pathway (37). Treatment of HemSCs with Velcade produced dose-dependent decrease in the cell viability. HemSCs were more sensitive to the cytotoxic effect of Velcade than BM-MSCs (Figure 4B). To verify that Velcade has an effect on NF-kB pathway in HemSCs, we analyzed the effect of Velcade on IκB phosphorylation. Velcade led to time-dependent increase in HemSC phosphorylated-IκB (p-IkB), similar to the effect of dexamethasone (Figure 4C).
Figure 4.
Velcade (Bortezomib) inhibits HemSCs proteosome activity and impairs HemSCs viability. Panel A, 20S proteasome chymotrypsin-like peptidase activity assay. HemSCs treated with Velcade, 1nM or 5nM, were lysed and assayed for 20S proteasome activity. Panel B, HemSCs treated with Velcade at the indicated doses were assayed for viability by CellTiter-Glo. Panel C, HemSCs were treated for 24 or 72 hours with Velcade (5nM) or with Dexamethasone (200nM) and then stimulated with or without medium containing full serum and growth factors. Cell lysates were subjected to Western blot analysis with anti-phosphorylated IκB and anti-β-actin.
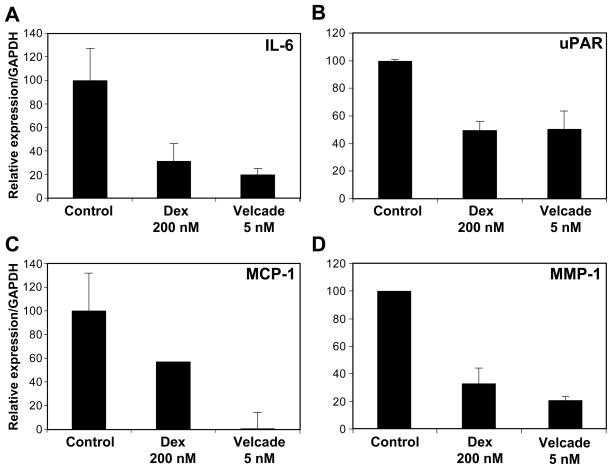
Velcade down-regulates the expression of MCP-1, uPAR, MMP-1 and IL-6 in HemSCs
We identified MCP-1, uPAR, MMP-1 and IL-6 as pro-angiogenic cytokines secreted by HemSCs and HemEC and regulated by corticosteroids (5). In order to investigate whether these cytokines are controlled by NF-κB in the specific cellular context of HemSCs, we tested the effect of Velcade on their expression in HemSCs. Consistent with our previous finding (5), dexamethasone led to a dose-dependent inhibition of MCP-1, uPAR, MMP-1 and IL-6 expression (Supplemental Figure 1). Similarly, by RT-qPCR, we found that Velcade down-regulated the expression of these cytokines in HemSCs (Figs. 5A–D).
Figure 5.
NF-κB inhibition by Velcade leads to down-regulation of IL-6, uPAR, MCP-1 and MMP-1 in HemSCs. HemSCs treated with Velcade (5nM) were assayed by RT-qPCR for the mRNA expression of IL-6 (A), uPAR (B), MCP-1 (C) and MMP-1 (D). Dexamethasone (Dex) (200nM) served as a positive control.
VEGF-A expression by HemSCs is regulated by NF-κB
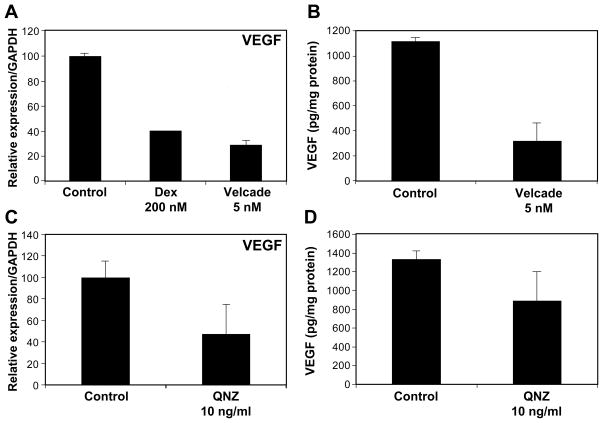
VEGF-A has been shown to be required for formation of hemangioma-like vessels in the murine model (5). Indeed, HemSCs secrete large amounts of VEGF-A, which is markedly suppressed by corticosteroids. We tested the effect of NF-κB inhibition on VEGF-A expression in HemSCs: Velcade treatment (5nM) down-regulated VEGF-A mRNA expression (Figure 6A). Similar results were observed at the protein level by ELISA (Figure 6B). Velcade, being a proteosome inhibitor, could exert its effect on VEGF by mechanisms other than direct NF-κB activity inhibition. In order to verify that NF-κB inhibition is involved, we used the specific NF-κB inhibitor 6-Amino-4-(4-phenoxyphenylethylamino) quinazoline (QNZ) (38). Similar to the effect of Velcade, QNZ suppressed VEGF-A in HemSCs at both the mRNA (Figure 6C) and the protein (Figure 6D) levels. In sum, these findings suggest that VEGF expression in HemSCs is regulated by NF-κB.
