Abstract
Objective:
Major histocompatibility complex (MHC) genes dominate genetic susceptibility factors in multiple sclerosis (MS). Given the general consensus that incidence and prevalence of MS has been rising and specifically in women, we evaluated MHC–gender interactions.
Methods:
In a large family-based cohort consisting of 7,093 individuals (2,127 affected individuals) from 1,055 MS families, we examined MHC transmission by family structure and gender stratified by genetic distance of affected relatives from the MS proband.
Results:
We found that affected individuals with HLA-DRB1*15-positive genotypes have higher female-to-male ratios as compared with affected individuals with HLA-DRB1*15-negative genotypes (χ2 = 9.97, p = 0.0015) with the exception of multiplex families with 3 or more affected across 2 generations. Transmission disequilibrium test results show that HLA-DRB1*15 transmission was more distorted in collateral families vs nuclear families (χ2 = 8.030, p = 0.0046), exclusively in affected female-female pairs (χ2 = 7.81, p = 0.0051), but not in mixed gender pairs (χ2 = 1.58, p = 0.21) or matched male pairs (Fisher p = 0.21).
Conclusions:
These observations implicate the MHC as the site of interactions and modifications mediating the female-to-male gender ratio in MS and its progressive increase. They further suggest this occurs via gene–environment interactions and epigenetic modifications in this region. The difference between collateral and nuclear families provides some insight into the inheritance, decay, and gender specificity of putative epigenetic marks.
The etiology of multiple sclerosis (MS) remains unclear; however, several lines of evidence suggest that MS is an acquired autoimmune disease and is triggered by environmental factors in genetically susceptible individuals.1
The main genetic contribution in MS comes from the major histocompatibility complex (MHC), and more specifically the human leukocyte antigen (HLA) Class II genes.2,3 Additional epistatic interactions and suppressor effects have also been observed within this locus.4,5
There is general consensus that the incidence and prevalence of MS has been rising with an increased penetrance among women,6 and excess monozygotic (MZ) twins concordance for MS is almost entirely female specific.7 Moreover, there is a maternal parent-of-origin effect8 with higher number of affected mother-daughter pairs and few father-son pairs.9
There is little known about how the MHC might interact with the observed gender bias in MS. Since HLA alleles carry the strongest genetic susceptibility factors, we reasoned that the reported gender predisposition could be MHC-mediated.10 Here we have used a large family-based cohort containing a range of relative pairs concordant for MS, which are characterized by their genetic distance from the MS proband. These affected relative pairs share a common genetic background, and are more homogeneous regarding exposure to environmental factors, which loom large in MS risk. They can also be differentiated by gender combinations that are either matching (female-female or male-male) or mixed (female-male), which are useful for dissecting gender-specific effects in MS susceptibility.
METHODS
Subjects.
We selected a large family-based cohort (family n = 1,055, total individual n = 7,093, affected individual n = 2,127) consisting of 6 different types of MS families (based on their family structure and affected relatives of MS proband), for which the methodology has been described previously.11 The 6 different types of MS families included affected sibling pair (ASP: family n = 358, total individual n = 2,935, affected individual n = 716), parent-child (PC: family n = 172, total individual n = 806, affected individual n = 344), sporadic (family n = 73, total individual n = 171, affected individual n = 73), aunt/uncle–niece/nephew (AUNN: family n = 217, total individual n = 1,671, affected individual n = 434), cousin (family n = 198, total individual n = 1,202, affected individual n = 396), and multiplex (MPX: family n = 37, total individual n = 308, affected individual n = 164). All families were Canadian and of European descent.
Parents, children, and full siblings share approximately 50% of their genes and are referred to as first-degree relatives. Aunts, uncles, nieces, and nephews share, on average, 25% of their genes and are referred to as second-degree relatives. First cousins share around 12.5% of their genes and are referred to as third-degree relatives. In this study, the selected families can be categorized into 2 types by the affected relative of the MS proband. They include nuclear families (n = 603) with affected first-degree relatives consisting of ASP, PC, and sporadic families, and collateral families (n = 452) with affected second- and third-degree relatives consisting of AUNN, cousin, and MPX families.
Nuclear (n = 530) and collateral (n = 415) families can be further stratified by the gender combinations of affected relative pairs; however, because of the family structure of sporadic and MPX families, they were excluded. Female-female pairs include affected daughter-daughter pairs in ASP families, affected mother-daughter pairs in PC families, affected aunt-niece pairs in AUNN families, and affected female-female cousins. Female-male pairs consist of affected daughter-son pairs in ASP families, affected mother-son pairs and father-daughter pairs in PC families, affected aunt-nephew pairs and uncle-niece pairs in AUNN families, and affected female-male cousins. Male-male pairs include affected son-son pairs in ASP families, affected father-son pairs in PC families, affected uncle-nephew pairs in AUNN families, and affected male-male cousins.
Standard protocol approvals, registrations, and patient consents.
This study obtained ethical approval from the relevant institutional review boards and written informed consent was received from all subjects.
HLA typing.
Genotyping of HLA-DRB1 was performed using either low- or high-resolution allele-specific PCR amplification method.4
Statistical methods.
The family pedigrees were first tested using the PEDCHECK program for the presence of any inconsistencies in Mendelian transmission.12 Transmission disequilibrium test (TDT) was performed using the TDTPHASE program of the UNPHASED software package.13
RESULTS
Gender ratio analysis.
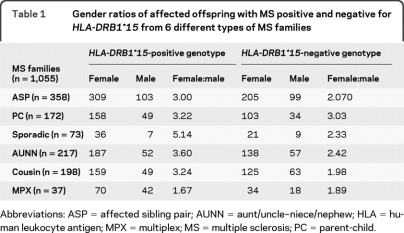
The gender ratio results of affected individuals either positive or negative for HLA-DRB1*15 are presented in table 1. The total ratio for all 6 types of MS families show that of cases with HLA-DRB1*15-positive genotypes, 919 were female and 302 were male (female:male = 3.04). Of cases with HLA-DRB1*15-negative genotypes, 626 were female and 280 were male (female:male = 2.24). The female-to-male ratio is higher in cases with HLA-DRB1*15 vs those without (χ2 = 9.97, p = 0.0015).
Table 1.
Gender ratios of affected offspring with MS positive and negative for HLA-DRB1*15 from 6 different types of MS families
Abbreviations: ASP = affected sibling pair; AUNN = aunt/uncle–niece/nephew; HLA = human leukocyte antigen; MPX = multiplex; MS = multiple sclerosis; PC = parent-child.
HLA-DRB1*15 transmission.
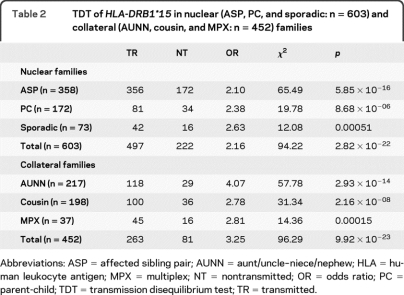
TDT analyses of HLA-DRB1*15 in nuclear (ASP, PC, and sporadic) and collateral (AUNN, cousin, and MPX) families are presented in table 2. The combined transmission of HLA-DRB1*15 in nuclear families was 497 times transmitted and 222 times not transmitted (χ2 = 94.22, p = 2.82 × 10−22). The pooled transmission of HLA-DRB1*15 in collateral families was 263 times transmitted and 81 times not transmitted (χ2 = 96.29, p = 9.92 × 10−23). Transmission of HLA-DRB1*15 was more distorted in collateral families vs nuclear families (χ2 = 6.27, p = 0.012).
Table 2.
TDT of HLA-DRB1*15 in nuclear (ASP, PC, and sporadic: n = 603) and collateral (AUNN, cousin, and MPX: n = 452) families
Abbreviations: ASP = affected sibling pair; AUNN = aunt/uncle–niece/nephew; HLA = human leukocyte antigen; MPX = multiplex; NT = nontransmitted; OR = odds ratio; PC = parent-child; TDT = transmission disequilibrium test; TR = transmitted.
HLA-DRB1 transmissions (from HLA-DRB1*15-negative parents).
To remove the confounding effect of the dominant HLA-DRB1*15 association, which results in secondary undertransmissions of other HLA-DRB1 alleles from the heterozygous parents, transmissions of other HLA-DRB1 alleles were examined from parents negative for HLA-DRB1*15. Because of the rarer nature of some of the HLA-DRB1 alleles in comparison with the more frequent HLA-DRB1*15 allele, their combined transmissions in the pooled nuclear families vs pooled collateral families were presented. No significant differences in the transmissions of other HLA-DRB1 alleles in the absence of HLA-DRB1*15 were observed between the nuclear vs collateral families (table e-1 on the Neurology® Web site at www.neurology.org).
HLA-DRB1 transmissions by gender of affected relative pairs.