Figure 6.
VEGF-A expression by HemSCs is regulated by NF-κB. Panel A, HemSCs treated with Velcade (5nM) for three days were assayed by RT-qPCR for VEGF-A transcripts. Panel B, an ELISA for VEGF-A protein was performed on conditioned media from HemSCs treated with Velcade. Panel C, HemSCs treated with quinazoline (QNZ), a specific inhibitor of NF-κB activity, were assayed for VEGF-A mRNA by RT-qPCR. Panel D, HemSCs treated with QNZ activity, were assayed for VEGF-A protein by ELISA.
Discussion
Our results indicate that NF-κB target genes are over-expressed in the proliferating versus the involuting phase of IH. In addition we show that corticosteroids suppress NF-κB activity in HemSCs and that this suppression leads to down-regulation of the pro-angiogenic cytokines MCP-1, uPAR, MMP-1 and IL-6. Moreover, we demonstrate that VEGF-A, a critical factor in IH, is regulated by NF-κB activity.
We found over-expression of MCP-1, uPAR and IL-6 in proliferating versus involuting IH. VEGF-A and urokinase plasminogen activator (uPA) are also NF-κB target genes previously reported to be differentially expressed(5, 39). Also, several genes that activate NF-κB have been shown to be over-expressed in proliferating versus involuting IH. Among them are: αvβ3 and αvβ1 integrins (40–42); Tumor necrosis factor (ligand) superfamily member 10 (TRAIL) (43, 44); VE-Cadherin(40, 45) ; Platelet-derived growth factor receptor β (PDGFR β) (40, 46). These findings suggest a role for the NF-κB system in the pathogenesis of IH. Further studies that employ direct activation or silencing of NF-κB in vivo are essential in order to establish a causative role for this signaling pathway in IH.
uPAR was found to be differentially expressed in proliferating versus involuting IH and to be a target of dexamethasone in HemSCs. The one involuting IH specimen that had detectable uPAR was in the early involuting phase, taken from a 14 month old infant. Our observation suggests a role for uPAR in the response to corticosteroids, as well as in natural involution. uPAR is a glycosylphosphatidylinositol anchored glycoprotein that is expressed by many tissues, especially during physiological and pathological tissue remodeling processes (26, 47). It has been reported to regulate cell adhesion, migration, proliferation, survival, and angiogenesis, through interaction with an array of proteins, including extracellular matrix molecules, cell adhesion receptors, G-protein coupled receptors, and tyrosine kinase receptors (26, 47). The uPAR ligand, the serine protease urokinase plasminogen activator (uPA), was shown to be expressed in both proliferating and involuting IH (39). Future knockdown or over-expression studies will be required to shed light on the function of uPAR in the life cycle of IH.
Dexamethasone suppressed NF-κB activity in HemSCs. Glucocorticoid effects are mediated through the intracellular glucocorticoid receptor (GR), which binds cognate ligands and regulates target gene expression positively or negatively. Distinct mechanisms are probably responsible for GR regulation of NF-κB activities (48). First, a physical interaction between NF-κB subunits and GR, similar to that shown for AP-1, has been reported (49) . Second, glucocorticoids have been demonstrated to induce transcription of the NF-κB inhibitor, IκBα (50). Finally, it has also been demonstrated that both NF-κB and GR can compete for interactions with coactivators, such as p300 and CREB-binding protein (CBP), which are present in limited amounts (51, 52). The occurrence and relative role of each mechanism seems to depend on both cellular and promoter contexts. Thus, it is of interest to further delineate the critical mechanisms responsible for the suppression of pro-angiogenic factors in HemSCs.