The HLA-DRB1 transmission analyses by affected relative pairs are presented in table 3. Difference of HLA-DRB1*15 transmission was observed between nuclear vs collateral families for the total affected relative pairs (χ2 = 8.030, p = 0.0046). This difference was most evident in affected female-female pairs (χ2 = 7.81, p = 0.0051), but not in affected female-male (χ2 = 1.58, p = 0.21) and affected male-male pairs (Fisher p = 0.21). Transmissions of other HLA-DRB1 alleles from HLA-DRB1*15-negative parents were also examined (table e-2).
Table 3.
Comparisons of HLA-DRB1*15 transmission stratified by gender of affected relative pairs in nuclear (ASP and PC: n = 530) and collateral (AUNN and cousin: n = 415) families
Abbreviations: ASP = affected sibling pair; AUNN = aunt/uncle–niece/nephew; HLA = human leukocyte antigen; NT = nontransmitted; OR = odds ratio; PC = parent-child; TR = transmitted.
Fisher exact test was used when the expected transmissions in any of the cells of the table were below 10.
DISCUSSION
In high prevalence areas, some 20% of MS cases have at least one affected relative.14 Risks are increased in first-degree relatives including parents, offspring, and siblings (2.0%–5.7% for relatives of female index cases; 2.5%–5.1% for relatives of male index cases), and also in distant relatives such as aunts, uncles, nieces, nephews, and cousins (1.0%–2.9% for relatives of female index cases; 1.5%–3.3% for relatives of male index cases).14 MS risk for half-siblings who have one parent in common and share a quarter of their genome is 1.9%.8 The risk for offspring of conjugal MS (both parents have MS) is as much as 200 times more than that of the general population,15 while the risk for adoptees is no different from that of the general population.16 The inheritance pattern of MS shows that PC risk (3.5%) is roughly equal to that of sibling risk (3.5%); however, the risks are diluted thereafter, with the half-sibling risk approximately half of the full-sibling risk. These observations show a pseudodominant inheritance pattern with almost no dominant variance. The rapid dilution of risk in descendants of patients with MS has suggested epigenetic marks and their subsequent decay in vertical transmission as one possible explanation.
The female-to-male ratio was near unity in North America at the turn of the 20th century,17 and today, a ratio of 2:1 is common despite regional variations. In Canada this ratio has been increasing for at least 60 years, and now surpasses 3.2:1,6 while in Scotland a change in sex ratio from unity to more than 3:1 has taken place since the 1950s.18 The exact cause of this increase remains unknown, but given the short duration over which this rise occurs, genetic factors can be ruled out and environmental changes would be the likely candidate, perhaps resulting from gene–environment interactions.6 Since HLA genes are the main genetic contributor to MS susceptibility, we hypothesized that gender-discrepant HLA-associated effects are possible. By using a large family-based cohort, we were able to address this question.
Gender ratio analysis revealed that affected individuals with HLA-DRB1*15 have a significantly higher female-to-male ratio as compared to those without. Most of the 6 types of MS families seem to share the same trend of HLA-associated female predominance in MS susceptibility. MPX families appear to be an anomaly, in which the gender ratio is actually lower in affected individuals positive for HLA-DRB1*15 as compared with those negative (1.67 vs 1.89, not significant). The reason for this discrepancy is yet to be determined but indicates genetic heterogeneity in these families. In MS, families with more than 3 or 4 affected cases and pedigrees with more than 2 or 3 consecutive affected generations are uncommon.19 Future research is needed with a special focus on these large multigenerational family pedigrees containing multiple affected individuals.
TDT results found that transmission of HLA-DRB1*15 is significantly more distorted in collateral families vs nuclear families. This finding suggests that differential transmission of the same haplotype in families with affected first-degree relatives vs those consisting of second- and third-degree relatives reflects the inheritance of putative epigenetic marks. In families with extended relatives concordant for MS, haplotype-specific epigenetic modifications could be more conserved (vertical vs collinear transmission). Differential risk carried by other HLA-DRB1 haplotypes could also exist. However, because of the rarer nature of these haplotypes and perhaps their effect size as compared with the more frequent HLA-DRB1*15, it will require much larger numbers of typed families to assess the patterns.