We have shown by different lines of evidence that the expression VEGF-A, a critical growth factor for IH vasculogenesis, is regulated by NF-κB in HemSCs. Our observations are in agreement with reports demonstrating a similar control process in other cells (53–56).. The molecular mechanisms leading to VEGF transcriptional control by NF-κB are largely elusive. A recent report demonstrated a critical role for NF-κB-induced JunB in VEGF regulation and tumor angiogenesis (57).
In summary, our findings of NF-κB target gene expression in IH and NF-κB regulation of VEGF-A expression by IH-derived stem cells may point to a role of this system in the IH pathogenesis. In an era of newly emerging, novel NF-κB –targeting drugs (58), these insights may lead to a new pharmacological therapy for IH.
Supplementary Material
Acknowledgments
We thank Debajit K. Biswas, Department of Cancer Biology, Dana-Farber Cancer Institute, Harvard Medical School, for invaluable advice and for providing NBD peptide for initial experiments. Supported by a NIH R01 HL096384 (JB), the Talpiot Medical Leadership Program, Sheba Medical Center, Israel (S.G.), Harvard Skin Diseases Pilot Study Grant (S.G.), and the John Butler Mulliken Foundation.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Mulliken J. Diagnosis and natural history of hemangiomas. In: Mulliken YAJB, editor. Vascular birthmarks: hemangiomas and malformations. Philadelphia: WB Saunders; 1988. [Google Scholar]
- 2.Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- 3.Frieden IJ, Eichenfield LF, Esterly NB, Geronemus R, Mallory SB. Guidelines of care for hemangiomas of infancy. American Academy of Dermatology Guidelines/Outcomes Committee. J Am Acad Dermatol. 1997;37:631–637. doi: 10.1016/s0190-9622(97)70183-x. [DOI] [PubMed] [Google Scholar]
- 4.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Dang J, Wang H, Allgayer H, Murrell GA, Boyd D. Identification of a novel nuclear factor-kappaB sequence involved in expression of urokinase-type plasminogen activator receptor. Eur J Biochem. 2000;267:3248–3254. doi: 10.1046/j.1432-1327.2000.01350.x. [DOI] [PubMed] [Google Scholar]
- 7.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 8.Karst AM, Gao K, Nelson CC, Li G. Nuclear factor kappa B subunit p50 promotes melanoma angiogenesis by upregulating interleukin-6 expression. Int J Cancer. 2009;124:494–501. doi: 10.1002/ijc.23973. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Hozawa S, Nakamura T, Nakano M, Adachi M, Tanaka H, Takahashi Y, Tetsuya M, Miyata N, Soma H, Hibi T. Induction of matrix metalloproteinase-1 gene transcription by tumour necrosis factor alpha via the p50/p50 homodimer of nuclear factor-kappa B in activated human hepatic stellate cells. Liver Int. 2008;28:1418–1425. doi: 10.1111/j.1478-3231.2008.01883.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Gaynor RB. Role of the NF–kappaB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- 13.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- 16.Tabruyn SP, Griffioen AW. A new role for NF-kappaB in angiogenesis inhibition. Cell Death Differ. 2007;14:1393–1397. doi: 10.1038/sj.cdd.4402156. [DOI] [PubMed] [Google Scholar]
- 17.Aurora AB, Biyashev D, Mirochnik Y, Zaichuk TA, Sanchez-Martinez C, Renault MA, Losordo D, Volpert OV. NF-{kappa}B balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2010;116:475–484. doi: 10.1182/blood-2009-07-232132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 20.Khan ZA, Melero-Martin JM, Wu X, Paruchuri S, Boscolo E, Mulliken JB, Bischoff J. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood. 2006;108:915–921. doi: 10.1182/blood-2006-03-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roelofs JJ, Rowshani AT, van den Berg JG, Claessen N, Aten J, ten Berge IJ, Weening JJ, Florquin S. Expression of urokinase plasminogen activator and its receptor during acute renal allograft rejection. Kidney Int. 2003;64:1845–1853. doi: 10.1046/j.1523-1755.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 22.Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038–1045. doi: 10.1158/0008-5472.CAN-06-2295. [DOI] [PubMed] [Google Scholar]
- 23.Isik FF, Rand RP, Gruss JS, Benjamin D, Alpers CE. Monocyte chemoattractant protein-1 mRNA expression in hemangiomas and vascular malformations. J Surg Res. 1996;61:71–76. doi: 10.1006/jsre.1996.0083. [DOI] [PubMed] [Google Scholar]