We further stratified the nuclear and collateral families by gender of affected relative pairs, which not only takes into account gender-specific effects, but also the degree of genetic sharing and environmental exposures. We found a significant increase of risk carried by HLA-DRB1*15 in collateral pairs vs nuclear pairs, which was female-female pair specific, but not in mixed gender pairs or matched male pairs. We have previously shown gender-specific intergenerational differences of HLA-DRB1*15 frequency in AUNN pairs, supported by the finding that MS nieces were more numerous and had a higher HLA-DRB1*15 allele frequency than their own affected aunts, clearly localizing gene–environment interactions mediated by putative epigenetic mechanisms to the MHC region.20 The results here further suggest that the molecular explanation for the increased penetrance of MS among women will have roots in female gender-specific epigenetic modifications of HLA Class II haplotypes.
How environmental factors interact with the genome to influence MS risk is being determined. There are now 5 discrete insights relevant to this interaction which include the maternal parent-of-origin effects,21 month of birth effects,22 presence of vitamin D responsiveness in a susceptibility gene,23 and transgenerational differences in allele frequency.20 Here, we add a fifth interaction with gender ratio. All of these localize to the MHC.
The MHC has been associated with nearly every autoimmune disease,24 and female predominance has been recognized for the majority of them.25 An earlier example reported gender ratios in HLA-related Sjögren syndrome with a strong association of HLA-DRB1*03 in female cases.26 Although studies have proposed the role of sex hormones in this bias,27 results here implicate epigenetics within the MHC as central to this propensity.
This study investigated gender-dependent inheritance of a genetic locus, but did not examine gender-dependent inheritance of epigenetic marks within the MHC region. An epigenetic mark that directly modifies the DNA is methylation, which has been associated with human autoimmunity.28 The determination of methylation differences in the MHC has been challenging because of single nucleotide polymorphisms (SNPs) being present every 5 to 10 base pairs. Furthermore, the relationship between genetic and epigenetic polymorphisms remains unclear. Two studies searched for possible links between SNPs and DNA methylation, and found that DNA sequence differences rarely affect methylation.29,30 However, a more recent study reported that the presence of CpGs at SNPs influences local DNA methylation status in cis.31 Given that the MHC is the most polymorphic region in the human genome plus the known effects of DNA methylation on mutation rate,32,33 there could be a unique interdependence between genetics and epigenetics within this region underlying disease susceptibility.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and their families who participated in this study.
- ASP
- affected sibling pair
- AUNN
- aunt/uncle–niece/nephew
- HLA
- human leukocyte antigen
- MHC
- major histocompatibility complex
- MPX
- multiplex
- MS
- multiple sclerosis
- MZ
- monozygotic
- PC
- parent-child
- SNP
- single nucleotide polymorphism
- TDT
- transmission disequilibrium test
Editorial, page 210
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Michael Chao.
DISCLOSURE
M. Chao reports no disclosures. Dr. Ramagopalan receives research support from the Multiple Sclerosis Society of Canada Scientific Research Foundation and the Multiple Sclerosis Society of the United Kingdom. Dr. Herrera and Dr. Orton report no disclosures. L. Handunnetthi has received support from the Medical Research Council UK (PhD studentship). Dr. Lincoln and Dr. Dyment report no disclosures. Dr. Sadovnick has received funding for travel and speaker honoraria from Bayer Canada, Teva Neurosciences, EMD Serono, Inc., and Biogen Idec; and has received research support from the MS Society of Canada Scientific Research Foundation. Dr. Ebers serves on the editorial boards of the International Multiple Sclerosis Journal and Multiple Sclerosis; has received a speaker honorarium from Roche; served as a consultant to UCB; and receives research support from Bayer Schering Pharma, the Multiple Sclerosis Society of the United Kingdom, and the Multiple Sclerosis Society of Canada Scientific Research Foundation.
REFERENCES
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000; 343: 938–952 [DOI] [PubMed] [Google Scholar]
- 2. Chao MJ, Barnardo MC, Lincoln MR, et al. HLA class I alleles tag HLA-DRB1*1501 haplotypes for differential risk in multiple sclerosis susceptibility. Proc Natl Acad Sci USA 2008; 105: 13069–13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lincoln MR, Montpetit A, Cader MZ, et al. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat Genet 2005; 37: 1108–1112 [DOI] [PubMed] [Google Scholar]
- 4. Dyment DA, Herrera BM, Cader MZ, et al. Complex interactions among MHC haplotypes in multiple sclerosis: susceptibility and resistance. Hum Mol Genet 2005; 14: 2019–2026 [DOI] [PubMed] [Google Scholar]
- 5. Ramagopalan SV, Morris AP, Dyment DA, et al. The inheritance of resistance alleles in multiple sclerosis. PLoS Genet 2007; 3: 1607–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 2006; 5: 932–936 [DOI] [PubMed] [Google Scholar]
- 7. Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA 2003; 100: 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebers GC, Sadovnick AD, Dyment DA, Yee IM, Willer CJ, Risch N. Parent-of-origin effect in multiple sclerosis: observations in half-siblings. Lancet 2004; 363: 1773–1774 [DOI] [PubMed] [Google Scholar]
- 9. Sadovnick AD, Bulman D, Ebers GC. Parent-child concordance in multiple sclerosis. Ann Neurol 1991; 29: 252–255 [DOI] [PubMed] [Google Scholar]
- 10. Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol 2008; 7: 268–277 [DOI] [PubMed] [Google Scholar]
- 11. Sadovnick AD, Risch NJ, Ebers GC. Canadian collaborative project on genetic susceptibility to MS, phase 2: rationale and method: Canadian Collaborative Study Group. Can J Neurol Sci 1998; 25: 216–221 [DOI] [PubMed] [Google Scholar]
- 12. O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 1998; 63: 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25: 115–121 [DOI] [PubMed] [Google Scholar]
- 14. Sadovnick AD, Baird PA, Ward RH. Multiple sclerosis: updated risks for relatives. Am J Med Genet 1988; 29: 533–541 [DOI] [PubMed] [Google Scholar]
- 15. Ebers GC, Yee IM, Sadovnick AD, Duquette P. Conjugal multiple sclerosis: population-based prevalence and recurrence risks in offspring: Canadian Collaborative Study Group. Ann Neurol 2000; 48: 927–931 [PubMed] [Google Scholar]
- 16. Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis: Canadian Collaborative Study Group. Nature 1995; 377: 150–151 [DOI] [PubMed] [Google Scholar]
- 17. Brain WR. Critical review: disseminated sclerosis. Q J Med 1930; 23: 343–391 [Google Scholar]
- 18. Murray S, Bashir K, Penrice G, Womersley SJ. Epidemiology of multiple sclerosis in Glasgow. Scott Med J 2004; 49: 100–104 [DOI] [PubMed] [Google Scholar]
- 19. Dyment DA, Cader MZ, Willer CJ, Risch N, Sadovnick AD, Ebers GC. A multigenerational family with multiple sclerosis. Brain 2002; 125: 1474–1482 [DOI] [PubMed] [Google Scholar]
- 20. Chao MJ, Ramagopalan SV, Herrera BM, et al. Epigenetics in multiple sclerosis susceptibility: difference in transgenerational risk localizes to the major histocompatibility complex. Hum Mol Genet 2009; 18: 261–266 [DOI] [PubMed] [Google Scholar]
- 21. Ramagopalan SV, Herrera BM, Bell JT, et al. Parental transmission of HLA-DRB1*15 in multiple sclerosis. Hum Genet 2008; 122: 661–663 [DOI] [PubMed] [Google Scholar]
- 22. Ramagopalan SV, Link J, Byrnes JK, et al. HLA-DRB1 and month of birth in multiple sclerosis. Neurology 2009; 73: 2107–2111 [DOI] [PubMed] [Google Scholar]
- 23. Ramagopalan SV, Maugeri NJ, Handunnetthi L, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 2009; 5: e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet 2008; 4: e1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med 1994; 96: 457–462 [DOI] [PubMed] [Google Scholar]
- 26. Foster H, Kelly C, Griffiths I. Sex ratios in HLA related autoimmune disease. Ann Rheum Dis 1991; 50: 969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2001; 2: 777–780 [DOI] [PubMed] [Google Scholar]
- 28. Strickland FM, Richardson BC. Epigenetics in human autoimmunity: epigenetics in autoimmunity: DNA methylation in systemic lupus erythematosus and beyond. Autoimmunity 2008; 41: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerkel K, Spadola A, Yuan E, et al. Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nat Genet 2008; 40: 904–908 [DOI] [PubMed] [Google Scholar]
- 30. Schalkwyk LC, Meaburn EL, Smith R, et al. Allelic skewing of DNA methylation is widespread across the genome. Am J Hum Genet 2010; 86: 196–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hellman A, Chess A. Extensive sequence-influenced DNA methylation polymorphism in the human genome. Epigenetics Chromatin 2010; 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horton R, Wilming L, Rand V, et al. Gene map of the extended human MHC. Nat Rev Genet 2004; 5: 889–899 [DOI] [PubMed] [Google Scholar]
- 33. Zingg JM, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis 1997; 18: 869–882 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.