- 24.Hasan Q, Tan ST, Gush J, Peters SG, Davis PF. Steroid therapy of a proliferating hemangioma: histochemical and molecular changes. Pediatrics. 2000;105:117–120. doi: 10.1542/peds.105.1.117. [DOI] [PubMed] [Google Scholar]
- 25.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–659. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 26.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 27.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–5655. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 28.Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2009;116:451–465. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- 29.Schwabe RF, Sakurai H. IKKbeta phosphorylates p65 at S468 in transactivaton domain 2. FASEB J. 2005;19:1758–1760. doi: 10.1096/fj.05-3736fje. [DOI] [PubMed] [Google Scholar]
- 30.Mattioli I, Geng H, Sebald A, Hodel M, Bucher C, Kracht M, Schmitz ML. Inducible phosphorylation of NF-kappa B p65 at serine 468 by T cell costimulation is mediated by IKK epsilon. J Biol Chem. 2006;281:6175–6183. doi: 10.1074/jbc.M508045200. [DOI] [PubMed] [Google Scholar]
- 31.Bredel M, Bredel C, Juric D, Duran GE, Yu RX, Harsh GR, Vogel H, Recht LD, Scheck AC, Sikic BI. Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24:274–287. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345–351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 33.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 34.Strickland I, Ghosh S. Use of cell permeable NBD peptides for suppression of inflammation. Ann Rheum Dis. 2006;65(Suppl 3):iii, 75–82. doi: 10.1136/ard.2006.058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 36.Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci. 2009;100:24–28. doi: 10.1111/j.1349-7006.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Hayashi H. A novel structural class of potent inhibitors of NF-kappa B activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg Med Chem. 2003;11:3869–3878. doi: 10.1016/s0968-0896(03)00438-3. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter MR, Dorrell MI, Edmonds J, Friedlander SF, Friedlander M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci U S A. 2002;99:7455–7460. doi: 10.1073/pnas.102185799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein S, de Fougerolles AR, Blaikie P, Khan L, Pepe A, Green CD, Koteliansky V, Giancotti FG. Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation. Mol Cell Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T, Hersey P. The role of NF-kappa B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol. 2001;166:5337–5345. doi: 10.4049/jimmunol.166.9.5337. [DOI] [PubMed] [Google Scholar]
- 44.Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174:1638–1649. doi: 10.2353/ajpath.2009.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrington-Myers J, Gao X, Kouklis P, Broman M, Rahman A, Vogel SM, Malik AB. Regulation of lung neutrophil recruitment by VE-cadherin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L764–771. doi: 10.1152/ajplung.00502.2005. [DOI] [PubMed] [Google Scholar]
- 46.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 47.Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types. APMIS. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 48.Tao Y, Williams-Skipp C, Scheinman RI. Mapping of glucocorticoid receptor DNA binding domain surfaces contributing to transrepression of NF-kappa B and induction of apoptosis. J Biol Chem. 2001;276:2329–2332. doi: 10.1074/jbc.C000526200. [DOI] [PubMed] [Google Scholar]
- 49.Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- 50.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 51.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 52.Lee SK, Kim HJ, Na SY, Kim TS, Choi HS, Im SY, Lee JW. Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits. J Biol Chem. 1998;273:16651–16654. doi: 10.1074/jbc.273.27.16651. [DOI] [PubMed] [Google Scholar]
- 53.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 54.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002;73:237–243. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 55.Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 56.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt D, Textor B, Pein OT, Licht AH, Andrecht S, Sator-Schmitt M, Fusenig NE, Angel P, Schorpp-Kistner M. Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis. EMBO J. 2007;26:710–719. doi: 10.1038/sj.emboj.7601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.